Abstract
The exact pathophysiology of the spaceflight-associated neuro-ocular syndrome (SANS) has so far not been completely elucidated. In this study we assessed the effect of acute head-down tilt position on the mean flow of the intra- and extracranial vessels. Our results suggest a shift from the external to the internal system that might play an important role in the pathomechanism of SANS.
Similar content being viewed by others
Introduction
The human cardiovascular system evolved to cope with the challenges imposed by terrestrial gravity. In healthy persons, cardiovascular control mechanisms maintain brain perfusion with changes in posture1. Moreover, fluid distribution between the vascular compartment and brain tissue is balanced, thus, preventing edema formation and intracranial pressure surges. Observations in astronauts affected by the spaceflight-associated neuro-ocular syndrome (SANS) suggest that this fine balance may be perturbed when humans leave terrestrial gravity for extended durations. SANS is associated with structural changes in the brain and optic disc edema consistent with cephalad fluid overload2,3. The condition is a major unresolved challenge to long-duration space missions. While neck vein congestion has been reported during spaceflight, suggesting an imbalance between arterial inflow and venous drainage4, however, the exact mechanisms and possible relationship to SANS are not fully understood. Strict head-down tilt (HDT) bedrest reproduces cephalad fluid shifts and optic disc edema akin to SANS5. A previous study showed reduced internal carotid artery flow following strict HDT bedrest6 with differences in the time course between persons with and without SANS-like responses. Therefore, we tested the effects of acute supine and HDT bedrest on, both, internal and external carotid and jugular flow to determine the effects of gravitational loading on arterial and venous circulation.
Methods
We measured cerebral blood flow in 11 healthy men in a cross-over designed study to test the effects of 21-hour bedrest in the −12° HDT posture versus supine posture (0°) (clinicaltrials.gov; identifier NCT 02976168). The study was approved by the ethics committee of the regional medical board (Ärztekammer Nordrhein). In accordance with the Declaration of Helsinki all subjects gave written informed consent before participation.
We chose −12° HDT because previous studies had shown that −12° HDT has stronger impact on cerebral perfusion than the more frequently used -6° HDT18. Prior to the 21-hour observation interval, subjects sojourned for one day and one night in the laboratory (:envihab facility, German Aerospace Center, Cologne, Germany) to allow for accommodation.
We measured blood flow in the vertebral arteries, internal and external jugular veins, and in the internal and external carotid arteries by cine phase contrast MRI (Voxel size: 0.8 × 0.6 × 4.0 mm, position: Isocenter) using a Biograph mMR 3-Tesla scanner (Siemens, Erlangen, Germany) with a 16-channel head-neck coil. We obtained measurements at baseline (supine posture), and at 1 and 21 h of exposure to bedrest in either the supine or −12° HDT posture. Then, we segmented vessels and quantified flows with the cvi42 software (www.circlecvi.com) and with custom-made Python scripts. For statistical analyses, we ran linear mixed effect models using the package nlme in R (www.r-project.org), with time and condition as fixed effects and subject ID as random effect. Mean flow rates comprised of the average of both sides for each vessel and timepoint, respectively. Additional mixed-effect models were fitted to accommodate any potential flow lateralization to the right or left vessel, as well as a side prioritization over time. Repeated within-subject identification of the external jugular veins was impeded due to the tortuous anatomy of the vessel in the investigated plane. They were, therefore, not included in the analysis.
Results
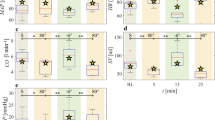
The mean flow rate (Table 1) in the external carotid arteries was stable during supine bedrest (baseline: 239.6 ± 18.7 ml/min; 1 h: 260.1 ± 21.1 ml/min, P = 0.34; 21 h: 249.1 ± 23.1 ml/min, P = 0.68). During −12° HDT bedrest, however, external carotid arterial flow increased by ~40% (1 h: 324.3 ± 20.5 ml/min, P < 0.001; 21 h: 343.9 ± 23.2 ml/min, P < 0.001). Internal jugular venous outflow was relatively stable during supine bedrest with a decrease on the second day (baseline: 480.0 ± 81.6 ml/min; 1 h: 455.4 ± 84.0 ml/min, P = 0.45; 21 h: 398.9 ± 83.8 ml/min, P = 0.01), however, it was elevated during −12° HDT bedrest (1 h: 589.6 ± 83.4 ml/min P < 0.001; 21 h: 612.5 ± 84.3 ml/min, P < 0.001). Mean blood flow of the internal carotid arteries was decreased during both body postures in this study (baseline: 490.7 ± 16.7 ml/min; 1 h −12° HDT: 453.1 ± 17.8 ml/min, P < 0.001; 21 h −12° HDT: 448.8 ± 18.3 ml/min, P < 0.001; 1 h 0° HDT: 457.0 ± 18.0 ml/min, P = 0.003; 21 h 0° HDT: 441.1 ± 18.0 ml/min, P < 0.001). The mean vertebral arterial flow was neither affected by HDT nor supine position (baseline: 173 ± 34.4 ml/min; 1 h −12° HDT: 171.9 ± 35.0 ml/min, P = 0.99; 21 h −12° HDT: 167.1 ± 35.3 ml/min, P = 0.99; 1 h 0° HDT: 171.2 ± 35.2 ml/min, P = 0.99; 21 h 0° HDT: 168.2 ± 35.2 ml/min, P = 0.99). The linear mixed model for lateralization showed main effects of side for the internal carotid artery and the internal jugular vein (P = 0.016 and P = 0.0090, respectively), yet no significant interaction effect (P > 0.80). This indicates that the mechanisms involved are not lateralized. Neither main nor interactions effects could be observed for the external carotid artery.
Discussion
Overall, our study suggests that HDT bedrest, a terrestrial model for weightlessness7, increases external carotid flow (Fig. 1). We propose a possible explanation for venous return, the outflow maybe simultaneously increased via the external jugular vein. As previously described, the minimum cross-sectional area of the external jugular vein, the minimum and maximum cross-sectional area of the internal jugular vein were significantly increased at −12° HDT during this study8. One possible explanation of these findings is a shifting from the superficial carotid system towards the internal jugular veins (Fig. 1). Cerebral arterial inflow is tightly regulated by autoregulatory mechanisms, which helps to balance intracranial volume and pressure according to the Monroe-Kelly boundary conditions. Yet, autoregulation in the external carotid arteries may be less effective at offsetting negative gravitational loading during HDT9 and possibly spaceflight. Increased blood flow in the external carotid arteries is compensated at least partly by increased internal jugular venous outflow. However, bypassing of internal carotid autoregulation may result in additional strain on jugular outflow, which is already hampered due the HDT-induced headward hydrostatic pressure gradient. This mechanism might also result in localized intraorbital effects due to a potential collateralization between the A. angularis and A. ophtalmica as it can be observed in patients with internal carotid artery stenosis10. While this effect could be one of the underlying causes for SANS, flow lateralization was not observed in our study, thus not supporting the asymmetric optic disc swelling associated with SANS3.
The numbers in panel a and b indicate the difference in flow in each respective position in comparison to the baseline data. The external system comprises the external carotid (red). The internal system includes the internal carotid artery (red) and jugular vein (blue). Algebraic signs refer to the physiological flow direction of each vessel. Figure panels c and d show anatomical MRI images of one of the subjects on the first interventional day in each of the respective body positions. †P < 0.05; *P < 0.001 (linear mixed effect models were used for statistical analysis). Panel a and b created with BioRender.com).
While we did not observe an effect of HDT on the frontal tissue thickness8, the puffy face observed in astronauts11 and long-term bedrest studies might be a direct consequence of the identified altered fascio-cerebral perfusion: Cerebral autoregulation ensures a cerebral tissue perfusion at arteriole level under varying pressure conditions12. Yet, the combined effect of headward volume and tissue shifting might negatively affect the autoregulatory compensation mechanisms. Thus, a flow shift towards the external system might countervail the increased blood volume, resulting in facial edema while maintaining cerebral autoregulation and adequate cerebral perfusion.
Further physiological effects caused by HDT, such as cardio-respiratory adaptions13,14 intertwine with cerebral perfusion. In addition, the general HDT-induced volume shift itself might play an important role in the observed flow rates. The increased diameter of the internal jugular vein along with the altered blood flow characteristics might suggest an increase in intracranial venous pressure, which is bound to reduce the absorption of cerebrospinal fluid and flow15,16. Since cerebrospinal fluid bulk flow is closely linked to venous flow17, the increase in internal jugular blood volume might also play a role in the pathophysiology of optic disc edema development during spaceflight and strict HDT bedrest.
Data availability
The raw data set was generated at the German Aerospace Centre (DLR), Cologne. The anonymized data supporting the findings of this study can be requested from the corresponding author AL Boschert.
References
Gelinas, J. C. et al. Influence of posture on the regulation of cerebral perfusion. Aviat. Space Environ. Med. 83, 751–757 (2012).
Lee, A. G. et al. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: a review and an update. npj Microgravity 6, 1–10 (2020).
Mader, T. H. et al. An overview of spaceflight-associated neuro-ocular syndrome (SANS). Neurol. India 67, 206 (2019).
Rosenberg, M. J. et al. Comparison of dural venous sinus volumes before and after flight in astronauts with and without spaceflight-associated neuro-ocular syndrome. JAMA Netw. Open 4, e2131465–e2131465 (2021).
Laurie, S. S. et al. Optic Disc Edema after 30 days of strict head-down tilt bed rest. Ophthalmology 126, 467–468 (2019).
Roberts, D. R. et al. Altered cerebral perfusion in response to chronic mild hypercapnia and head-down tilt Bed rest as an analog for Spaceflight. Neuroradiology 63, 1271–1281 (2021).
Ong, J., Lee, A. G. & Moss, H. E. Head-down tilt bed rest studies as a terrestrial analog for spaceflight associated neuro-ocular syndrome. Front. Neurol. 12, 648958 (2021).
Boschert, A. L. et al. Sleep is compromised in −12° head down tilt position. Front. Physiol. 10, 397 (2019).
Ogoh, S., Sato, K., de Abreu, S., Denise, P. & Normand, H. Arterial and venous cerebral blood flow responses to long-term head-down bed rest in male volunteers. Exp. Physiol. 105, 44–52 (2020).
Ogoh, S., Sato, K., de Abreu, S., Denise, P. & Normand, H. Effect of jump exercise training on long-term head-down bed rest-induced cerebral blood flow responses in arteries and veins. Exp. Physiol. 106, 1549–1558 (2021).
Kirsch, K. A. et al. Fluid shifts into and out of superficial tissues under microgravity and terrestrial conditions. Clin. Investig. 71, 687–689 (1993).
Silverman, A. & Petersen, N. H. Physiology, Cerebral Autoregulation. In StatPearls (StatPearls Publishing, 2022).
Segizbaeva, M. O., Pogodin, M. A., Lavrova, I. N., Balykin, M. V. & Aleksandrova, N. P. Effect of head-down tilt on respiratory responses and human inspiratory muscle activity. Hum. Physiol. 37, 171–177 (2011).
Traon, P.-L., Heer, M., Narici, M. V., Rittweger, J. & Vernikos, J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986– 2006). Eur. J. Appl. Physiol. 101, 143–194 (2007).
Zahid, A. M., Martin, B., Collins, S., Oshinski, J. N. & Ethier, C. R. Quantification of arterial, venous, and cerebrospinal fluid flow dynamics by magnetic resonance imaging under simulated micro-gravity conditions: a prospective cohort study. Fluids Barriers CNS 18, 1–9 (2021).
Kramer, L. A. et al. Intracranial effects of microgravity: a prospective longitudinal MRI study. Radiology 295, 640–648 (2020).
Dreha-Kulaczewski, S. et al. Identification of the upward movement of human CSF in vivo and its relation to the brain venous system. J. Neurosci. 37, 2395–2402 (2017).
Marshall-Goebel, K. et al. Effects of short-term exposure to head-down tilt on cerebral hemodynamics: a prospective evaluation of a spaceflight analog using phase-contrast MRI. J. Appl. Physiol. 120, 1466–1473 (2016).
Acknowledgements
We thank the whole team of the Muscle and Bone Metabolism Department, Institute of Aerospace Medicine, German Aerospace Centre Cologne for preparation and conduct of the study. Additionally, we are much obliged to the study participants. Without them, the study would not have been possible. The study was funded by the German Aerospace Centre—Institute of Aerospace Medicine Department: Space Physiology.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to data analysis, interpretation, and manuscript finalization. Hypothesis development, study design, and conduction were carried out by A.L.B., P.G., B.J., Z.L., D.E., A.B., D.G., J.Z., and J.R. P.G. and J.R. supervised the study. A.L.B. wrote the first draft of the manuscript and designed the graphs.
Corresponding author
Ethics declarations
Competing interests
JJ has served as a consultant for Novartis, Boehringer-Ingelheim, and Novo-Nordisk and is co-founder of Eternygen GmbH (Modest relationship). All other authors have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boschert, A.L., Gauger, P., Bach, A. et al. External to internal cranial perfusion shifts during simulated weightlessness: Results from a randomized cross-over trial. npj Microgravity 9, 25 (2023). https://doi.org/10.1038/s41526-023-00267-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41526-023-00267-2