Abstract
The combination of atezolizumab and nab-paclitaxel is recommended in the EU as first-line treatment for PD-L1-positive metastatic triple-negative breast cancer (mTNBC), based on the results of phase III IMpassion130 trial. However, ‘real-world’ data on this combination are limited. The ANASTASE study (NCT05609903) collected data on atezolizumab plus nab-paclitaxel in PD-L1-positive mTNBC patients enrolled in the Italian Compassionate Use Program. A retrospective analysis was conducted in 29 Italian oncology centers among patients who completed at least one cycle of treatment. Data from 52 patients were gathered. Among them, 21.1% presented de novo stage IV; 78.8% previously received (neo)adjuvant treatment; 55.8% patients had only one site of metastasis; median number of treatment cycles was five (IQR: 3–8); objective response rate was 42.3% (95% CI: 28.9–55.7%). The median time-to-treatment discontinuation was 5 months (95% CI: 2.8–7.1); clinical benefit at 12 months was 45.8%. The median duration of response was 12.7 months (95% CI: 4.1–21.4). At a median follow-up of 20 months, the median progression-free survival was 6.3 months (95% CI: 3.9–8.7) and the median time to next treatment or death was 8.1 months (95% CI: 5.5–10.7). At 12 months and 24 months, the overall survival rates were 66.3% and 49.1%, respectively. The most common immune-related adverse events included rash (23.1%), hepatitis (11.5%), thyroiditis (11.5%) and pneumonia (9.6%). Within the ANASTASE study, patients with PD-L1-positive mTNBC treated with first-line atezolizumab plus nab-paclitaxel achieved PFS and ORR similar to those reported in the IMpassion130 study, with no unexpected adverse events.
Similar content being viewed by others
Introduction
Triple-negative breast cancer (TNBC) represents 15–20% of all breast cancers (BCs); it is characterized by the lack of expression of estrogen receptor (ER), progesterone receptor (PR), and the absence of HER2 gene amplification1,2. Compared with other BC subtypes, TNBCs are often histologically high-grade tumors characterized by strong invasiveness and higher rates of relapse and mortality3,4. Unlike other BC subtypes that harbor therapeutic targets, such as ER or HER2, in the metastatic TNBC (mTNBC) subtype, systemic chemotherapy remains the standard of care. However, new targeted therapies, such as PARP inhibitors, immune checkpoint inhibitors (ICIs), and antibody−drug conjugates (e.g., sacituzumab−govitecan or trastuzumab deruxtecan in HER2 low BC) are now available5,6,7,8.
However, patients with metastatic mTNBC have a median overall survival (OS) of less than 18 months with standard chemotherapy, making mTNBC a clinical challenge to treat, highlighting the need for more effective targeted therapies or combinations6,9,10,11,12.
TNBC is more likely to have increased expression of the PD-L1 in the tumor microenvironment, making it an ideal candidate for targeted therapy with ICIs6,12,13,14. Initial trials with ICIs in BC were conducted as monotherapy, but because of the limited benefit observed, research shifted to testing combinatorial approaches5. In particular, the combination of atezolizumab and nab-paclitaxel, within the randomized phase III IMpassion130 study, demonstrated a benefit for patients with mTNBC and PD-L1-positive by Ventana SP142 assay; in particular, the study met its co-primary progression-free survival (PFS) endpoint in the intention-to-treat population and patients with PD-L1-positive in ≥1% immune cells (IC+). Improved activity of a such combination was observed only in patients whose tumors were PD-L1-positive, and in these patients, a clinically meaningful OS improvement was also observed15. On these bases, the combination of atezolizumab and nab-paclitaxel has been approved in Europe as a first-line treatment option for PD-L1-positive unresectable locally advanced or mTNBC16, thus setting a new standard of care.
However, ‘real-world’ data on both the efficacy and safety of this combination are limited. To fill this gap, we designed the multicentre, real-world ANASTASE study, which aimed to evaluate the therapeutic effectiveness and safety of atezolizumab plus nab-paclitaxel in a cohort of Italian patients with PD_l1-positive metastatic or unresectable locally advanced TNBC enrolled in the Compassionate Use Program (CUP).
Results
Patient characteristics
Data from 52 patients were gathered. The clinical features of the study population are summarized in Table 1. The median age at the initial diagnosis was 52 years (IQR: 45–63 years), and 65.4% (n = 34) of patients were in postmenopause. At diagnosis, 11 patients (21.1%) presented de novo stage IV, and BRCA mutation was identified in eight patients (15.4%) among 37. Of the 41 (78.8%) non-metastatic patients at their first diagnosis, most of them had previously received neoadjuvant (n = 15, 36.6%) or adjuvant (n = 18, 43.9%) or both (n = 7, 17.1%) treatments, including a taxane-based and anthracycline-based chemotherapy regimen in 65.4% and 69.2% of cases, respectively; the median disease free-interval was 20 months (IQR: 13–50). Concerning the number and the site of metastases at the diagnosis of metastatic disease, 29 (55.8%) patients had only one site of metastasis, six (11.5%) had three or more metastatic sites, 31 (59.6%) had visceral metastases, and two (3.8%) had brain metastases.
Exposure to drugs
Treatment exposure and features are summarized in Table 2. The median number of nab-paclitaxel and atezolizumab cycles was five (IQR: 2–6 cycles) and six (IQR: 3–8), respectively. Overall, 50 (96.2%) patients discontinued nab-paclitaxel, and 45 (86.5%) patients discontinued atezolizumab, mainly due progressing disease in 66% and 80% of cases, respectively. Treatment discontinuation rates due to AEs were 14% and 13.3% for nab-paclitaxel and atezolizumab, respectively. Regarding maintenance, eight patients (15.4%) received atezolizumab monotherapy for a median number of six cycles (IQR: 3–8). No differences in terms of baseline characteristics were reported between patients receiving atezolizumab maintenance therapy compared with no maintenance treatment (Supplementary Table 1). A trend for patients with single metastatic site in favour of receiving atezolizumab maintenance compared to multiple metastatic sites was reported (Supplementary Table 1). Finally, eight patients (15.4%) were still on treatment at the analysis time.
Activity results
All the patients were evaluable for time to treatment discontinuation (TTD), while a total of 48 patients out of 52 were evaluable for response (two patients were not evaluable due to missing data, and two patients did not complete the first treatment cycle due to AEs). Response outcomes are reported in Table 3. The objective response rate (ORR) was obtained in 22 patients (42.3%; 95% CI: 28.9–55.7%), including 5.8% (n = 3) of complete response and 36.5% (n = 19) of partial response. A total of 16 (30.8%) patients had progressive disease. Stable disease was reported in 10 (19.2%) patients.
The median TTD was 5 months (95% CI: 2.8–7.1 months; Fig. 1A) for the overall population. There was no difference in terms of median TTD between patients treated with or without previous anthracyclines regimens in early disease (5 months [95% CI: 2.5–7.5 months) and 4.9 months (95% CI: 0–10.9 months). The clinical benefit at 6 and 12 months was 54.2% and 45.8%, respectively.
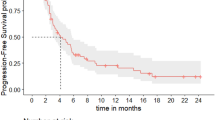
A Kaplan–Meier curve of the time to treatment discontinuation (TTD) of anastase study population. Median TTD was 5.0 months (95%CI: 2.8–7.1). B Kaplan–Meier curve of the progression free survival (PFS) of anastase study population. Median PFS was 6.3 months (95%CI: 3.9–8.7). C Kaplan–Meier curve of the time to next treatment or death (TNT-D) of anastase study population: Median TNT-D was 8.1 months (95%CI: 5.5–10.7).
Concerning the DoR, the median time was 12.7 months (95% CI: 4.1–21.4 months) with a median cycle to best response of 3 months (95% CI: 1–7 months) (Table 3).
Efficacy results
At a median follow-up of 20 months (IQR: 16–24 months), the median PFS was 6.3 months (95% CI: 3.9–8.7 months) (Fig. 1B). When analyzing the TNT-D, the median time was 8.1 months (95% CI: 5.5–10.7 months) (Fig. 1C). At 12 months and 24 months, the OS rates were 66.3% and 49.1% (Supplementary Fig. 1), respectively. No statistically significant difference was reported in terms of ORR, PFS, and TNT-D according to different subgroups such as stage at diagnosis, number and type of metastatic sites, BRCA1–2 mutational status, disease-free interval, ECOG PS, HER2 status (0 vs low) and previous treatment (Supplementary Tables 2–4).
Outcome after atezolizumab plus nab-paclitaxel progression
Among the 43 patients with progressive disease after atezolizumab plus nab-paclitaxel, thirty-one patients (59.6%) received second-line therapy; among them, eight (25.8%) and seven (22.6%) patients received regimens including carboplatin and capecitabine, respectively. Median PFS on second-line therapy was 6.9 months (IQR: 3.1–10.7 months). The second-line treatments are summarized in Table 4.
Safety
All 52 patients were available for safety. The most common AEs of any grade included neutropenia (57.7%), anemia (53.8%), lymphocytopenia (46.2%), asthenia (46.2%), liver toxicity (40.4%), nausea and vomiting (32.7%) (Table 5).
The most common potential immune-related AEs of any grade included: rash (23.1%), thyroiditis (11.5%), hepatitis (11.5%) and pneumonia (9.6%). Grade 3 or 4 of these events occurred only in one patient with hepatitis (Table 5).
Discussion
Our paper reports data on atezolizumab plus nab-paclitaxel in PD-L1-positive mTNBC patients enrolled in the Italian Compassionate Use Program within the ANASTASE study. Tumor heterogeneity and the long-standing paucity of effective therapies other than chemotherapy have contributed to TNBC being the subtype with the least favorable outcomes3,4,17. In recent years, advances in -omics technologies have shed light on the relevance of the TNBC microenvironment heterogeneity, unveiling a close dynamic relationship with cancer cell features17. In particular, TNBC resulted as the most immunogenic BC subtype, with higher PD-L1 expression levels and more tumor-infiltrating lymphocytes18,19,20. These assumptions have led to the development of novel targeted agents, including ICIs, revolutionizing the therapeutic landscape and providing new therapeutic opportunities6.
Patients with PD-L1-positive TNBC are the most likely to benefit from ICIs21. Notably, the IMpassion130 trial established the utility of adding atezolizumab to nab-paclitaxel as the first-line treatment for mTNBC, with most of the clinical benefit realized in the PD-L1-positive subgroup15,22. On this basis, the results of the IMpassion130 trial led to the accelerated approval of atezolizumab in combination with nab-paclitaxel for patients with unresectable locally advanced tumors or mTNBC whose tumors express PD-L1. This represents a major breakthrough in BC treatment because of the novelty of immunotherapy in BC and the improved outcome benefit compared with chemotherapy alone16. Despite atezolizumab indication has been withdrawn from the USA given negative findings in the IMpassion 131 trial, the combination of atezolizumab and nab-paclitaxel is currently authorized in Europe as a first-line treatment for PD-L1-positive mTNBC16.
Within our cohort, 19% of patients were de novo metastatic. Compared with the IMpassion130 study, a higher percentage of patients (65% ANASTASE vs 51% IMpassion130 study) had adjuvant therapy with taxanes; otherwise, the disease burden was similar, with most patients reporting between zero and three metastatic sites15. We observed an ORR of 42.3%, which is a lower rate than in the IMpassion130 study (58.9%) (Supplementary Table 5)15. TTD, describing the period from the treatment initiation to discontinuation or death, has been proposed as a potential effectiveness endpoint for real-world studies where imaging assessment is less structured and standardized23. In our study, the median TTD was 5 months (95%, CI: 2.8–7.1), slightly shorter than the median PFS (6.3 months; 95% CI: 3.9–8.7 months). These data align with recent findings from a patient-level correlation analysis comprising 18 clinical trials in advanced non-small-cell lung cancer23. This analysis showed that with ICI therapy, the median PFS is slightly longer than the median TTD, with both early and late TTD cases23. This suggests that, in some cases, patients terminated the ICI treatment because of immune-mediated AEs and continued to have sustained benefits after treatment discontinuation.
Comparable results in terms of PFS (6.3 vs 7.5 months) were observed with the IMpassion130 trial (Supplementary Table 5)15. Otherwise, a longer median DoR was observed in our study, compared with the IMpassion130 (12.7 months vs 8.5) (Supplementary Table 5)15. The selection of the most treatment-responsive patients remains unresolved to date, and the definition of biomarkers to optimize both patient and treatment selection is still an unmet need24. As mentioned above, a recent sub-study from the IMpassion130 trial reported that a clinical benefit was observed only in PD-L1 IC+ patients. However, the combination treatment was more efficacious in patients with richer tumor immune microenvironments22. Therefore, our finding supports the observation that patients sensitive to combined treatment have a prolonged benefit, highlighting the importance of patient selection to improve the clinical benefit of treatments.
The analysis of TNT-D represents interesting information in a population with poor prognosis after progression from first-line treatment, such as triple-negative patients. In particular, an attractive characteristic of TNT-D is its ability to capture the treatment-free interval from the end of index therapy to the date of initiation of a subsequent line of treatment or death25. Within the ANASTASE study, we analyze the TNT-D in a TNBC population treated with ICI for the first time, reporting a median of 8.1 months (95% CI: 5.5–10.7 months). These data further support the benefit of ICI therapy through a prolonged treatment effect in clinical practice. At 12 months and 24 months, the OS rates were 66.3% and 49.1%, respectively.
In 2020, the FDA granted accelerated approval of pembrolizumab, another PD-1 inhibitor, in combination with chemotherapy for locally recurrent unresectable and metastatic PD-L1-positive TNBC, based on the results of phase III Keynote-355 trial26. In particular, this trial showed a statistically significant PFS benefit with the addition of pembrolizumab to chemotherapy in patients with a combined positive score ≥10. This benefit was more pronounced if pembrolizumab was associated with a taxane regimen26. However, PD-L1 positivity was defined by two different tests in the IMpassion130 and Keynote-355 trials: Ventana SP142 and Dako 22C3 assays, respectively. Utilizing the Dako 22C3 assay to select PD-L-1-positive tumors on the biobank from IMpassion130, considering a combined positive score ≥10, the median OS was 22 months with atezolizumab versus 18.7 months (hazard ratio, HR: 0.77), compared with 25 months with atezolizumab versus 18 months (HR: 0.71) via SP142 assay27. For these reasons, when using the Ventana SP142 assay, atezolizumab and nab-paclitaxel are recommended as standard of care for patients with mTNBC whose tumors have a ≥1% PD-L1-positive score in Europe28. In particular, this combination represents the only first-line treatment registered and reimbursed in Italy for TNBC patients with PD-L1 ≥ 1% (Ventana SP142 assay). Consistent with observations from other atezolizumab–chemotherapy combination trials, no unexpected AEs were observed15,29,30. Of note, previous literature evidence has shown that the unique spectrum of AEs associated with ICIs requires supplementary monitoring and treatment practices more than those required for chemotherapy31. In our cohort, no high incidence of severe toxicity was reported; one patient reported severe hepatitis leading to treatment discontinuation. Only two cases of grade 2 pneumonia were observed: one achieved after the first cycle, treated with antibiotic and steroid therapy, and recovered in 1 month without further treatment interruption; the other patient presented this AE after five cycles of therapy and temporarily stopped treatment until there was an improvement to grade 1.
This study presents some limitations, such as the relatively small sample size and the retrospective and real-world nature. However, the compassionate use programs, such as the ANASTASE study, are characterized by some relevant values: offer a controlled system of access to new experimental drugs before the commercialization and completely outside of clinical trial, commonly to patients with life‐threatening diseases and with no therapeutic options or in case of highly active drugs also in early therapeutic approach32. Furthermore, the compassionate use can also permit to clinicians to have more confidence with new drugs or regimens in terms of toxicity management. Since the ANASTASE study represented the first experience for clinicians of the compassionate use of immunotherapy in TNBC, toxicity management was not based on previous clinical experiences. Moreover, our study provides real-world evidence, which is of increasing interest to support clinical decision-making, as it could fill gaps by supporting the generation of evidence for subsequent indications, optimal dosing, and studying special populations, as well as providing information on the management of toxicities in the manner and scope of the real-world clinical practice23. On this regard, a series of previous compassionate use experience has been reported in the context of BC32,33,34. We would like also to specify that although the CUP excluded all patients with TFI <12 months, in relation to the inclusion and exclusion criteria of the IMpassion130, it was considered important to also include patients with TFI <12 months in the ANASTASE study to gather preliminary information on the activity of combination in the real world in this subsetting of patients. The combination of atezolizumab plus chemotherapy in the first-line mTNBC PD-L1-positive patients with DFI <12 months is also currently being studied in the prospective phase III study Impassion 132 (NCT03371017).
Recently, the Keynote-522 study showed that neoadjuvant pembrolizumab plus chemotherapy, followed by adjuvant pembrolizumab after surgery, resulted in significantly longer event-free survival than neoadjuvant chemotherapy alone in early TNBC35. To date, the combination of pembrolizumab and chemotherapy is the standard of care for high-risk, early-stage TNBC, regardless of PD-L1 status. However, the neoadjuvant use of pembrolizumab and chemotherapy results, upon disease progression, in a degree of uncertainty regarding the use of ICI in the first line, due to the lack of data on patients progressing after neoadjuvant therapy with ICI and treated in the first-line setting with this regime. Consequently, new data from this patient setting are awaited.
Our findings suggest that PD-L1- positive mTNBC patients treated with first-line atezolizumab plus nab-paclitaxel substantially achieved, in a ‘real-word’ context, a similar PFS to that reported in the IMpassion130 study, despite a lower response rate. The combination of atezolizumab and nab-paclitaxel appeared safe, with no unexpected AEs. A longer median DoR was observed in our study, compared with the IMpassion130, highlighting the importance of patient selection to improve the clinical benefit of the treatment. In addition, we provided the first evaluation of TTD and TNT-D, suggesting that ICI may find benefits even after treatment discontinuation in clinical practice.
Patients and methods
Study design and setting
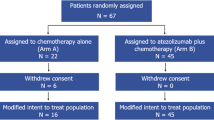
ANASTASE study was a retrospective, multicenter, observational trial conducted in 29 Italian oncology centers to evaluate the therapeutic effectiveness and safety of the combination of atezolizumab plus nab-paclitaxel in a real-life context. The study involved PD-L1-positive metastatic or locally advanced TNBC adult patients who completed at least the first cycle of atezolizumab and nab-paclitaxel treatment within the CUP AL41712 (active from November 2019 to August 2020). No prior chemotherapy, experimental or targeted systemic therapy for mTNBC was allowed. Prior chemotherapy (including anthracyclines and taxanes) in the neoadjuvant or adjuvant setting was allowed if treatment was completed ≥12 months prior to the start of atezolizumab plus nab-paclitaxel treatment. Atezolizumab plus nab-paclitaxel were administered as follows: atezolizumab 840 mg intravenous (iv), on days 1 and 15 associated with nab-paclitaxel 100 mg/m2 iv on days 1, 8, and 15, every 28 days, at the same dose and frequency as the IMpassion130 study15. The treatment continued until disease progression, unacceptable toxicity, or a patient’s or physician’s request to discontinue. Grade 3 or 4 toxic effects were managed by dose modifications. Concomitant treatments that did not interfere with both drugs, including the use of bisphosphonates, were admitted. The study was conducted in accordance with the ethical standards of the Declaration of Helsinki and its subsequent amendments and within the protocol approved by the ethics committee of Fondazione Policlinico Universitario A Gemelli IRCCS of Rome (Italy; protocol number 25493/22). All participants provided written informed consent to the use of medical records for research purposes. ClinicalTrials.gov identifier: NCT05609903.
Study measures
The primary objectives were to describe the overall population, including patients who completed at least the first cycle of treatment, estimate the time-to-treatment discontinuation (TTD, defined as the time from initiation of therapy to discontinuation of treatment for any reason), the ORR, using RECIST v1.1, the assessment of clinical benefit at 6 and 12 months, and assess the safety-evaluable population (including all patients who received at least one cycle of the study drug).
The secondary objectives were to estimate the duration of response (DoR) among patients with an objective response, defined according to the clinical practice, the median PFS (time to the start of treatment to the first progression of disease), time to next treatment or death (TNT-D, intended as the time to the start of the therapy to the date of next subsequent systemic treatment initiation or death, whichever occurs first), and OS rate, as well as to describe second-line therapy after atezolizumab plus nab-paclitaxel progression. The incidence of adverse events (AEs) suggestive of potential immune-related etiology was also assessed.
Data retrieval
Demographics, medical history, BC history, and tumor biology were collected within the CUP, active in Italy, from November 2019 to August 2020. The primary data source was the medical record of the patient. The expression of conventional biological factors, such as ER and PR, HER2 status, and Ki-67 proliferation index, was obtained from pathology reports. Triple-negative subtype was defined as ER- and PR-negative, and HER2-negative. The ER-negative and PR-negative status was defined if the percentage of positive nuclei by immunohistochemical (IHC) method was <1%. The HER2-negative status was defined if a 0, 1+ or 2 + IHC score was found with non-amplified in situ hybridization, according to the ASCO-CAP 2018 guidelines36. PD-L1-positive tumor status was defined as PD-L1 expression ≥1% on tumor-infiltrating immune cells as a percentage per tumor area, assessed by the Ventana PD-L1 (SP142) assay based on the status of the primary tumor and/or the biopsy of metastatic disease before starting treatment. Samples should have been evaluated by a qualified laboratory, and different assays are not acceptable. Response to atezolizumab plus nab-paclitaxel was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria37. Toxicity was evaluated by the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.02) and according to Italian laws38,39,40. All AEs and serious AEs considered related to atezolizumab, and nab-paclitaxel were documented in the source data.
Statistical analysis
Data were summarized using absolute counts and percentages when considering categorical variables and median values, and interquartile range (IQR) when referring to quantitative items. Differences in ORR between subgroups were assessed using the chi-square test. Survival times were estimated by the Kaplan-Meier method, and median values were reported with their 95% CIs. Differences between the curves were evaluated with the log-rank test. The sample size was not determined previously, as this analysis was performed on patients participating in the Expanded Access Program for atezolizumab according to their clinician’s decision. A subgroup analysis was planned; p-values are to be considered in an exploratory approach. IBM SPSS Statistics for Windows v.28.0 (Armonk, NY) was used for analysis.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Change history
30 October 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41523-023-00596-1
05 February 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41523-024-00619-5
References
Garrido-Castro, A. C., Lin, N. U. & Polyak, K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 9, 176–198 (2019).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J. Clin. Oncol. 36, 2105–2122 (2018).
Li, X. et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res. Treat. 161, 279–287 (2017).
Zhu, Y., Zhu, X., Tang, C., Guan, X. & Zhang, W. Progress and challenges of immunotherapy in triple-negative breast cancer. Biochim. Biophys. Acta Rev. Cancer 1876, 188593 (2021).
Kwa, M. J. & Adams, S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): where to go from here: checkpoint inhibitors in TNBC. Cancer 124, 2086–2103 (2018).
Bianchini, G., De Angelis, C., Licata, L. & Gianni, L. Treatment landscape of triple-negative breast cancer — expanded options, evolving needs. Nat. Rev. Clin. Oncol. 19, 91–113 (2022).
Abdou, Y. et al. Immunotherapy in triple negative breast cancer: beyond checkpoint inhibitors. NPJ Breast Cancer 8, 121 (2022).
Gupta, T., Vinayak, S. & Telli, M. Emerging strategies: PARP inhibitors in combination with immune checkpoint blockade in BRCA1 and BRCA2 mutation-associated and triple-negative breast cancer. Breast Cancer Res. Treat. 197, 51–56 (2023).
Cardoso, F. et al. 4th ESO-ESMO international consensus guidelines for Advanced Breast Cancer (ABC 4)†. Ann. Oncol. 29, 1634–1657 (2018).
Yardley, D. A. et al. nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: results from the tnAcity trial. Ann. Oncol. 29, 1763–1770 (2018).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer, V5.2020. J. NatL. Compr. Cancer Netw. 2020. Available at: https://www2.tri-kobe.org/nccn/guideline/breast/english/breast.pdf.
Garufi, G. et al. Updated neoadjuvant treatment landscape for early triple negative breast cancer: immunotherapy, potential predictive biomarkers, and novel agents. Cancers (Basel) 14, 4064 (2022).
Cimino-Mathews, A. et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum. Pathol. 47, 52–63 (2016).
Papadimitriou, M., Liakouli, Z. & Papadimitriou, C. A. The role of immune checkpoint inhibitors in triple-negative breast cancer: recent developments and future perspectives. J. Cancer Metastasis Treat. 7, 63 (2021).
Schmid, P. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med 379, 2108–2121 (2018).
European Medicines Agency. Tecentriq 840 mg concentrate for solution for infusion: EU summary of product characteristics. 2019. https://www.ema.europa.eu.
Zong, Y. & Pegram, M. Research advances and new challenges in overcoming triple-negative breast cancer. Cancer Drug Resist. 4, 517–542 (2021).
Haricharan, S., Bainbridge, M. N., Scheet, P. & Brown, P. H. Somatic mutation load of estrogen receptor-positive breast tumors predicts overall survival: an analysis of genome sequence data. Breast Cancer Res. Treat. 146, 211–220 (2014).
Budczies, J. et al. Classical pathology and mutational load of breast cancer - integration of two worlds. J. Pathol. Clin. Res. 1, 225–238 (2015).
Mittendorf, E. A. et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2, 361–370 (2014).
Thomas, R., Al-Khadairi, G. & Decock, J. Immune checkpoint inhibitors in triple negative breast cancer treatment: promising future prospects. Front. Oncol. 10, 600573 (2021).
Emens, L. A. et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann. Oncol. 32, 983–993 (2021).
Blumenthal, G. M. et al. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small-cell lung cancer. Ann. Oncol. 30, 830–838 (2019).
Atkins, M. B. et al. Maximizing the value of phase III trials in immuno-oncology: a checklist from the Society for Immunotherapy of Cancer (SITC). J. Immunother. Cancer 10, e005413 (2022).
Branchoux, S. et al. Time to next treatment or death as a candidate surrogate endpoint for overall survival in advanced melanoma patients treated with immune checkpoint inhibitors: an insight from the phase III CheckMate 067 trial. ESMO Open 7, 100340 (2022).
Cortes, J. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396, 1817–1828 (2020).
Rugo, H. S. et al. Performance of PD-L1 immunohistochemistry (IHC) assays in unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC): post-hoc analysis of IMpassion130. ESMO 2019 Congress. Ann. Oncol. 30, v851–v934 (2019).
Heeke, A. L. & Tan, A. R. Checkpoint inhibitor therapy for metastatic triple-negative breast cancer. Cancer Metastasis Rev. 40, 537–547 (2021).
Socinski, M. A. et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med 378, 2288–2301 (2018).
Adams, S. et al. Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up: a phase 1b clinical trial. JAMA Oncol. 5, 334–342 (2019).
Brahmer, J. R. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline. J. Clin. Oncol. 36, 1714–1768 (2018).
Patil, S. Early access programs: Benefits, challenges, and key considerations for successful implementation. Perspect. Clin. Res. 7, 4–8 (2016).
Capri, G. et al. An open-label expanded access study of lapatinib and capecitabine in patients with HER2-overexpressing locally advanced or metastatic breast cancer. Ann. Oncol. 21, 474–480 (2010).
Battisti, N. M. L. et al. Palbociclib and endocrine therapy in heavily pretreated hormone receptor-positive HER2-negative advanced breast cancer: the UK compassionate access programme experience. Breast Cancer Res. Treat. 174, 731–740 (2019).
Schmid, P. et al. Datopotamab deruxtecan + durvalumab as first-line treatment for unresectable locally advanced/metastatic triple-negative breast cancer: Initial results from BEGONIA, a phase 1b/2 study. Presented at: ESMO Breast Cancer Congress 2022. Abstract 166MO. May 4, (2022).
Wolff, A. C. et al. HER2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update summary. J. Oncol. Pract. 14, 437–441 (2018).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Schoen, M. W. et al. Software for administering the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events: usability study. JMIR Hum. Factors 16, e10070 (2018).
Italian Medicines Agency. Implementation of Directive 2001/83/EC (and subsequent amending directives) relating to a Community code concerning medicinal products for human use, as well as Directive 2003/94/EC (2006).
Italian Medicines Agency. Guideline for the classification and managing of observational studies on drugs (2008). www.gazzettaufficiale.it/eli/id/2008/03/31/08A02109/sg.
Acknowledgements
This study was funded by the Italian minister of health – Ricerca Corrente 2022. This study was also unconditionally supported by Roche. We would like to thank the following Investigators: Gambaro Anna (Luigi Sacco Hospital, ASST Fatebenefratelli Sacco, Milan, Italy); Montesarchio Vincenzo, Leo Luigi (AORN dei Colli Hospital, Napoli, Italy); Spagnoletti Ilaria (Sacro Cuore di Gesù Hospital, Fatebenefratelli, Benevento, Italy); Piacentini Federico (University of Modena and Reggio Emilia, Italy); Bonura Salvatore (ASL5 Bassa Friulana, Gorizia, Italy); Stani Simonetta (Santo Spirito Hospital, Rome, Italy); Fioroni Iacopo, Bruno Vincenzi (University Campus Bio-Medico Rome, Italy); Corsi Domenico Cristiano (San Giovanni Calibita Fatebenefratelli Hospital, Rome, Italy); Tagliabue Paola (Vimercate Hospital, ASST della Brianza, Vimercate, Italy); Berardi Rossana (AOU Ospedali Riuniti Di Ancona, Ancona, Italy); Leonardi Vitabaldassarra (Ospedale Civico, Palermo, Italy); Fiorio Elena (Azienda Ospedaliera Universitaria Integrata, Verona, Italy); Pizzirani Cinzia (Azienda Ospedaliera-Univesitaria Policlinico Sant’Orsola IRRCS, Bologna, Italy); Orlandi Armando (Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy). Editorial and graphical assistance was provided by Simonetta Papa, PhD, Massimiliano Pianta, Aashni Shah, and Valentina Attanasio (Polistudium SRL, Milan, Italy). This assistance was supported by internal funds.
Author information
Authors and Affiliations
Contributions
A.F., L.C., D.G., G.S.: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing, Visualization. A.F., L.C., A.B., I.P., P.F., M.C.S., N.L.V., C.S., R.P., S.G., G.C., C.C., M.R., A.B., A.F., M.R.V., F.V., A.F., E.R.H., A.C., A.D.L., G.T., D.G., G.S.: Investigation, review & editing.
Corresponding author
Ethics declarations
Competing interests
The Authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fabi, A., Carbognin, L., Botticelli, A. et al. Real-world ANASTASE study of atezolizumab+nab-paclitaxel as first-line treatment of PD-L1-positive metastatic triple-negative breast cancer. npj Breast Cancer 9, 73 (2023). https://doi.org/10.1038/s41523-023-00579-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-023-00579-2