Abstract
Nanotechnology shows potential to promote sustainable and productive agriculture and address the growing population and food demand worldwide. However, the applications of nanotechnology are hindered by the lack of knowledge on nanoparticle (NP) transformations and the interactions between NPs and macromolecules within crops. In this Review, we discuss the beneficial and toxicity-relieving transformation products of NPs that provide agricultural benefits and the toxic and physiology-disturbing transformations that induce phytotoxicities. Based on knowledge related to the management of NP transformations and their long-term effects, we propose feasible design suggestions to attain nano-enabled efficient and sustainable agricultural applications.
Similar content being viewed by others
Introduction
Nanotechnology provides promising opportunities to establish efficient and sustainable agricultural systems1, which is important because food demands are continuously increasing worldwide due to population and consumption growth2; in addition, crop yields are low due to climate change, shrinking arable land, drought, disease, inefficient overuse of applied agrochemicals3 and environmental contamination (especially persistent organic pesticides4). Nanoparticles (NPs) are easy to functionalize, are small enough to break through the size exclusion limit of the plant cell wall (5–20 nm)5 and can enter crops. Some NPs can serve as nanofertilizers due to their nutrient composition6. Some NPs, such as Ag-, Ti- and Cu-based NPs, can be applied as nanopesticides due to their strong antimicrobial activity7. Other NPs, such as carbon-based NPs (carbon dots (CDs))8, polymer-based (nanocapsules, nanospheres and nano(hydro)gels made of natural chitosan and cellulose, etc.) and clay-based NPs (mesoporous silica NPs and montmorillonite)6,7, can serve as nanocarriers with various properties and application approaches to deliver agrochemicals precisely. NPs can also be used for plant genetic engineering9; for example, NPs can deliver genetic material into plants to silence genes in insects and pathogens, including viruses10. In addition, nanotechnology can be used to manage environmental stress11 and monitor crop health12.
Given the complex and constantly changing environments of the rhizosphere and plant interior, NPs often undergo transformations. The transformation of NPs is accompanied by changes in surface morphology, chemical structure and species, which affect the transport ability, bioavailability, and distribution of NPs13 and impact the effects and applications of NPs within crops (Fig. 1). On the one hand, some NPs applied in agriculture rely on the effects of particle transformation products. For example, multiple kinds of released beneficial ions, such as Ni14, Mo15, Zn16, Cu17, Se18, and La19 ions, from corresponding NPs exhibit growth-promoting, antimicrobial, and disease-suppressing effects on crops. On the other hand, toxic transformation products generated from NPs pose threats to crop ecosystems and even human health through potential trophic transfer20 and biomagnification21, and preventing the generation of these products is very important. Thus, studies on the transformation of NPs in crops are fundamental for managing their ecological risks and application efficiency.
NPs and crops interact with each other. Additionally, the types and product doses of the transformations of NPs in crop systems play decisive roles in their effects on crops. Beneficial transformation products and transformations relieving the initial phytotoxicity of NPs and their transformation products have positive effects on crops. Toxic transformation products and NP transformations disturbing physiological processes in plants induce harmful phytotoxicity.
The routes of NP exposure to plants in laboratory studies mainly involve roots, foliar and seed treatments11; these experiments simulate the methods used in agricultural nanobiotechnology, such as soil application, foliar spraying and seed priming. The transport of NPs inside crops from the applied tissue to working tissues is an important foundation for effective applications and has been well studied, mainly through apoplastic and symplastic pathways and vasculature loading22. In addition, studying the transformation of NPs through physical and chemical changes and interactions with macromolecules is essential for understanding the process by which NPs enter crop systems to generate effects. Additionally, determining the intermediate transformation products of NPs will help researchers develop nano-enabled agricultural applications with comprehensive precise designs and long-term systematic uses. However, compared with the beneficial23 and toxic effects24 of original NPs in crop systems, the transformations of NPs and nano-effects of transformation products have not received sufficient research attention.
Here, we provide a comprehensive review of nanoparticle-specific transformations and their influence on nano-effects in crop systems and propose practical suggestions for nano-enabled agricultural applications. We focus on the differences in the performance of commonly used metal (including silicon25 and selenium26 metalloids)- and carbon-based NPs. We review the processes and mechanisms of typical NP transformations inside plants and at the root and leaf interfaces. Then, we specify and summarise the NP transformation-controlled plant effects and describe studies in non-crop plant systems to provide a reference for possible behaviours in crops. Finally, we provide specific recommendations for the future prospects of utilizing NP transformations to optimize nano-enabled agricultural applications, achieve sustainable agricultural development and promote human interests.
Transformation of NPs in and around plants
Many varieties of NP transformations occur in plant-related systems27,28,29, including inside plants, in the rhizosphere microzone and at leaf interfaces (Fig. 2). In this study, the transformations of NPs at leaf interfaces mainly involve processes that occur at the epidermis of leaves after foliar applications. Through advanced analysis techniques, such as synchrotron radiation- and mass spectrometry-based techniques (Box 1), the speciation and contents of NP transformation products can be studied. These transformations can be mediated by both abiotic and biotic factors. In this work, we specifically summarise the transformations mediated by biotic factors in plant-related systems.
Many kinds of NP transformations occur inside plants, in the rhizosphere microzone and at leaf interfaces. In this scheme, the transformations of NPs at leaf interfaces mainly involve processes that occur at the epidermis of leaves after foliar applications. Mn+ represents n-valent ions, mainly metals and metalloids but also contains small amounts of nonmetals.
Aggregation
Aggregation is a typical physical transformation that occurs in all kinds of NPs, increasing the aggregate size. Aggregation occurs in two main forms: homoaggregation between the same NPs and heteroaggregation between a type of NP and other particles in the environment29. According to the extended Derjaguin–Landau–Verwey–Overbeak (XDLVO) theory, aggregation occurs if the sum of van der Waals attraction, electrostatic double layer repulsive force, macromolecular coating-induced steric repulsion force and other forces is attractive30.
After NPs are absorbed into roots, homoaggregation occurs inside root tissues31 and in the vasculature and leaf tissues after root-to-leaf translocation32. In addition, homoaggregation occurs in the rhizosphere microzone33. For example, multi-walled carbon nanotubes (MWCNTs), graphene nanoplatelets, and carbon black aggregate in aqueous extracts of soil planted with soybean34. Some carbon-based NPs have large surface areas, potentially exhibit superior adsorption properties, and induce heteroaggregation and homoaggregation; there are examples such as heteroaggregation of MWCNTs with CuO NPs35, and graphene oxide (GO) with Al2O3 NPs36.
Corona formation on the surface of the NPs
The ubiquitous and multitudinous macromolecules in plant-related systems inevitably and selectively37 cover NPs and form a dynamic surface corona. In plant tissues, binding with biomolecules (proteins, lipids, carbohydrates, peptides, nucleic acids, etc.) can form biomolecular coronas on NPs38. In areas outside the plant, including at the rhizosphere microzone and leaf interface, an ecological corona (eco-corona) can be formed consisting of biomolecules secreted from plants and microorganisms, such as extracellular polymeric substances and metabolites. Additionally, non-biomolecular natural organic matter (NOM) in the environment adsorbed on NPs can form an environmental corona39. The corona changes the surface physicochemical properties of NPs, further altering their biological identity and plant response40. Examples of surface coronas formed from the biomolecules of plants are described as follows.
Biomolecular coronas have been mostly investigated by in vitro exposure because extracting NPs with surface coronas from plant tissues is difficult. After incubation with endogenous substances extracted from leaf tissue, a protein corona on AuNPs41 and a corona enriched with flavonoids and lipids on TiO2 NPs38 were found in vitro. Extracellular polymeric substances in algal exudates can form eco-corona on CdSe/ZnS quantum dots (QDs)42 and graphene family NPs (GO, reduced graphene oxide (rGO) and graphene)43. Limited in vivo studies have shown that surface organic ligands and possible biomolecular coronas are formed on the TiO2 NPs inside lettuce leaves after foliar application, according to inconsistent XANES spectra with uncoated TiO2 NPs but consistent with those obtained for humic acid-coated anatase44.
Dissolution into ions
In plant systems, dissolution of metal-based NPs into ions is a common and elementary transformation process and involves a proton-promoted process and a ligand-assisted dissolution process33. Taking CuO NPs as an example, dissolution via a proton-promoted process is induced and promoted by H+ (Eqs. 1 and 2)45, which is provided by organic acids inside tissues46 and in the rhizosphere microzone. For the ligand-assisted dissolution process, Cu from CuO NPs react with surrounding ligands to form Cu-ligand complexes and detach ions into solution; simultaneously, the original copper oxygen bonds are polarized, become weak and break. The Cu-ligand complexes decrease the number of free ions in the ambient environment and further promote NP dissolution33.
Dissolution has been reported to occur inside plants. For example, dissolved Ag47, Pt48, and Ni14 ions were detected in AgNP-, PtNP- and NiO NP-exposed plant tissues, including roots, shoots and seeds, after root exposure. Soil-exposed MoS2 nanoparticles and nanosheets (500 mg kg−1) were biotransformed to molybdate and SO42− in soybean roots, shoots and nodules after 90 days of exposure49.
Dissolution also occurs in the rhizosphere microzone45,50,51, which follows first-order kinetics52. MoS2 nanoparticles and nanosheets were transformed to 14% and 43% molybdate in soybean rhizosphere soils after 60 days49. Se species in Arabidopsis thaliana leaves sprayed with SeNPs (500 μg per plant) included 53.4% SeO32− after 10 days of exposure53. Another study revealed that foliar-treated SiO2 NPs entered A. thaliana leaves and slowly released soluble orthosilicic acid, Si(OH)4, in cells54.
Complexation
Ions released from metal-based NPs can complex with ligands, mainly organic acids (acetate, citrate and oxalate) and thiols (cysteine and GSH), both inside the plant tissues and in the rhizosphere soil45,55.
Inside plant tissues, CuO and CeO2 NPs form similar acetate complexes in diverse plant species, including Cu-Ac and Cu(I)-Ac in Eichhornia crassipes and rice45,55, Ce acetate (Ce(Ac)3) in shoots (13.6%), leaves (21.5%) in cucumber46, and shoots (5–9%) and roots (4–9%) in rice56. In addition, the two types of NPs can form specific complexes, such as Cu-Cit and Cu(I)-Cys for CuO NPs45,55 and Ce-Ox for CeO2 NPs57. Zn-Cit is an important Zn complex produced from ZnO NPs, which was detected in root-exposed soybean grains58. Additionally, molybdate dissolved from MoS2 NPs chelated to form Mo-RCOOH and Mo-thiol in soybean roots, shoots and nodules after soil exposure49. After AgNPs were applied to lettuce leaves, Ag-GSH were produced inside the epidermis and parenchyma59. In rhizosphere soil, CuO NPs occur in the form of Cu(I)-cys45, and CeO2 NPs generated Ce(Ac)3 in rice rhizobag soils56. Mo from MoS2 NPs could be transformed to MoCl5 in soybean rhizosphere soils49.
Precipitation
Metal-based NPs can be transformed to sulphide particles. Thiols from metallothioneins, L-cysteine and L-methionine may play roles in the sulphidation process55,60. CuS is generated in vacuoles of CuO NP-exposed A. thaliana60. Precipitation of Cu2S was detected in CuO NP-exposed Eichhornia crassipes55 and rice paddy soils45.
Formations of chloride and oxide particles are important precipitation types for metal-based NPs. Cu(I)Cl can be generated from CuO NPs sequestered in the root cell vacuoles of A. thaliana60. After foliar exposure to AgNPs, AgCl precipitates were found in the leaf epidermis59. For the generation of oxide particles, Cu2O is produced from root-exposed CuO NPs in the submerged leaves of Eichhornia crassipes (3.8%)55 and the grains (42.1%) and roots (15.7%) of rice45. Nanoscale zerovalent iron (nZVI) generates ferric citrate and iron (oxyhydr)oxides in the roots and shoots of cucumber61. Cu2O was detected in CuO NP-exposed rice rhizosphere soil45.
Besides, metal-based NPs can form phosphate particles. Rare earth ions (RE3+) can be easily captured by PO43− and form insoluble REPO4 (Ksp = 10−24–10−26) in plant-related systems. For example, CeO2 NPs easily produce CePO4 precipitates, which were needle-like clusters46. The Ce3+ released from corresponding NPs can be immobilized in rice potted soil and on the root surface, absorbed into the roots and fixed in the intercellular space56, cell walls, cytoplasm, and vacuoles62 by PO43−. Besides rare earth ions, other metal ions also can generate phosphate precipitates. Root-exposed CuO NPs and foliar-applied ZnO NPs produced Cu2(OH)PO4 inside Eichhornia crassipes55 and Zn3(PO4)2 (70%–80%) in field-grown wheat grains63, respectively.
In addition, some metal-based NPs reprecipitate after dissolution. PtNPs absorbed into rice roots might be dissolved into ions, which are deposited on the particle surface, induce recrystallization and generate large spherical PtNPs with a smooth surface48. Similar reprecipitation processes after dissolution of AgNPs were supposed during the uptake by lettuce root and trophic transfer from lettuce to terrestrial snails according to the alteration of particle size distribution20.
Transformation to organic forms
Metal-based NPs can be transformed to organic forms in plants. After foliar spraying was applied to A. thaliana, SeNPs were transformed into selenoamino acids, including selenomethionine (SeMet, 44.4% on day 10) in exposed leaves and additional selenocystine (SeCys2) and methylselenocysteine (MeSeCys) in shoots or roots. In addition, two selenium-binding proteins were found in plant tissues53. GSH-coated CdSe/ZnS QDs were biotransformed to volatile alkyl selenides, including dimethyl selenide (DMSe), dimethyl diselenide (DMDSe) and sulphur-containing alkyl selenides, through chalcogenide exchange, methylation, and demethylation reactions mediated by rice plants and rhizosphere microorganisms64. Sulphur from soil-exposed MoS2 NPs was assimilated to thiol compounds (R-SH) and sulphoxide in soybean49.
Reduction/oxidation
Reduction/oxidation reactions are common for metal- and carbon-based NPs. For metal-based NPs, redox reactions are always difficult to distinguish from other transformation processes, such as dissolution, complexation and precipitation. Redox reactions are usually mediated by reducing substances (e.g., reducing sugars such as glucose and fructose, ascorbic acid (Vc), catechol46,65, and reductases60, especially biomolecules with O-containing electron-rich moieties substituted structures55) and oxidizing substances in the atmosphere and water environment (including pore water in the rhizosphere)66. For example, CeO2 NPs adsorbed to the root surface of cucumbers was reduced, and Ce3+ was released with the assistance of reducing Vc and citric acid46,62. The CuNPs and surface oxidation product, CuO, were dissolved and accompanied by a redox reaction to form Cu(0), Cu(I) and Cu(II) in synthetic root exudates66. Additionally, foliar-applied AgNPs produced Ag-GSH at the leaf interface, which was accompanied by oxidation59. The precipitation of Cu2S inside CuO NP-exposed plants was accompanied by the reduction of Cu(II) to Cu(I)55.
The surface oxidation of carbon-based NPs in a plant system via the cleavage of carbon‒carbon bonds led to the formation of defects and holes in the lattice, which were attributed to enzymatic catalysis (especially the plant enzyme horseradish peroxidase (HRP))67 and reactive oxygen species (ROS).
Enzymatic catalysis by HRP leads to the oxidation of carbon-based NPs. The reactive site of HRP is the heme, in which the essential positively charged residue is Arg17868. HRP is initially inactive, and the heme active site is in an iron oxidation state. In the presence of H2O2, the heme active site undergoes protein-assisted transformation into a ferryl oxo iron (Fe4+=O) and a porphyrin π cation radical. During the catalysis reaction, ferryl oxo iron is reduced back to the ferric state, carbon-based NPs are oxidized and the σ carbon‒carbon bonds of the tertiary hydroxyl and epoxide groups are cleaved69. The proximity of the NPs to the heme active site of HRP was necessary for NP degradation. For example, the oxygen-containing groups (C–O and C = O) and surface negative charges of CDs orient them towards the positively charged Arg178 at the HRP active site, resulting in the degradation of CDs70.
ROS are able to cause the oxidation of carbon-based NPs. ROS are ubiquitous in plant tissues and are produced as by-products of metabolic pathways or by dedicated oxidases for signalling71. Few-layer graphene NPs can be degraded by ·OH in rice leaves, generating structural defects72. In an in vitro study73, carboxylated MWCNTs were decarboxylated through reactions between ·OH and surface carboxylated carbonaceous fragments and the graphitic sidewall, leading to defects in functional groups and vacancies.
In the rhizosphere microzone, electrons produced during microbial respiration can be transferred by mineral and organic matter to O2 and produce ROS74. The degradation of carbon-based NPs mediated by ROS in the rhizosphere may involve mechanisms similar to those inside plant tissues. However, this topic has not received enough research attention until now.
After surface oxidation and structural defects, carbon-based NPs generate multiple degradation species through enzymatic catalysis and reactions with ROS; these species include incomplete degradation products, such as oxidized aromatic hydrocarbons, and complete product CO2 gas68,70,72. Specifically, some identified products of HRP-degraded carboxylated single-walled CNTs (SWCNTs), such as benzaldehyde and 1,2-benzenediol, are similar to bioremediation products of polycyclic aromatic hydrocarbons (PAHs)68. Products of HRP-degraded CDs in rice in the presence of H2O2 were identified as plant-hormone analogues containing aromatic, ether, and carboxyl groups70. Moreover, carboxylated SWCNTs and CDs generated a large amount of CO2 through degradation by HRP68,70. 14C-labelled few-layer graphene (~9%) was also degraded to CO2 in rice72.
Three-phase-like transformation behaviours of NPs in plants
By analogy with metabolite patterns in animal liver, the transformation of exogenous compounds in plants has been conceptualized in three phases75. In the “green liver” model in plant, phase I transformations generally increase the water solubility and reactivity of substances. Phase II reactions are conjugations between the substances and biomolecules, increasing their water solubility and reducing their toxicity. Although plants do not have active excretion mechanism like animals, plants can similarly sequester the conjugated products in nonmetabolic tissues, which is phase III reactions (compartmentalization). These substances are sequestered mainly through storage in the cell vacuole or apoplast and covalent binding to cell walls76. The “green liver” model has rarely been applied in studies on exogenous NP transformation in plants. In one previous study, the surface modification of QDs was postulated as exogenous compounds with potential three-phase transformation reactions in plants50.
From our perspective, the overall transformation of exogenous NPs in plants can be classified and summarised as three-phase-like transformation reactions, according to the changes they induce in NPs and NP behaviours (Fig. 3). The dissolution of metal-based NPs into corresponding ions accompanied by oxidation or reduction, increasing their water solubility, and the oxidation of carbon-based NPs to form surface defects and oxidized aromatic hydrocarbons are phase I transformations. The complexation of dissolved ions with ligands, transformation of selenium-containing NPs to selenoamino acids, and surface corona formation are phase II transformations.
The main reactions are shown in black text. Typical examples of NPs and their transformation products are listed in red text. For sequestration, the original NPs are not presented in italics, and the transformation products are shown in italics. Mn+ represents n-valent ions, mainly metals and metalloids but also contains small amounts of nonmetals.
NPs or their phase II products undergo phase III transformation and are isolated from harmful metabolic processes, including precipitation, sequestration and excretion. (i) Inert precipitation, including reprecipitation, decreases the mobility, reactivity and toxicity61 of exogenous NPs in plant tissues. (ii) Sequestration of NPs in the cell wall, vacuoles or apoplast could prevent adverse effects. Cell walls, especially those with high levels of pectins and fucosylated xyloglucans, are conducive to the sequestration of NPs77. PbS NPs reportedly enter the maize root cell wall65, and CuO NPs are stored on the in vitro exposed plant cell wall78. CNTs aggregate on the internal surface of cell walls in maize secondary root phloem79. The CePO4 transformation product of CeO2 NPs is fixed in cucumber root cell walls62. Storage in vacuoles is also an important detoxification pathway for exogenous NPs80. CuO NPs and their transformation products (CuCl and CuS) could be internalized and sequestered in root cell vacuoles60. γ-Fe2O3 NPs81 and biomineralization aggregates of nZVI with chainlike structures61 are deposited in the vacuoles of corn root and cucumber leaf parenchymal cells, respectively. Vacuoles are also the main accumulation sites for CePO4 transformation product of CeO2 NPs62, CNTs82, and graphene-based NPs80. NPs often appear in the intercellular apoplastic space, especially NPs that undergo apoplastic transportation. PbS NPs65, CuO NPs55, CeO2 NPs and their transformation products CePO446 were reportedly distributed in the root intercellular space. (iii) Excretion from plants occurs through phytovolatilization of volatile transformation products and through root exudation to the rhizosphere. Se-containing NPs are biotransformed to volatile alkyl selenides, which easily escape from the plant system64. Carbon-based NPs can be completely degraded to CO2 and phytovolatilized68,70,72. AuNPs with sizes smaller than 50 nm could be exuded into the wheat rhizosphere soil after foliar exposure83.
The categorization and summary of diverse transformations of NPs associated with plants in three-phase-like reactions are conducive to understanding the relationships among transformations in different sequences (phases). More importantly, three-phase-like reactions are plant responses against the disturbance of exogenous NPs; these responses maintain plant homeostasis and detoxify plants through the action of reactive biomolecules and transfer inside tissues. Identifying the underlying factors and internal connection of multiple NP transformations contributes greatly to the effective prediction of NP behaviours and effects in plant systems. Notably, the three-phase-like transformation processes do not always occur sequentially, and some processes are intertwined and skip steps.
Factors affecting NP transformations associated with plants
Rhizosphere microorganisms play important roles in transformations of NPs. Many diverse microorganisms live in the rhizosphere and benefit plants by helping them to acquire nutrients from the soil and suppressing pathogenic invasions84. On the one hand, microorganisms can mediate the transformation of NPs13; for example, the rhizospheric symbiotic microorganism Bacillus subtilis mediates the complete sulphidation of AgNPs85, volatile biomethylation of CdSe/ZnS QDs64, and dissolution of CuO NPs with decreased particle size45. On the other hand, extracellular polymeric substances produced by microorganisms interact with NPs, change their surface physicochemical properties, and lead to transformations. Thiol-containing proteins and peptides in the secretome of Bacillus subtilis induce surface sulphidation of AgNPs with the formation of a Ag2S coating85.
Root exudates and vasculature sap affect the transformation behaviours of NPs. Living plant roots secrete complex mixtures of soluble organic substances, including sugars, amino acids, organic acids, enzymes, etc86. Root exudates modify rhizosphere chemistry and impact the transformation of NPs, such as increasing soluble species52, inhibiting aggregation33 and inducing redox reactions13. Different vasculature sap compositions also alter the behaviours of NPs. Inorganic solutes in synthetic tree sap accelerated the dissolution of AgNPs; in contrast, organic components exhibited an inhibitory effect87. Moreover, the compositions of xylem sap and root exudates vary by plant species, which results in different plant-associated transformations of NPs88.
Properties and concentrations of the NPs determine the degree of their transformations. The aggregation of NPs is affected by their surface charges and coatings. For example, compared to negatively charged corresponding same-species NPs, positively charged AuNPs89 and amine-functionalized MWCNTs (MWCNT-NH2)90 more easily clustered on negatively charged roots. In addition, compared with citrate and polyvinylpyrrolidone coatings, the maximum-molecular-weight gum Arabic coating more effectively stabilized the AgNPs from aggregation in synthetic tree sap with high ionic strength87. Furthermore, HRP enzyme-catalysed degradation of carbon-based NPs depends on the proximity of the particle to the heme active site of HRP, which is strongly affected by the surface coating. Compared to rGO69 and pristine SWCNTs91, GO and carboxylated SWCNTs with closer proximity to the active site of HRP achieved better enzymatic oxidation.
Besides surface properties, the transformation speed and degree also depend on the shape57, size48 and crystal phase49 characteristics of NPs. The transformation proportions from MoS2 nanosheets, MoS2 nanoparticles and MoS2 bulk to MoCl5 and molybdate in soybean rhizosphere soils decreased successively by more than 60%, 33–45%, and 0%, respectively; these decreases occurred because greater surface areas of nanomaterials promoted the adsorption of organic ligands from soils, aggregation barriers formed, NP dissolution was increased and MoCl5 and molybdate were produced. Compared with the 2H phase, the 1T crystal phase of MoS2 results in higher solubility and greater oxidation rates, and the MoS2 nanosheets presented the highest 1T/2H phase ratio and the strongest environmental reactivity among the three kinds of MoS249. The concentrations of NPs impact their transformations. Carbon-based NPs, including MWCNTs, graphene nanoplatelets, and carbon black, agglomerate more rapidly at high concentrations (300 mg L−1) in aqueous soil extracts of soybean than at low concentrations (10 mg L−1)34.
Environmental conditions impact NP transformations. Increasing ionic strength accelerated the aggregation of NPs in root exudates33. Anaerobic flooded soil conditions decreased the soil redox potential (Eh), changed the soil pH, reduced the dissolution of CuO NPs45, promoted the reduction reaction and transformation of the Cu species of CuO NPs from the easily extractable form (Cu adsorbed on humic acid) to the stable form (Cu2S)45 and the Ce species from CeO2 NPs to CePO4 and Ce(Ac)356.
The dynamic nature of NP transformations
NP transformations in plant systems are constantly changing and evolving. First, when NPs in the environment approach plants, are absorbed by a plant, or are transported to different plant tissues, cells or organelles, the transformation types and degree of transformation change in response to diverse biomolecule compositions and metabolic conditions (e.g., ionic strength, Eh and pH)92. Second, the evolution of NP transformations in specific locations is time-dependent due to ongoing reaction processes. Third, further transportation and transformation of NP transformation products in plant systems changes the transformation balance of NPs and is intertwined with the behaviour of the NPs.
For example, dissolution of NPs and reprecipitation are often associated. Intertransformation between Ag+ ions and AgNPs occurs in AgNP-exposed rice systems through roots; these reactions include dissolution outside roots, reduction of Ag+ ions to AgNPs in the roots and oxidation of AgNPs to Ag+ ions in the shoots47. In addition, the surface corona of NPs is highly dynamic and exhibits changes in composition and abundance in a timely manner41. One in vitro study revealed that carbohydrates in cucumber xylem fluid adsorbed on CuO NPs could be removed during rinsing with ultrapure water for 3 h, suggesting that carbohydrate‒CuO interactions were reversible37. Similar to the dynamic nature of the protein corona in non-plant biological systems93, hard coronas are strongly attached and long-lasting; however, soft coronas are weakly attached to particles inside and outside plants, which are rapidly exchanged and greatly depend on the local macromolecule compositions. The bioavailability and transport behaviours of NPs are constantly changed by these highly dynamic transformations, leading to variations in the long-term effects of NPs on plants.
Impacts of NP transformations on plants
NPs disturb the plant homeostasis and affect plants at the molecular, biochemical, and physiological levels and can cause DNA damage, variations in nutritional composition, ROS induction, the expression of antioxidant enzymes, and changes in photosynthesis, plant morphology, growth, yield, and seed germination94. The inhibition of pathogens by NPs can also affect plant growth17,18,95. In addition to the dose and effect of original NPs, the type of their transformation and the concentration of products play decisive roles in the final plant response. Here, we summarise the adverse and beneficial impacts of NP species-dependent transformations on plants; in addition, we provide insight into how the dose of NPs and their transformation products control the balance between the toxicity and agricultural benefits of NPs (Fig. 4).
Metal- and carbon-based NPs have common and specific transformations. Typical examples of transformation products that induce changes in effects are listed. The blue up and red down arrows next to each “effect” represent beneficial up-regulation and adverse down-regulation impacts on plants, respectively. Mn+ represents n-valent ions, mainly metals and metalloids but also contains small amounts of nonmetals.
Adverse effects of NP transformation on plants
The negative effects of the transformation of metal- and carbon-based NPs on plants mainly result from the release of toxic ions via the Trojan horse mechanism96 and disturbances in the physiological processes of plants by particle aggregation, respectively.
Metal-based NPs that release toxic metal ions lead to heavy metal stress in the plant system. For example, the addition of maize root exudates (20 mg L−1) increased the dissolution of 25 mg L−1 CuO NPs (10.96%) by 13.9% within 72 h, which promoted and led to excessive accumulation of Cu in root tissues and reduced the seedling growth rate from 4.82% to 1.89% after 7 days of exposure33. In another study, ZnO NPs (800 mg kg−1) inhibited young corn photosynthesis, which decreased by 12% on day 20. The authors supposed that the toxicity occurred due to ZnO NPs and released Zn ions. On the one hand, the photosynthetic membranes underwent Zn-induced lipid peroxidation. On the other hand, the central chlorophyll atoms were replaced by Zn, which inhibited light harvesting. However, further experimental evidence is needed to clarify the contribution of Zn ions to the toxic effects and the mechanisms involved, and control groups such as ionic state exposure may be helpful97.
The Fe2+ and Cu2+ released from NPs can produce excessive ROS via Fenton reaction (Eqs. 3–5) and Fenton-like reaction, leading to oxidative stress. OH radicals in nZVI (0.5 g L−1)-exposed A. thaliana cause root cell walls to loosen, decrease the thickness and elongate root by 150–200% compared with those of the control; these processes result in an asymmetrical distribution of tensional strength and increased endocytosis98. O2·− generated due to Cu2+ ions from CuO NPs (100 mg L−1) induced acute toxicity (96 h) in A. thaliana, with fatty acid saturation increasing in plant cells; this toxicity affected membrane properties and even resulted in cell apoptosis, which is consistent with the response of plants to Cu2+ stress (1.2 mg L−1)60. Moreover, CuO NP-released Cu ions catalyse H2O2 to generate ·OH, which causes DNA damage in radish seeds with the accumulation of formamidopyrimidine lesions (FapyAde and FapyGua) at an exposure dose of 10–1000 mg L−1 99.
For carbon-based NPs, aggregation is the main transformation type that results in phytotoxicity by disturbing normal physiological processes and destroying the cell structure. MWCNTs (diameters of <10 nm) significantly inhibited algal growth via several combined effects, including oxidative stress; the agglomeration of MWCNTs with algal cells, which was accompanied by physical interactions; and shading effects. Quantitative analysis revealed that each factor accounted for approximately 50%, 25% and 25% of the response at 50 mg L−1 (approximately 96 h IC50)100.
In addition to aggregation, redox reactions of carbon-based NPs may accelerate phytotoxicity. GO can be reduced to rGO by plants101; however, compared with GO, more rGO translocated from the pea roots to the leaves, inhibited the activity of photosystem II (PSII) via oxidative stress and the destroyed oxygen-evolving-complex on the donor side of PSII in the exposure concentration range from 0.04 to 2.0 g L−1, as reported by one study102.
Beneficial effects of NP transformation on plants
For both metal- and carbon-based NPs, the positive effects of NP transformation on plants result from beneficial transformation products and reductions in the reactivity or bioavailability of harmful NPs and transformation products via aggregation, surface corona formation, complexation and precipitation.
Nutritional metal ions released from metal-based NPs facilitate related physiological processes in plants. Compared with that of unprimed seeds (72%), ZnO NP priming (10 mg L−1) improved the wheat seed germination rate to 100% due to the dissolved Zn2+, which played a key role in the biochemical processes of seed germination, such as dormancy breaking, inhibitor hydrolysis, and enzyme activation. A greater impact was achieved than that of bulk ZnSO4 priming (88%) due to the nanometric size and more gradual release of Zn2+ 16. Recent studies have shown that Ni2+ released from NiO NPs (50 mg kg−1) increased soybean seed yield by 39% compared with that of unexposed controls because nitrogen assimilation was increased, and NiO NPs are much more effective than conventional Ni2+ sources (NiSO4)14. Another study56 reported that CeO2 NP exposure (100 mg kg−1) increased photosynthesis in rice and supposed that released Ce3+ contributed to this improvement. These authors provided evidence of a positive correlation between the photosynthesis and the Ce(III) contents in the shoots under different soil water regimes; however, direct evidence for the release of Ce3+ is still lacking.
Additionally, both aloe vera extract gel-biosynthesized SeNPs (50 mg kg−1) and their released Se ions (2.75%) exhibited antifungal activity, which repressed Fusarium-induced wilt disease and increased the yield of lettuce by 61.6%18. Cu2+ from copper-based NPs showed a similar ability to repress fungal disease in plants17. Recent studies revealed that foliar application of La10Si6O27 nanorods (100 mg L−1) suppressed sheath blight (Rhizoctonia solani) in rice by 62.4%, which was 2.7-fold greater suppression than that of a commercial pesticide (thifluzamide). La ions released from La10Si6O27 nanorods with properties similar to those of Ca can induce calmodulin activity and enhance the systemic acquired resistance of plants19. Moreover, released Ce3+ can capture and scavenge ROS via Eqs. (6) – (9)56,103. Ce3+ from leaf-infiltrated CeO2 NPs (50 mg L−1) reduced leaf ROS by 52% and protected A. thaliana photosynthesis from excess light, heat, and dark chilling, with carbon assimilation rates increasing by 67%103.
The typical transformation products of carbon-based NPs in plants usually have beneficial effects. CO2 transformed from CDs (0.56 mg mL−1) can be converted into carbohydrates through the Calvin cycle during photosynthesis. Rice plant growth was accelerated by CO2 and incomplete products of hormone analogues, resulting in a 14.5% increase in yield70.
As described above, chelation and stable precipitation, typical phase II and III reactions, greatly decrease the reactivity and mobility of NPs; in addition, these processes usually reduce the phytotoxicity of NPs and their dissolved toxic metal ions. Complexing with chelators, such as phytochelatins45 and metallothioneins, can detoxify metal ions104. One study included treatment groups with the addition of citric acid or removed phosphate nutrients from silica sand for plant culture. The results revealed that Ce carboxylate chelation occurred outside the roots and a stable precipitate of CePO4 formed on the root surface and in the intercellular spaces and vacuoles of the roots. These processes significantly reduce the translocation of released Ce3+ from roots to shoots under exposure to CeO2 NPs (2000 mg kg−1) and relieve the toxicity from decreased biomass in lettuce105. This study used a simplified single-factor simulation system with silica sand, which is helpful for determining the specific mechanisms underlying nano-effects. However, these findings are not applicable to real soil environments, which involve complicated chemical compositions, microbial activity and interactions with soils. Thus, studying exposure in soil systems is essential for understanding what occurs in a realistic environment.
The aggregation of harmful NPs outside plants greatly decreases their bioavailability and toxicity. CuO NPs (25 mg L−1) aggregate more easily in the rhizosphere microzone without added root exudates than with exudates, which diminishes the contact, uptake and accumulation of NPs in roots and decreases the toxicity to maize growth33. Similar effects were observed for carbon-based NPs. MWCNTs, graphene nanoplatelets, and carbon black showed accelerated aggregation at concentrations of 1000 mg kg−1 (for each), and the bioavailability and toxicity to soybean aboveground part growth were reduced compared with those at 0.1 mg kg−1 34. Moreover, GO-Al2O3 heteroaggregation suppressed GO (25 mg L−1)-induced algal membrane damage36.
The surface coronas of either metal- or carbon-based NPs mitigate the effects of toxic NPs, which hinders their surface activity and reduces direct contact with organisms. Recent studies demonstrated that the eco-corona on the surface of CdSe/ZnS QDs (20 nmol L−1) promoted attachment and aggregation among the particles and reduced the subsequent bioavailability, which mitigated the toxicity of pristine QDs on algal cell viability42. The barrier effect of the eco-corona also diminished the toxicity to algal growth induced by graphene family NPs (GO, rGO and graphene; 1 mg L−1)43.
Dose balance between the toxicity and benefits of NPs
In addition to the species of NP transformation products, their doses, which are highly dependent on the dose of parent NPs, contribute greatly to the balance of harmful and beneficial agricultural effects. Typical examples in literature are summarised in Fig. 5. There are three main types of dose-dependent nano-effects, including toxic effects of transformation products at high concentrations, no or beneficial effects of those at low doses. For example, CuO NPs (500 mg Cu kg−1) applied to wheat soils for 14 days as micronutrient amendments or fungicides did not cause visual toxicity. However, compared with the unaged treatment (223 mg kg−1), the same dose of CuO NPs, which was aged 28 days prior to planting, increased the labile Cu fraction in the soils (305 mg kg−1) due to dissolution; this process led to a decrease in the maximal length of the roots by 6.6 cm compared with that of the control52. Another study showed that Si(OH)4 released from foliar-treated SiO2 NPs can activate the salicylic acid (SA)-dependent defence pathway and enhance systemic acquired resistance to the bacterial pathogen Pseudomonas syringae in Arabidopsis plants. SiO2 NP treatments (100–800 mg L−1) reduced bacterial growth to greater than 90%, and higher concentrations exceeding 1600 mg L−1 decreased the bacterial inhibition efficiency. The concentration of the active substance, the transformation product Si(OH)4, should be in a suitable range because excessive concentrations of Si(OH)4 (equivalent to 2560 mg SiO2 L−1) were ineffective at inducing systemic acquired resistance and had detrimental effects54. Mo released from MoS2 nanoparticles (500 mg kg−1) was found at 640 mg kg−1 in roots and enhanced soybean nitrogenase activity by 122%. However, the Mo concentration in the roots of soybean plants exposed to MoS2 nanosheets (500 mg kg−1) was greater than 1000 mg kg−1, which was greater than the Mo concentration from MoS2 NPs. An excessive amount of Mo diminished the nodule function and yield of soybean, which occurred because the MoS2 nanosheets transformed more easily and to a greater extent than the NPs. In addition, more sulphate generated from MoS2 nanosheets accumulated in plant tissues, leading to levels approximately 1.40 times greater than that in the nodules of control at 90 d49. The doses of the transformation products significantly balance the beneficial and adverse effects.
The dots and bars indicate the literature dose points and ranges of exposed NPs, respectively. The details of the studies, including exposed NPs, plant species, exposure routes, and nano-effect targets, are stated and separated by “–”. References are marked in the form of superscript. The transformation products of NPs that cause nano-effects are shown in italics in parentheses. Ref. 52,54 give the doses of the NP transformation products; the dose and corresponding effects of the transformation products on plants are shown in separate rows. Different doses induce three kinds of effects, including no obvious effects (grey), toxic effects (red) and beneficial effects (blue). The doses of NPs and transformation products in the soil and hydroponic matrix are uniform to mg L−1.
Perspectives and future outlook
The transformation of NPs greatly impacts new-generation agricultural applications based on nanobiotechnology. Here, we propose practical suggestions based on changes in plants induced by NP transformations to optimize application design. Additionally, we provide future development strategies to systematically understand and effectively manage the transformation of NPs and their ecological risks for developing efficient, safe, and sustainable agricultural systems (Fig. 6).
First, transformations of NPs can be fully utilized for desired applications by controlling the material chemistry at the design and synthesis stage. On the one hand, NPs that generate beneficial transformation products for crops show potential for development as controlled-release fertilizers, which maintain extended supply periods with moderate and safe release doses. On the other hand, NPs with harmful transformation products should be replaced with other safe NPs or applied with secure packages until they can be recycled. In both situations, the speed and substance of release must be precisely regulated by adjusting the size, aspect ratio (the ratio between the height and width), morphology, crystal phase and multi-element compositions of the materials. Furthermore, applying phytotoxic NPs to crops at concentrations below the toxicity threshold can trigger the defence system of the crop and facilitate systemic acquired acclimation. For example, an excellent review11 recently proposed that ROS-triggering NPs can be applied for increasing crop disease or stress tolerance. In addition, the macromolecules naturally adsorbed by NPs through the corona when the NPs are identified or delivered by plant cells can be applied as engineering coatings of functional NPs for targeted delivery in precision agriculture. Additionally, the composition and charge of surface ligands and coatings should be optimized to control preferential biomolecule binding106 and appropriate adsorption to soils and plant tissues, for example, to achieve the desired transport route. Moreover, the transformation ability of plants can be used for the renewable and eco-friendly synthesis of NPs, such as rGO101 and AgNPs107.
In addition, systematic transformation studies of NPs in crop systems should be performed before these NPs are applied in the field. Investigating the transformations that coexist simultaneously and the dynamic NP transformation processes during transport from entering to leaving the crop system is critical. Thus, there is a great need to develop analysis techniques for in situ and real-time investigations of the microscopic location and speciation of NPs and their transformation products in living organisms. In addition, the compositions and roles of plant biological molecules, ROS and ubiquitous plant-associated microorganisms in the transformation of NPs inside plants and at plant interfaces should be clearly identified.
Furthermore, the long-term effects of NPs and their transformation products on the overall ecosystem are noteworthy. Nano-effects, which occur throughout the life cycle of crops from their germination to full maturity and are potentially passed to the next generation, are highly important for crop security. Researchers have focused on this area. A recent study108 demonstrated that ROS-generating AgNPs through combined seed and leaf treatments enhanced the resistance of rice, and this trait was transferred transgenerationally and resulted in offspring seeds with improved germination performance. Furthermore, in addition to crops, other dependent components of an ecosystem, such as the surrounding microbiome, pollinators, soil, groundwater, the atmosphere, and higher trophic organisms that feed on plants (biomagnification), should be investigated for biosphere health assessments. Moreover, research on the nano-effects should focus on realistic application situations, such as with relatively low environmental doses and complex soil systems, and investigate nano-enabled mixtures for practical applications, such as nanocarriers together with their delivered agrochemicals and composite nanosensors of multiple NPs.
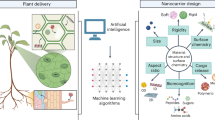
Finally, studying the plant behaviours and effects of every NP is unrealistic. Therefore, it is necessary to effectively predict and optimize the possible uptake, delivery, transformation, and effects of NPs for application in agricultural systems using the power of computational approaches, such as mathematical models109, artificial intelligence (AI)110, and machine learning111. Establishing a sufficiently detailed database of reported interactions between NPs and plants with as much detail as possible is necessary. Additional attention should be paid to some unconventional key physicochemical characteristics of NPs, such as their redox activity and surface properties, which are directly related to their subsequent behaviours27. In addition, automated tools should be used to harvest experimental data from published research articles and data from public databases and to pre-process the data; then, these data should be directly entered into AI or machine learning models to predict parameters for precision agriculture.
References
Lowry, G. V., Avellan, A. & Gilbertson, L. M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 14, 517–522 (2019).
White, J. C. & Gardea-Torresdey, J. Achieving food security through the very small. Nat. Nanotechnol. 13, 627–629 (2018).
Lew, T. T. S. et al. Species-independent analytical tools for next-generation agriculture. Nat. Plants 6, 1408–1417 (2020).
Goulson, D. Pesticides linked to bird declines. Nature 511, 295–296 (2014).
Schwab, F. et al. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants – critical review. Nanotoxicology 10, 257–278 (2016).
Kah, M., Kookana, R. S., Gogos, A. & Bucheli, T. D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 13, 677–684 (2018).
Wang, D. et al. Nano-enabled pesticides for sustainable agriculture and global food security. Nat. Nanotechnol. 17, 347–360 (2022).
Oliveira, L. S. B. L. & Ristroph, K. D. Critical review: uptake and translocation of organic nanodelivery vehicles in plants. Environ. Sci. Technol. 58, 5646–5669 (2024).
Zhang, H. et al. Nanoparticle cellular internalization is not required for RNA delivery to mature plant leaves. Nat. Nanotechnol. 17, 197–205 (2022).
Hofmann, T. et al. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food 1, 416–425 (2020).
Zhao, L. et al. Nanobiotechnology-based strategies for enhanced crop stress resilience. Nat. Food 3, 829–836 (2022).
Giraldo, J. P., Wu, H., Newkirk, G. M. & Kruss, S. Nanobiotechnology approaches for engineering smart plant sensors. Nat. Nanotechnol. 14, 541–553 (2019).
Zhang, P. et al. Nanomaterial transformation in the soil–plant system: implications for food safety and application in agriculture. Small 16, 2000705 (2020).
Zhou, P. et al. Nickel oxide nanoparticles improve soybean yield and enhance nitrogen assimilation. Environ. Sci. Technol. 57, 7547–7558 (2023).
Li, M. et al. Molybdenum nanofertilizer boosts biological nitrogen fixation and yield of soybean through delaying nodule senescence and nutrition enhancement. ACS Nano 17, 14761–14774 (2023).
Rai-Kalal, P. & Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 160, 341–351 (2021).
Borgatta, J. et al. Copper based nanomaterials suppress root fungal disease in watermelon (Citrullus lanatus): role of particle morphology, composition and dissolution behavior. ACS Sustain. Chem. Eng. 6, 14847–14856 (2018).
Shang, H. et al. Aloe vera extract gel-biosynthesized selenium nanoparticles enhance disease resistance in lettuce by modulating the metabolite profile and bacterial endophytes composition. ACS Nano 17, 13672–13684 (2023).
Cao, X. et al. Lanthanum silicate nanomaterials enhance sheath blight resistance in rice: mechanisms of action and soil health evaluation. ACS Nano 17, 15821–15835 (2023).
Wu, J., Sun, J., Bosker, T., Vijver, M. G. & Peijnenburg, W. J. G. M. Toxicokinetics and particle number-based trophic transfer of a metallic nanoparticle mixture in a terrestrial food chain. Environ. Sci. Technol. 57, 2792–2803 (2023).
Werlin, R. et al. Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain. Nat. Nanotechnol. 6, 65–71 (2011).
Lv, J., Christie, P. & Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: recent advances and methodological challenges. Environ. Sci.: Nano 6, 41–59 (2019).
Zhao, L. et al. Nano-biotechnology in agriculture: use of nanomaterials to promote plant growth and stress tolerance. J. Agric. Food Chem. 68, 1935–1947 (2020).
Ma, C., White, J. C., Dhankher, O. P. & Xing, B. Metal-based nanotoxicity and detoxification pathways in higher plants. Environ. Sci. Technol. 49, 7109–7122 (2015).
Prasad, T. N. V. K. V. et al. Nanoparticulate silica internalization and its effect on the growth and yield of groundnut (Arachis hypogaea L.). Environ. Sci. Technol. 57, 5881–5890 (2023).
Chasteen, T. G. & Bentley, R. Biomethylation of selenium and tellurium: microorganisms and plants. Chem. Rev. 103, 1–26 (2003).
Valsami-Jones, E. & Lynch, I. How safe are nanomaterials? Science 350, 388–389 (2015).
Qu, G. et al. Property-activity relationship of black phosphorus at the nano-bio interface: from molecules to organisms. Chem. Rev. 120, 2288–2346 (2020).
Lowry, G. V., Gregory, K. B., Apte, S. C. & Lead, J. R. Transformations of nanomaterials in the environment. Environ. Sci. Technol. 46, 6893–6899 (2012).
Hotze, E. M., Phenrat, T. & Lowry, G. V. Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 39, 1909–1924 (2010).
Deng, Y. et al. Multiple method analysis of TiO2 nanoparticle uptake in rice (Oryza sativa L.) plants. Environ. Sci. Technol. 51, 10615–10623 (2017).
Wang, Z. et al. Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ. Sci. Technol. 46, 4434–4441 (2012).
Shang, H. et al. Maize (Zea mays L.) root exudates modify the surface chemistry of CuO nanoparticles: altered aggregation, dissolution and toxicity. Sci. Total Environ. 690, 502–510 (2019).
Wang, Y. et al. Agglomeration determines effects of carbonaceous nanomaterials on soybean nodulation, dinitrogen fixation potential, and growth in soil. ACS Nano 11, 5753–5765 (2017).
Fang, R. et al. The combined toxicity and mechanism of multi-walled carbon nanotubes and nano copper oxide toward freshwater algae: Tetradesmus obliquus. J. Environ. Sci. 112, 376–387 (2022).
Zhao, J. et al. Toxicity of GO to freshwater algae in the presence of Al2O3 particles with different morphologies: importance of heteroaggregation. Environ. Sci. Technol. 52, 13448–13456 (2018).
Borgatta, J. R. et al. Biomolecular corona formation on CuO nanoparticles in plant xylem fluid. Environ. Sci.: Nano 8, 1067–1080 (2021).
Kurepa, J., Shull, T. E. & Smalle, J. A. Metabolomic analyses of the bio-corona formed on TiO2 nanoparticles incubated with plant leaf tissues. J. Nanobiotechnol. 18, 28 (2020).
Wheeler, K. E. et al. Environmental dimensions of the protein corona. Nat. Nanotechnol. 16, 617–629 (2021).
Monopoli, M. P., Åberg, C., Salvati, A. & Dawson, K. A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 7, 779–786 (2012).
Prakash, S. & Deswal, R. Analysis of temporally evolved nanoparticle-protein corona highlighted the potential ability of gold nanoparticles to stably interact with proteins and influence the major biochemical pathways in Brassica juncea. Plant Physiol. Biochem. 146, 143–156 (2020).
Chakraborty, D., Ethiraj, K. R., Chandrasekaran, N. & Mukherjee, A. Mitigating the toxic effects of CdSe quantum dots towards freshwater alga Scenedesmus obliquus: role of eco-corona. Environ. Pollut. 270, 116049 (2021).
Debroy, A. et al. EPS-corona formation on graphene family nanomaterials (GO, rGO and graphene) and its role in mitigating their toxic effects in the marine alga Chlorella sp. Environ. Pollut. 341, 123015 (2024).
Larue, C. et al. Fate of pristine TiO2 nanoparticles and aged paint-containing TiO2 nanoparticles in lettuce crop after foliar exposure. J. Hazard. Mater. 273, 17–26 (2014).
Peng, C. et al. Fate and transformation of CuO nanoparticles in the soil–rice system during the life cycle of rice plants. Environ. Sci. Technol. 51, 4907–4917 (2017).
Zhang, P. et al. Biotransformation of ceria nanoparticles in cucumber plants. ACS Nano 6, 9943–9950 (2012).
Yang, Q. et al. Uptake and transformation of silver nanoparticles and ions by rice plants revealed by dual stable isotope tracing. Environ. Sci. Technol. 53, 625–633 (2019).
Zhou, Y. et al. Effects of platinum nanoparticles on rice seedlings (Oryza sativa L.): size-dependent accumulation, transformation, and ionomic influence. Environ. Sci. Technol. 57, 3733–3745 (2023).
Li, M. et al. Dynamic Transformation of nano-MoS2 in a soil−plant system empowers its multifunctionality on soybean growth. Environ. Sci. Technol. 58, 1211–1222 (2024).
Wang, J. et al. Uptake, translocation, and transformation of quantum dots with cationic versus anionic coatings by Populus deltoides × nigra cuttings. Environ. Sci. Technol. 48, 6754–6762 (2014).
Kong, W. et al. Accumulation, translocation, and transformation of two CdSe/ZnS quantum dots in rice and pumpkin plants. Sci. Total Environ. 864, 161156 (2023).
Gao, X. et al. CuO nanoparticle dissolution and toxicity to wheat (Triticum aestivum) in rhizosphere soil. Environ. Sci. Technol. 52, 2888–2897 (2018).
Wang, Y. et al. Incorporation of selenium derived from nanoparticles into plant proteins in vivo. ACS Nano 17, 15847–15856 (2023).
El-Shetehy, M. et al. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 16, 344–353 (2021).
Zhao, J. et al. Uptake, distribution, and transformation of CuO NPs in a floating plant Eichhornia crassipes and related stomatal responses. Environ. Sci. Technol. 51, 7686–7695 (2017).
Zhang, P. et al. Growing rice (Oryza sativa) aerobically reduces phytotoxicity, uptake, and transformation of CeO2 nanoparticles. Environ. Sci. Technol. 55, 8654–8664 (2021).
Zhang, P. et al. Shape-dependent transformation and translocation of ceria nanoparticles in cucumber plants. Environ. Sci. Technol. Lett. 4, 380–385 (2017).
Hernandez-Viezcas, J. A. et al. In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max). ACS Nano 7, 1415–1423 (2013).
Larue, C. et al. Foliar exposure of the crop Lactuca sativa to silver nanoparticles: evidence for internalization and changes in Ag speciation. J. Hazard. Mater. 264, 98–106 (2014).
Yuan, J., He, A., Huang, S., Hua, J. & Sheng, G. D. Internalization and phytotoxic effects of CuO nanoparticles in Arabidopsis thaliana as revealed by fatty acid profiles. Environ. Sci. Technol. 50, 10437–10447 (2016).
Dwivedi, A. D. et al. Uptake, distribution, and transformation of zerovalent iron nanoparticles in the edible plant Cucumis sativus. Environ. Sci. Technol. 52, 10057–10066 (2018).
Ma, Y. et al. Where does the transformation of precipitated ceria nanoparticles in hydroponic plants take place? Environ. Sci. Technol. 49, 10667–10674 (2015).
Zhang, T. et al. Using synchrotron-based approaches to examine the foliar application of ZnSO4 and ZnO nanoparticles for field-grown winter wheat. J. Agric. Food Chem. 66, 2572–2579 (2018).
Wei, L. et al. Rice seedlings and microorganisms mediate biotransformation of Se in CdSe/ZnS quantum dots to volatile alkyl selenides. Environ. Sci. Technol. 57, 20261–20271 (2023).
Ullah, H., Li, X., Peng, L., Cai, Y. & Mielke, H. W. In vivo phytotoxicity, uptake, and translocation of PbS nanoparticles in maize (Zea mays L.) plants. Sci. Total Environ. 737, 139558 (2020).
Huang, Y., Zhao, L. & Keller, A. A. Interactions, transformations, and bioavailability of nano-copper exposed to root exudates. Environ. Sci. Technol. 51, 9774–9783 (2017).
Zhao, J., Wang, Z., White, J. C. & Xing, B. Graphene in the aquatic environment: adsorption, dispersion, toxicity and transformation. Environ. Sci. Technol. 48, 9995–10009 (2014).
Allen, B. L. et al. Mechanistic investigations of horseradish peroxidase-catalyzed degradation of single-walled carbon nanotubes. J. Am. Chem. Soc. 131, 17194–17205 (2009).
Kotchey, G. P. et al. The enzymatic oxidation of graphene oxide. ACS Nano 5, 2098–2108 (2011).
Li, H. et al. Impacts of carbon dots on rice plants: boosting the growth and improving the disease resistance. ACS Appl. Bio Mater. 1, 663–672 (2018).
Mittler, R., Zandalinas, S. I., Fichman, Y. & Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 23, 663–679 (2022).
Huang, C. et al. Transformation of 14C-labeled graphene to 14CO2 in the shoots of a rice plant. Angew. Chem. Int. Ed. 57, 9759–9763 (2018).
Qu, X., Alvarez, P. J. J. & Li, Q. Photochemical transformation of carboxylated multiwalled carbon nanotubes: role of reactive oxygen species. Environ. Sci. Technol. 47, 14080–14088 (2013).
Dai, H., Wu, B., Chen, B., Ma, B. & Chu, C. Diel fluctuation of extracellular reactive oxygen species production in the rhizosphere of rice. Environ. Sci. Technol. 56, 9075–9082 (2022).
Sandermann, H. Higher plant metabolism of xenobiotics: the ‘green liver’ concept. Pharmacogenetics 4, 225–241 (1994).
Burken, J. G. Uptake and Metabolism of Organic Compounds: Green-Liver Model. (John Wiley & Sons, Inc., Hoboken, New Jersey, 2003).
Liné, C., Manent, F., Wolinski, A., Flahaut, E. & Larue, C. Comparative study of response of four crop species exposed to carbon nanotube contamination in soil. Chemosphere 274, 129854 (2021).
Dai, Y. et al. Interaction of CuO nanoparticles with plant cells: internalization, oxidative stress, electron transport chain disruption, and toxicogenomic responses. Environ. Sci.: Nano 5, 2269–2281 (2018).
Wang, X. et al. The impact of carbon nanotubes on bioaccumulation and translocation of phenanthrene, 3-CH3-phenanthrene and 9-NO2-phenanthrene in maize (Zea mays) seedlings. Environ. Sci.: Nano 3, 818–829 (2016).
Hu, X. et al. Humic acid acts as a natural antidote of graphene by regulating nanomaterial translocation and metabolic fluxes in vivo. Environ. Sci. Technol. 48, 6919–6927 (2014).
Li, J. et al. Uptake, translocation and physiological effects of magnetic iron oxide (γ-Fe2O3) nanoparticles in corn (Zea mays L.). Chemosphere 159, 326–334 (2016).
Serag, M. F. et al. Trafficking and subcellular localization of multiwalled carbon nanotubes in plant cells. ACS Nano 5, 493–499 (2011).
Avellan, A. et al. Nanoparticle size and coating chemistry control foliar uptake pathways, translocation, and leaf-to-rhizosphere transport in wheat. ACS Nano 13, 5291–5305 (2019).
Ling, N., Wang, T. & Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 13, 836 (2022).
Eymard-Vernain, E. et al. Impact of a model soil microorganism and of its secretome on the fate of silver nanoparticles. Environ. Sci. Technol. 52, 71–78 (2018).
Trivedi, P., Leach, J. E., Tringe, S. G., Sa, T. & Singh, B. K. Plant–microbiome interactions: from community assembly to plant health. Nat. Rev. Microbiol. 18, 607–621 (2020).
Su, Y. et al. Delivery, fate, and mobility of silver nanoparticles in citrus trees. ACS Nano 14, 2966–2981 (2020).
Zhang, P. et al. Plant species-dependent transformation and translocation of ceria nanoparticles. Environ. Sci.: Nano 6, 60–67 (2019).
Avellan, A. et al. Nanoparticle uptake in plants: gold nanomaterial localized in roots of Arabidopsis thaliana by X-ray computed nanotomography and hyperspectral Imaging. Environ. Sci. Technol. 51, 8682–8691 (2017).
Zhai, G., Gutowski, S. M., Walters, K. S., Yan, B. & Schnoor, J. L. Charge, size, and cellular selectivity for multiwall carbon nanotubes by maize and soybean. Environ. Sci. Technol. 49, 7380–7390 (2015).
Peng, Z. et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: a review. Environ. Int. 134, 105298 (2020).
Avellan, A. et al. Critical review: role of inorganic nanoparticle properties on their foliar uptake and in planta translocation. Environ. Sci. Technol. 55, 13417–13431 (2021).
Miclăuş, T. et al. Dynamic protein coronas revealed as a modulator of silver nanoparticle sulphidation in vitro. Nat. Commun. 7, 11770 (2016).
Prakash, V., Peralta-Videa, J., Tripathi, D. K., Ma, X. & Sharma, S. Recent insights into the impact, fate and transport of cerium oxide nanoparticles in the plant-soil continuum. Ecotoxicol. Environ. Saf. 221, 112403 (2021).
Parra-Torrejón, B. et al. Multifunctional nanomaterials for biofortification and protection of tomato plants. Environ. Sci. Technol. 57, 14950–14960 (2023).
Hsiao, I. L., Hsieh, Y. K., Wang, C. F., Chen, I. C. & Huang, Y. J. Trojan-horse mechanism in the cellular uptake of silver nanoparticles verified by direct intra- and extracellular silver speciation analysis. Environ. Sci. Technol. 49, 3813–3821 (2015).
Zhao, L. et al. Monitoring the environmental effects of CeO2 and ZnO nanoparticles through the life cycle of corn (Zea mays) plants and in situ μ-XRF mapping of nutrients in kernels. Environ. Sci. Technol. 49, 2921–2928 (2015).
Kim, J. H. et al. Exposure of iron nanoparticles to Arabidopsis thaliana enhances root elongation by triggering cell wall loosening. Environ. Sci. Technol. 48, 3477–3485 (2014).
Atha, D. H. et al. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ. Sci. Technol. 46, 1819–1827 (2012).
Long, Z., Ji, J., Yang, K., Lin, D. & Wu, F. Systematic and quantitative investigation of the mechanism of carbon nanotubes’ toxicity toward algae. Environ. Sci. Technol. 46, 8458–8466 (2012).
Ismail, Z. Green reduction of graphene oxide by plant extracts: A short review. Ceram. Int. 45, 23857–23868 (2019).
Chen, L. et al. Chemical reduction of graphene enhances in vivo translocation and photosynthetic inhibition in pea plants. Environ. Sci.: Nano 6, 1077–1088 (2019).
Wu, H., Tito, N. & Giraldo, J. P. Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano 11, 11283–11297 (2017).
Rylott, E. L. & Bruce, N. C. Plants to mine metals and remediate land. Science 377, 1380–1381 (2022).
Zhang, P. et al. Phytotoxicity, uptake and transformation of nano-CeO2 in sand cultured romaine lettuce. Environ. Pollut. 220, 1400–1408 (2017).
Voke, E., Pinals, R. L., Goh, N. S. & Landry, M. P. In planta nanosensors: understanding biocorona formation for functional design. ACS Sens. 6, 2802–2814 (2021).
Pradeep, M., Kruszka, D., Kachlicki, P., Mondal, D. & Franklin, G. Uncovering the phytochemical basis and the mechanism of plant extract-mediated eco-friendly synthesis of silver nanoparticles using ultra-performance liquid chromatography coupled with a photodiode array and high-resolution mass spectrometry. ACS Sustain. Chem. Eng. 10, 562–571 (2022).
Chen, S. et al. Engineering climate-resilient rice using a nanobiostimulant-based “stress training” strategy. ACS Nano 17, 10760–10773 (2023).
Wong, M. H. et al. Lipid exchange envelope penetration (LEEP) of nanoparticles for plant engineering: A universal localization mechanism. Nano Lett. 16, 1161–1172 (2016).
Zhang, P. et al. Nanotechnology and artificial intelligence to enable sustainable and precision agriculture. Nat. Plants 7, 864–876 (2021).
Wang, X., Liu, L., Zhang, W. & Ma, X. Prediction of plant uptake and translocation of engineered metallic nanoparticles by machine learning. Environ. Sci. Technol. 55, 7491–7500 (2021).
Acknowledgements
This work was jointly supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0750100) and the National Natural Science Foundation of China (22193051, 22136007).
Author information
Authors and Affiliations
Contributions
J.L. and G.J. conceptualized this review; L.W. and J.L. discussed and wrote the review. L.W. produced the figures. J.L. and G.J. critically reviewed and edited this review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wei, L., Liu, J. & Jiang, G. Nanoparticle-specific transformations dictate nanoparticle effects associated with plants and implications for nanotechnology use in agriculture. Nat Commun 15, 7389 (2024). https://doi.org/10.1038/s41467-024-51741-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51741-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.