Abstract
Many lung immune cells are known to respond to inhaled particulate matter. However, current known responses cannot explain how particles induce thrombosis in the lung and how they translocate to distant organs. Here, we demonstrate that lung megakaryocytes (MKs) in the alveolar and interstitial regions display location-determined characteristics and act as crucial responders to inhaled particles. They move rapidly to engulf particles and become activated with upregulation in inflammatory responses and thrombopoiesis. Comprehensive in vivo, in vitro and ex vivo results unraveled that MKs were involved in particle-induced lung damages and shed particle-containing platelets into blood circulation. Moreover, MK-derived platelets exhibited faster clotting, stronger adhesion than normal resting platelets, and inherited the engulfed particles from parent MKs to assist in extrapulmonary particle transportation. Our findings collectively highlight that the specific responses of MKs towards inhaled particles and their roles in facilitating the translocation of particles from the lungs to extrapulmonary organs for clearance.
Similar content being viewed by others
Introduction
Particulate matters (PMs) in polluted air are consistently linked to cardiopulmonary diseases1 such as thrombus formation, myocardial infarction, and acute heart failure2. Growing evidence suggests that these health threats are caused by fine PMs entering the respiratory tract and traveling to extrapulmonary organs via the blood circulation3. Inhaled particles such as silica (SiO2), carbon black (CB) and other inorganic/organic particles can spontaneously adsorb proteins to form a unique corona4 that enables the particles to cross the air-blood barrier and enter the systemic circulation. Once entering the blood stream, particles have been shown to reach sensitive sites such as the liver, spleen, heart, kidney, lymph nodes, brain and even the knee5,6. The underlying mechanisms for how particles traverse biological barriers and distribute to different organs remain unclear.
In most cases, however, inhaled particles are quickly engulfed and cleared by immune cells in the lungs such as monocytes and macrophages7 –the previously reported first responders that lead the changes in spatial and temporal distribution of particles in the lung. Nanoparticles camouflaged with biomembranes derived from blood cells such as immune cells, erythrocytes and platelets, have been shown to evade immune clearance, circulate longer and cross barriers8,9, further demonstrating the role of the immune system in particle clearance and biodistribution. While epidemiological studies have linked the uptake of particles by immune cells10 to disorders in extrapulmonary organs and the cardiovascular system, it remains unclear which resident lung immune cells are critical and how these cells recognize and aid the translocation of invading particles.
Recently, megakaryocytes (MKs), one of many resident cells in the lung, were shown to undergo thrombopoiesis11,12. These cells were also reported to express multiple cytokines and antigen presentation-related molecules such as toll-like receptors13 and major histocompatibility complex II (MHC II)14 that allow them to process and present antigens like immune cells, and promote leukocyte and endothelial activation and adhesion. Moreover, in vitro studies have shown that PMs can promote megakaryocytic maturation and thrombopoiesis in a mitochondrial phosphorylation-dependent manner15. Based on these findings, we posit that MKs in the lung could play a major role in the capture, transport, and distribution of inhaled PMs.
Here, we found lung MKs in mice rapidly engulfed inhaled airborne PMs and shed particle-containing platelets that travel to extrapulmonary organs. Using multiple fluorescent imaging and mice models, we found that resident lung MKs located in the interstitial and alveolar regions moved quickly towards and engulfed inhaled particles faster than other immune cells. Once engulfing particles, MKs were activated to produce proinflammatory cytokines and involve in other immune cell responses. Enhanced thrombopoiesis by particle-activated lung MKs increased the number of activated platelets in the blood circulation. Importantly, the engulfed particles were transferred to nascent platelets and transported to extrapulmonary organs. Animal models further revealed that deficiency in the MK-platelet axis compromised particle clearance and relative inflammatory cell responses. Our findings have uncovered the distinct functions of lung MKs in particle clearance, transport and distribution, offering new insights on particle-induced injuries seen in distant organs.
Results
Lung MK accumulate rapidly to inhaled particles
To investigate how pulmonary immune cells respond to airborne PMs, we treated mice with 2 mg/kg body weight of carbon black particles by oropharyngeal aspiration (o.p.a.) for 6, 12, and 24-h and thereafter, assessed the immune cell composition in the bronchoalveolar lavage fluid (BALF). We used CB particles because it is the main component of airborne PMs16. The dosage was converted from measurements obtained during the serious polluted periods when PM concentration was >300 μg/m3 (ref. 17).
Consistent with previous studies18, CB particles increased alveolar macrophage (AM), dendritic cell (DC), neutrophil (NE), monocyte (MO), MK, basophil (Baso) and eosinophil (Eosin) populations in the alveoli (Fig. 1a, b). Although the proportion of CD41+Hoechst+ MKs in naïve mice was <0.6% (nearly 61 per 1.0 × 104 cells in BALF, excluding anucleate platelets, Supplementary Fig. 1a), the number of MKs in the alveoli increased by 2.0- and 6.4-fold at 6 h and 12 h post inhalation (hpi) relative to untreated control, respectively (Fig. 1b and Supplementary Fig. 1b, P < 0.001). Immunofluorescent images of lung sections showed that the number of platelet factor 4 (PF4)+CD41+DAPI+MKs increased by 80% after exposure to CB particles for 24 h (Fig. 1c, P < 0.001), and these MKs were positively double stained with anti-mouse CD41 and CD42b antibodies (Abs) (Supplementary Fig. 1c). Moreover, the percentage increase in the number of MKs at most time points relative to 0 hpi was much higher than the other immune cells (Fig. 1b, P < 0.001). The 2.3-6.4-fold increase in MKs relative to 1.1–1.3- fold increase in AM (the main sentinel against particles7) indicated that MKs were more responsive to particle intrusion than thought previously that lung macrophages constitute the first line of defense against particles19.
a Number of AM, DC, NE, MO, MK, Baso and Eosin in the BALF increased at 6, 12, and 24 h hpi of CB particles (n = 4 mice per group). b Ratio of the cumulative increase of different cells (1-7) in BALF of treated mice in a relative to untreated control. Horizontal dotted line shows a ratio of 1. Ratio in each group is the mean values of 3 independent experiments. c Immunofluorescent images (left) and PF4+CD41+DAPI+ cell counts (right) show lung sections from mice post 24 h-exposure had more PF4+CD41+DAPI+ MKs (white arrow) than untreated control (n = 6 mice per group). d Fluorescent images of lung sections from naïve PF4-mTmG mice showing resident and crawling MKs (green) in the Alv. and Int. region (red, inside dotted line). e 3D fluorescent imaging of lung sections from untreated PF4-mTmG mice showing MK resident in Alv. wall. f Giemsa-Wright stained image of an isolated CD41+ Alv. MKs with the classical hyperchromatic nucleus and granular cytoplasm. g Fluorescent images of Alv. MKs (CD41+CD42b+ Hoechst+) obtained from BALF through magnetic bead sorting. h Fluorescence analysis of the percentage of Alv. MKs and Int. MKs in the whole lung from untreated PF4-mTmG mice (n = 3 mice per group). i Venn diagram showing the gene signature of Alv. and Int. MKs. Numbers represent number of genes. j Heatmap of the enriched pathways for genes expressed in Alv. and Int. MKs. k 3D fluorescent images showing accumulated PF4+GFP+MKs (green) and platelets (green) in lung sections from PF4-mTmG mice treated with CB particles for 24 h. l H&E and Masson stained images of lung sections from mice exposed to CB particles for 24 h show infiltration of inflammatory cells (blue arrows) and fiber deposition in alveoli (red arrows) and capillaries (green arrowheads). Data are presented as the mean values of 3 independent experiments ± SEM (a, c and h), P-value relative to 0 hpi (a) and control (c and h) by two-side student’s t-test.
To understand how MKs accumulate and respond so quickly to inhaled particles, we used PF4-mTmG reporter mice11 to visualize the movement of green fluorescent protein (GFP)-labeled MKs relative to tomato red+ interstitial cells (Fig. 1d). This animal model has been widely applied as a more effective lineage tracing reporter for MKs and platelets20 than other staining methods that apply exogenous labeling, as it vindicates that staining MKs with PF4 is sufficient for in vivo observation11. Fluorescent imaging showed that in naïve animals, GFP+ MKs were found in both pulmonary interstitium and alveolar lumen. Benefited from the advantages of FDISCO method21 combined with quantitative light sheet fluorescence microscopy (LSFM) in fluorescence preservation of PF4+GFP+ MKs and platelets, and effective clearing of organs without size reductions, we obtained high-resolution images showing the 3-dimensional (3D) structure of alveoli with lung-resident alveolar (Alv.) and interstitial (Int.) MKs (Fig. 1e, Supplementary Fig. 2a and Supplementary Movie 1). The location of Alv. MKs was confirmed by immunofluorescence analysis of whole-mount lung sections (Supplementary Fig. 2b), and BALF cells stained with phycoerythrin (PE)-labeled CD41 Abs dropping with intra-alveolar microinjection method (Supplementary Fig. 2c–e). Because only a few (1.6%) MKs were found in the interstitial sample after Ab dripping, ruling out MKs in BALF shedding from the lung interstitium (Supplementary Fig. 2d, e). These alveolar cells displayed classical hyperchromatic nucleus and granular cytoplasm characteristic of MKs (Fig. 1f), different from isolated CD170 (Siglec F)+ monocyte/macrophage cells (Supplementary Fig. 2f). Dual staining with CD41 and CD42b Abs reconfirmed that the observed cells were MKs, but not resident AMs (Fig. 1g). Based on the location characters of MKs, nearly 25% PF4+GFP+DAPI+ MKs were residentially alveolar (Fig. 1h). These results unveiled that lung residing MKs strategically positioned in the alveolar lumen, and this is likely the reason for their rapid response as sentinel cells.
We further evaluated MKs’ ability to respond to CB particles by comparing the RNA sequence (RNA-seq) analyses of resident Alv. MKs and Int. MKs isolated from naïve mice. Because Alv. MKs have not previously been clarified and these reported markers for MKs are not well-supported to differentiate Alv. MKs from Int. MKs22, we optimized the isolation process of MKs based on the principle of resident location determined-intrinsic characters14. Alv. and Int. MKs were sorted from the single-cell suspension of BALF and lung parenchymal tissues, respectively, with a high purity of 92.5% (Supplementary Fig. 3a). Furthermore, in-depth analyses of these RNA-Seq data against known MK datasets confirmed the expression of 45 typical MK markers (Supplementary Fig. 3b), especially most highly expressed Apoe, CD41 and CD42b (Supplementary Table 1). There were a total of 13,309 common genes expressed between Alv. and Int. MKs, with slightly different expression patterns (Fig. 1i and Supplementary Table 2). These common genes were enriched in 5 functional categories associated with cell motility, metabolism, hematopoietic process, secreted protein synthesis and host defense (Fig. 1j), suggesting both MKs are capable of thrombopoiesis (Supplementary Fig. 3c) and immune responses (Supplementary Fig. 3d). In support of these observations, 3D and 2D fluorescent images of the lung indicated the increased PF4+GFP+DAPI+ MKs in the collapsed alveoli lumen after CB exposure (Fig. 1k and Supplementary Fig. 3e). Additionally, hematoxylin & eosin (H&E)- and Masson-stained lung sections displayed perivascular accumulation of inflammatory cells and thickened alveolar wall. Furthermore, compared to untreated control, 57% more collagen fibers, resembling fibrin aggregates formed by MK-derived platelets23, were deposited in the parenchyma and blood vessel regions of treated animals (Fig. 1l and Supplementary Fig. 3f). Moreover, to simulate human breathing, mice were exposed to suspended fine particulate matter (PM2.5) collected from the atmospheric environment in an atmospheric chamber (Supplementary Fig. 3g). The total amount of inhaled particles after 3 days of exposure was as low as ~750 μg/kg body weight24. Consistent with pathological results from CB-exposed mice, lung sections of PM2.5-exposed animals showed higher infiltration of inflammatory cells (Supplementary Fig. 3h), including 55% more CD41+DAPI+ MKs than untreated control (Supplementary Fig. 3i). Together, our results demonstrated that lung MKs in the pulmonary interstitium and alveolar lumen rapidly increased in response to inhaled particles.
Lung MKs actively engulf inhaled particles
The rapid accumulation of MKs in BALF inspired us to investigate whether and how MKs respond to inhaled particles. Because CB particles are difficult to trace in the lung7, we used Cy5-labeled fluorescent polystyrene microspheres (Cy5-PSs) with similar particle-induced immune responses to CB particles instead to characterize the interactions with PF4+GFP+ DAPI+MKs (Fig. 2a).
a Schematic showing the interactions between fluorescently labeled Cy5-PSs (red) and Alv. or Int. MKs (green). b Imaging using two-photon intravital microscopy (2PIVM) shows lung MKs (green) migrating to engulf Cy5-PSs (pink) in PF4-mTmG mice treated with 2 mg/kg body weight Cy5-PSs through o.p.a. for 2 h. c Fluorescent images of Alv. MKs (green) crawling towards and engulfing Cy5-PSs (yellow arrows) deposited in the alveoli. Nuclei are stained with DAPI (blue). d Intravital two-photon imaging of lung MK (green) squeezing into and out of 2 bordering alveoli possibly through the pore of Kohn (white arrow). e Flow cytometry images show that CD41+ MKs (yellow) from the lungs of mice treated with Cy5-PSs for 12 h contain Cy5-PSs (red). Nuclei are stained with Hoechst (blue). f Fluorescent images showing engulfed Cy5-PSs (orange) inside CD41+ MKs sorted from mice and Dami cells treated with 200 ng/mL Cy5-PSs for 12 h. White dotted line outlines a cell. g TEM image of lung MKs treated with Cy5-PSs for 12 h. Red arrow in the insert image indicates engulfed particles. h Flow cytometer analysis of engulfed particles in lung MKs of mice treated with Cy5-PSs after 6 and 12 h (n = 10 mice per group). N.D. not detected. i Quantification of internalized Cy5-PSs in Alv. MKs, Int. MKs and AMs from mice in h after 6 h (n = 10 mice per group). Data are presented as the mean values of 3 independent experiments ± SEM (h, i), one-way analysis of variance (ANOVA). j, k RNA-seq analyses on Int. MKs (j) and Alv. MKs (k) show significant mRNA enrichment in thrombopoiesis and immune regulatory functions, respectively. The statistical analyses were performed using Metascape, one-side Fisher’s exact test (j, k). Figure 2a, created with BioRender.com, released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.
Intravital two-photon imaging11 to real-time visualize the alveolar compartment of the lungs and behavior of MKs for 2 h after particle inhalation showed that Alv. MKs migrated to engulf Cy5-PSs within 10 min (Fig. 2b and Supplementary Fig. 4a). Like AMs that crawl long distances through the alveoli and engulf foreign matters25, MKs were also seen to crawl along the Alv. wall (Fig. 2c) and sequentially squeeze into and out of 2 bordering alveoli (Fig. 2d and Supplementary Movie 2). In details, Cy5-PSs were present inside the cytoplasm of isolated MKs even after extensive washing during single-cell extraction from the BALF and total lung tissue (Fig. 2e and Supplementary Fig. 4b). Phagocytosed Cy5-PSs were also identified in human megakaryocytic cell line-Dami26 and Meg-01 cell27 upon particle exposure, similar to particles inside sorted MKs (Fig. 2f and Supplementary Fig. 4c). Besides, transmission electron microscope (TEM) images of particle-treated isolated MKs revealed that particles were encapsulated within membranous vesicle-like structures (Fig. 2g). We quantified the fluorescence intensity of Cy5-PSs inside MKs and found continuous increase in phagocytosis in MKs by 2.5- and 6.5-fold after 6 and 12 h of Cy5-PS administration relative to 0 hpi, respectively (Fig. 2h and Supplementary Fig. 4d, P < 0.001). While AMs were the most phagocytic cells as expected, Alv. MKs displayed more than half (60%) of content of intracellular particles relative to that of AMs (Fig. 2i, P < 0.001). Importantly, Alv. MKs displayed higher phagocytic activity, as evidenced by the percentage of particle-bearing Alv. MKs and content of engulfed particles in Alv. MKs were 4.3-fold and 2.3-fold higher than that of Int. MKs (Fig. 2i and Supplementary Fig. 4e, f, P < 0.001). Compared to Int. MKs enriching in thrombocytopoiesis-related pathways (Fig. 2j), RNA-seq analysis showed that mRNAs in Alv. MKs were enriched in immune regulatory functions, including immunoreceptors in endocytosis, phagosome, lysosome, and B cell receptor signal (Fig. 2k), corroborating the phagocytic activity seen in Fig. 2e–g. Transcriptome sequencing further uncovered immune-related properties in lung MKs that enabled them to accumulate around and engulf particles. Overall, these results suggested that lung MKs could quickly move towards and engulf inhaled particles.
Lung MKs involve in CB-induced inflammatory responses
To understand the particle-initiated responses in MKs, we isolated CD41+ MKs from mice exposed to CB particles for 24 h, and subjected them to RNA-seq analysis. Considering the similarities in immune and migration potentials of MKs caused aggregation of Alv. MKs and Int. MKs, to be more accurate, we examined the responses of the entire population to particle stimuli. RNA-seq results showed 4,116 differentially expressed genes in MKs upon CB exposure. Genes associated with the phagocytic lysosome28 (e.g., lysosomal associated membrane protein 3 (Lamp3) and CD44), chemokine29 (e.g., chemokine (C-X-C motif) ligand 14 (Cxcl14)) and platelet activation30 (e.g., integrin subunit alpha 6 (Itga6) and CD62p) were significantly upregulated (Fig. 3a, |log2 fold change | ≥ 0.8 and P < 0.05). The bubble chart of KEGG pathway enrichment analyses further revealed that these upregulated genes were mainly enriched in actin cytoskeleton organization, inflammatory response, and endocytosis signaling pathways31,32,33 (Fig. 3b). This close association between immune and thrombocytopoiesis functions was corroborated by heatmap showing the upregulation of crucial genes in platelet production34 (e.g., Itga6, Tek), phagolysosome associated signal35 (e.g., Lamp3, CD40) and monocyte chemotaxis (e.g., cadherin 2 (Cdh2), platelet and endothelial cell adhesion molecule 136 (Pecam1)) after CB particle administration (Fig. 3c). The gene set enrichment analysis (GSEA) in MKs showed that genes reflecting intracellular metabolism processes including thermogenesis and oxidative phosphorylation were upregulated (Supplementary Fig. 5a), suggesting that the activation of immune and thrombocytopoiesis signal-associated pathways in MKs was accompanied with increased macromolecule synthesis and energy requirements37. Because pro- interleukin-1 beta (IL-1β) is abundant inside MK granules38, and mature IL-1β could increase vascular endothelial permeability for immune cell across during the inflammation period39, we took the activation of Caspase-1/NLRP3/IL-1β pathway as an example of the sensitive signals for invaded particles40, to examine immune responses in MKs. Here, 1.5-, 1.3- and 1.5-fold increase of Caspase-1, Nalp3 and IL-1β mRNA levels, and 2-, 2.5- fold increase of Caspase-1, IL-1β protein content (Fig. 3d, e and Supplementary Fig. 5b–d, P < 0.05) were detected in MKs sorted from CB-treated mice, respectively, relative to untreated control. A similar increment was observed in CB-treated Dami MKs (Supplementary Fig. 5e), indicating that CB particles activated MKs to secrete cytokines per se.
a–c RNA-seq analysis of CD41+ MKs from lung single-cell suspension obtained from mice treated with 2 mg/kg body weight CB particles for 24 h. The volcano plots show the Log2 fold change of gene expression in CB group relative to control group (Ctrl). a Volcano plot showing upregulated and downregulated genes in lung MKs, two-tailed Wilcoxon rank-sum test. b KEGG pathway enrichment analysis of annotated upregulated genes in CB particle-treated lung MKs were associated with thrombocytosis, immunity, and cell regulation signals, one-side Fisher’s exact test. c Heatmap showing differentially expressed genes in lung MKs after CB particle treatment, categorized by biological functions. Columns represent samples (n = 3). d, e RT-qPCR analyses of Caspase-1 (d) and IL-1β (e) mRNA expression in lung MKs sorted from mice treated with CB particles for 24 h (n = 6 biological replicates per group). f H&E-stained images of lung sections show that c-mpl-/- mice treated with CB particles for 24 h had fewer inflammatory foci (red arrows) than treated WT animals. g Flow cytometer analysis of lung single-cell suspension from animals in f (n = 6 per group). h ELISA assay of IL-1β content in sera and BALF from WT and c-mpl-/- mice upon CB particles for 24 h (n = 6 per group). i H&E (top) and Masson (bottom) stained images of lung sections from WT and c-mpl-/- mice at 72 h post-CB administration show accumulated inflammatory cells (red arrows) and collagen deposition (blue arrows). j Immunofluorescent images of vascular junction with CD31+ cells in lung sections from WT and c-mpl-/- mice at 72 h post-CB particle exposure. White arrowheads indicate damaged junctions. White dash-dots outline the vessel. Inset: magnified view of vascular junction. k, l Immunofluorescent images (k) and counts per field (l) of neutrophils (MPO+Ly6G+ cells, white arrowheads) in lung sections from WT and c-mpl-/- mice at 72 h after CB exposure (n = 6 mice per group). m Immunofluorescence-stained images of lung sections show that mice receiving MK transplant promoted inflammatory responses and recruited significantly more neutrophils than control mice without transplants. n ELISA assay of IL-1β content in sera of mice with (+) and without (-) MK transplant at 24 h post 2 mg/kg body weight CB exposure (n = 6 mice per group). o Schematic shows c-mpl-/- mice displayed greater lung damage and 1–2 days slower recovery from particle-triggered inflammation than WT mice. Box and whisker plots indicate median, interquartile range and 10th to 90th percentiles of the distribution (h, n). Data are presented as the mean values of 3 independent experiments ± SEM (d–e, g, h, l and n), two-side student’s t-test (d, e and g) and one-way ANOVA (h, l and n). N.S, not significant.
Typical membrane proteins were also involved in the priming and activation of MKs. We found that the percentage of CD44+ cells within MKs isolated from CB-exposed mice increased from 11% (6 h) to 160% (24 h) (Supplementary Fig. 5f, P < 0.05). Meanwhile, the percentage of P-selectin (CD62p)+ cells, and MHCII+ cells within MKs increased by 22% and 34%, 45% and 230% than untreated control, respectively (Supplementary Fig. 5g, h, P < 0.001). The high expression pattern of CD44 and CD62p could enhance MK adhesion to endothelial cells and immune cells (e.g., leukocytes), and mediate heterogeneous cell-cell interactions, as described41,42. Analogously, higher expression of antigen presentation cell-like marker MHCII in MKs substantiated the activated state of MKs with the ability to present antigens to adaptive immune cells14.
To further determine whether MKs are a requisite for particle-triggered immune responses in the lung, we used an MK-deficient (c-mpl−/−) mouse model43. These animals lack MKs and platelets throughout the body, while other blood cell counts, pulmonary vascular integrity and extrapulmonary tissue remain normal (Supplementary Fig. 6a–d). Although the three most recognized methods for targeting deletion of MKs including the use of PF4Cre-iDTR mice44, injecting CD42 Abs14 and c-mpl−/− model have limitations in analyzing the specific contribution of MKs or platelets43, c-mpl−/− model considerably harbors advantages in ensuring minimal influence on other immune cells and ensuring long-lasting depletion for observation, which can help define the role of MKs in inflammatory responses towards particles45. Unlike wild type (WT) mice with intact c-mpl (Fig. 1), the lungs of c-mpl−/− animals exposed to CB particles for 24 h had fewer inflammatory foci (Fig. 3f), and 35% fewer polymorphonuclear neutrophils (PMN) (Fig. 3g, P < 0.05). Aside from this, IL-1β and IL-6 protein content in the sera and BALF collected from CB-treated c-mpl−/− mice were respectively 45% and 34% lower than WT mice (Fig. 3h and Supplementary Fig. 7a, P < 0.05), implying that the lack of MK-platelet axis could compromise particle-induced immune responses.
Because immune cells are critical for particle clearance and tissue injuries25, we followed the animals up to the recovery period of 72 h after particle exposure46. C-mpl−/− animals displayed atelectasis (collapsed alveoli) and abnormal alveoli structure (Fig. 3i) after 72 h, along with increased blood vessel (CD31+) permeability (Fig. 3j). This delayed recovery of inflammation in c-mpl−/− mice was also reflected by the 135% higher fibrillar collagen deposition (Supplementary Fig. 7b), ~1.6-fold greater number of myeloperoxidase (MPO)/lymphocyte antigen 6 complex, locus G (Ly6G)47 positive neutrophils (Fig. 3k–l), 1.4-fold higher percentage of PMNs and macrophages, and 1.2-fold greater number of peripheral blood inflammatory cells than treated WT animals (Supplementary Fig. 7c–f, P < 0.05). Fluorescent image analyses also showed that c-mpl−/− mice had 47% more particles (Supplementary Fig. 7g, h) at the terminal bronchiole and interstitium region than WT mice. Moreover, most particles distributed randomly in the interstitium, being engulfed by the immune cells rather than by epithelial cells (Supplementary Fig. 7i), indicating that the worse vascular leak in exposed c-mpl−/− mice was caused by slower recovery from particle-induced inflammation and pairing poorer clearance of deposited particles. Notably, using the MK transplant model14, compromised inflammatory responses in c-mpl−/− mice could be reversed after pre-reinfusion with isolated MKs through o.p.a. route 2 days before CB treatment (Supplementary Fig. 7j). Phagocytosed particles were found in the transplanted MKs after 24 h-exposure (Supplementary Fig. 7k). MK-receiving mice showed 1.5-fold more MPO+Ly6G+ neutrophils in lungs (Fig. 3m and Supplementary Fig. 7l) and 32.2% increase of IL-1β in the sera (Fig. 3n) than mice without MK transplant. These multiple lines of evidence together indicated that MKs were important cells in inflammatory response towards particles (Fig. 3o).
Particles activate MKs to release platelets
Airborne particle pollution promoted biomarkers of thrombosis in healthy young adults48. However, it remains unclear whether a certain part of the circulating platelets is directly generated from lung MKs upon particle exposure. To investigate this, we treated PF4-mTmG mice with Cy5-PSs and examined thrombocytosis of MKs. Whole-mount sections of lungs collected from mice exposed to Cy5-PSs for 24 h revealed that MKs with internalized particles produced cytoplasmic extensions that formed proplatelets (Fig. 4a), consistent with previous observations that mature MKs released platelets by remodeling their cytoplasm into long, and branched cytoplasmic projections49. Against the pulmonary capillary, these particle-bearing MKs were seen to move from the alveoli to the interstitium region and accumulate beside the capillaries (Fig. 4b), implying that these MKs are primed to release platelets into the blood circulation by protruding extended cytoplasm into the nearby endothelial wall.
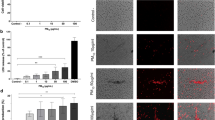
a Fluorescent images of lung sections from PF4-mTmG mice treated with 2 mg/kg body weight Cy5-PSs for 24 h and untreated control. White arrow pointing to MKs with engulfed particles releasing platelets (white arrowhead). b Fluorescent image of lung MK releasing a nascent platelet (green arrow) into a Alexa Fluor 647-dextran dye -stained blood capillary (red arrow). c Number of resting and activated platelets in peripheral blood of CB particle-treated mice (n = 8). d Flow cytometer analysis plots of activated platelets (488SSC-405SSC+/lowCD41+CD62P+) in peripheral blood of control and CB particle-treated animals from c. e Scanning electron microscope images of resting and activated platelet from CB-treated animals. f Peripheral blood of mice (n = 8) treated with CB for 24 h clotted faster than untreated control. g Bright-field images and counts per field show more platelets (blue arrowheads) from platelet-rich plasma (PRP) of CB particle-treated mice attached on the collagen matrix than untreated control (n = 10 randomly captured images). h KEGG pathway enrichment analysis of upregulated genes associated with MK development and platelet production, one-side Fisher’s exact test. MKs were isolated from mice treated with CB particles for 24 h. i Ex vivo maturation assay involves culturing isolated CD41+ MKs in TPO/SCF/IL-6 medium. Fluorescent images of membrane-stained matured MKs and nascent platelets after 2 days. j Live-cell imaging of isolated MKs with engulfed Cy5-PSs using LSFM features an extended cytoplasm and membrane shedding nascent platelet clusters (white dotted lines). k, l Flow cytometer analysis of the number of total platelets (CD41+Hoechst-, k) and activated platelets (CD41+CD62p+Hoechst-, l) in culture medium. MKs were pretreated with 200 ng/mL Cy5-PSs and cultured for 2 days (n = 10 biological replicates). m Schematic showing that particle inhalation induces greater activated platelets in circulation than physiological conditions. Box and whisker plots indicate median, interquartile range and 10th to 90th percentiles of the distribution (f, g). Data are presented as the mean values of 3 independent experiments ± SEM (c, f, g, k and l), two-side student’s t-test (c, f, g, k and l). Figure 4i and m, created with BioRender.com, released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.
After CB exposure, the number of total platelets and activated platelets (CD41+CD62p+) in the peripheral blood increased by ~30% and 39% than untreated control, respectively (Fig. 4c, d, P < 0.05). Importantly, platelets from CB-treated mice transformed from a disc (2–5 μm in diameter) to an irregular shape with pseudopod-like structures (Fig. 4e). Because of increased number and aggressive shape of platelets, CB-treated animals showed 28% shorter bleeding time and 20% faster clotting time compared to untreated control (Supplementary Fig. 8a and Fig. 4f), consistent with previous results demonstrating that activated platelets accelerated coagulation and increased the risk of thrombosis in peripheral organs50. Collagen adhesion assays showed that peripheral blood from CB-treated mice caused 2-fold more platelets attaching to the collagen matrix than blood from untreated control (Fig. 4g and Supplementary Fig. 8b, P < 0.001). Ex vivo maturation assay confirmed that platelets from particle-treated Dami MKs had 1.5-fold higher adhesion than those from untreated MKs (Supplementary Fig. 8c). Collectively, these results demonstrated that CB particles could trigger MKs to release activated platelets bearing features conducive to faster clotting and enhanced adhesion.
To further track the activated state of platelets in vivo, we challenged the animals once or twice with CB particles and afterwards examined peripheral blood through flow cytometry. There were 2-fold greater activated platelets found in CB-treated mice than in untreated control, and these platelets remained in the blood circulation up to 3 days (Supplementary Fig. 8d, e, P < 0.05), supporting that CB exposure increased number of MK-derived activated platelets, leading to disorders in the balance between clearance and production of platelets in circulation, and a systemic hypercoagulable state. To confirm the origin of these activated platelets, we quantified MKs and MK progenitors (MKps) in lung, bone marrow and spleen, which are main sites for platelet production and recovery11,51. MKps did not proliferate after particle exposure (Supplementary Fig. 9a), hinting their incapacitation in participating in emergency thrombopoiesis within 24 h. Strikingly, there were 32% and 65% more lung MKs after 12- and 24-h exposure, however, only 34% more bone marrow MKs post 24 h (Supplementary Fig. 9b) compared to untreated control, implying that lung MKs are more sensitive responders to particles than bone marrow MKs. Moreover, these upregulated genes in lung MKs isolated from CB-treated mice were enriched in signaling pathways associated with MK proliferation and platelet production process (Fig. 4h and Supplementary Fig. 9c), including Ras-proximate-1(Rap1) signal52, Hippo signal53 and actin cytoskeleton, supporting that the rapid increase of platelets in such a short time was more likely triggered by lung MKs. Considering most upregulated genes, such as TAL bHLH transcription factor 1 (Tal1), TEK receptor tyrosine kinase (Tek) and myosin light chain kinase (Mylk), were implicated in platelet production (Supplementary Fig. 9c), we performed an ex vivo maturation assay by culturing isolated CD41+ cells in thrombopoietin (TPO)/stem cell factor (SCF)/IL-6 medium54 (Fig. 4i, the left panel) to look into the stimulation of particles on platelet production from MKs seen in vivo (Fig. 4a). After 2-days in culture, isolated lung MKs displayed typical cytoplasmic extensions, large pseudopod-like structure and expansive membrane system (Fig. 4i, right panel). Additionally, real-time live-cell images revealed that these proplatelets separated from extended membrane of MKs within 3 min (Fig. 4j). Compared to untreated control, the number of nascent platelets and CD41+CD62p+ activated platelets (Fig. 4k, l and Supplementary Fig. 9d, P < 0.001) were respectively 28% and 59% higher in culture medium of particle-pulsed MKs. Our comprehensive in vivo and ex vivo experiments together unraveled that CB particles activated lung MKs to release platelets into blood, partly contributing to 60% more activated platelets in circulation (Fig. 4m). This increased platelet circulation likely explains the platelet accumulation-caused fibrous capillary network seen in particle-treated animals (Fig. 1l).
MK-derived platelets inherit particles from parental MKs
To determine the in vivo fate of particles engulfed by MKs, we examined the offspring-platelets because platelets are known to inherit most membrane and cytoplasmic components of their parental MKs during thrombopoiesis55. Intriguingly, Cy5-PSs were observed in the cytoplasm of CD41+ platelets sorted from the circulating blood of particle-treated mice (Fig. 5a). Using pulse-labeling circulating platelet method, about 15% of newborn platelets presented the Cy5-PS fluorescence signal were found after 24 h-exposure (Supplementary Fig. 10a–f), and the mean fluorescence intensity (MFI) of Cy5-PS signal in newborn platelets was much higher than that of the pre-existing platelets (MFI 120 vs 10, Supplementary Fig. S10h). To exclude whether these intracellular particles were phagocytized by platelets, PSs ranging from 0.08 to 2 μm diameter, simulating the inhaling PMs56, were severally incubated with blood-derived platelets. TEM images showed that there were no engulfed PSs in platelets (Supplementary Fig. 10i), partly echoing that platelets lack a sufficient number of full functional mitochondria to supply energy for positive phagocytosis57, especially in rapid blood flow. To simulate their free absorption towards PMs in circulation, isolated platelets mixed with Cy5-PSs were transplanted into c-mpl−/− mice through o.p.a. route (Fig. 5b). These complexes were found be mainly swallowed by CD45+ white blood cells in the lung interstitium once deposition (Fig. 5d). Moreover, although very few Cy5-PSs were accumulated in livers, they were attached onto the platelet surface rather than being internalized after 72 h-transplantation (Fig. 5c), suggesting that these platelet-borne particles found in circulation were transferred from their parental MKs.
a Immunofluorescent images of CD41+ platelets from peripheral blood of mice exposed to 2 mg/kg body weight Cy5-PSs for 72 h show platelet colocalized with fluorescent particles (red). b Schematic shows o.p.a. transplantation of 1.0 ×107 platelets and 50 ng Cy5-PS mixture into c-mpl-/- mice. c Fluorescent images of liver sections from recipient c-mpl-/- mice in b 72 h after transplantation showing Cy5-PSs adhered onto platelets (Green). d Fluorescent images of lung sections in recipient mice showing platelets (green) and Cy5-PSs (red) inside CD45+ immune cells (yellow) at 72 h post platelets-Cy5-PS mixture transplantation. e Intravital two-photon imaging shows lung MK (green) releasing nascent platelets containing Cy5-PSs (pink). Red dotted square is magnified in bottom right. f Fluorescent images of Dami cells treated with 200 ng/mL Cy5-PSs for 12 h showing endoplasmic distribution of engulfed particles. Phalloidin-stained cytoskeleton (green, left panel), carbocyanine-stained membrane (red, right panel) and engulfed fluorescent particles. g Continuous imaging analysis of MKs every 3 min. Nascent platelet clusters released from Cy3-labeled MKs (yellow) are shown. h Flow cytometry images of Cy5-PSs (red)-laden platelets (CD41+, green) collected from MK induction medium. Figure 5b, created with BioRender.com, released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.
We afterwards applied two-photon intravital microscopy to track the particles-laden MKs. After 12 h, MKs with internalized particles accumulated beside capillaries, where MKs released platelets into blood circulation by protruding extended cytoplasm into the nearby endothelial wall (Fig. 5e and Fig. 4b), consistent with the previous finding that the entire sequence of platelet releasing from MKs varied from ~20 to 60 min in the lung11. In detail, the engulfed particles were mostly trapped within the focal tubular membrane early in the maturation of MKs (Fig. 5f). Real-time live-cell imaging analyses showed that proplatelets shedding from particle-laden MKs involved 5 steps: cytoplasmic maturation, cytoplasmic protrusion, cytoplasmic fracture, fracture enlargement and proplatelet shedding (Fig. 5g and Supplementary Movie 3). Within 12 min, particle-bearing platelets (2–5 μm diameter) were released into the culture medium (Fig. 5h), consistent with in vivo observation of activated particle-laden platelets in Fig. 4. Together, these observations signified our findings that MKs engulfed particles and transferred particles from mature MKs to nascent platelets during thrombopoiesis.
MK-derived platelets transport particles to extrapulmonary organs
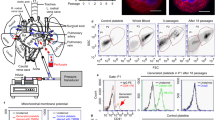
Next, we examined how MK-derived platelets contribute to particle transport by transplanting particle-laden CD41+GFP+ donor MKs into homologous c-mpl−/− recipient mice (Fig. 6a). The mass of MK-borne particles was rigorously equivalent to those in transplantation of particle and platelet mixture in Fig. 5. These transplanted MKs stably colonized the lungs of recipient mice for >72 h (Fig. 6b). Thereafter, panoramic scan analysis was performed to track donor MK-derived platelets (CD41+GFP+DAPI−) in extrapulmonary organs 72 h post-transplantation. Whole-mount sections of the liver, kidney and spleen showed that donor MK-derived platelets were present in these organs, and nearly 70% of the GFP+DAPI− platelet signals overlapped with particles (Fig. 6c, d and Supplementary Fig. 11a, b). Relative fluorescence intensity (RFI) analysis found 2.3-fold more platelet-bound particles in the liver and spleen than diffused platelet-free particles, and most GFP-labeled platelets in the liver were seen mainly in the vessel lumen (Fig. 6e), indicating that particles were substantially transported by MK-derived platelets delivered by circulating blood (Fig. 6d, P < 0.001). To test alternative routes such as lymph and exosomal vesicles (EVs) in particle and platelet delivery, we examined the mediastinal and abdominal lymph sections and found there were no platelet-bound particles (Supplementary Fig. 11c, d). Moreover, particle-laden EVs (with the average size <1 μm) were also barely observed in anti-CD63 (a classical marker of platelet EVs58) Abs-stained liver, spleen and kidney sections of mice receiving MK transplantation (Fig. 6f). Together, our results indicated that MK-derived platelets inherited and transported particles from parental MKs to the liver, spleen and kidney through blood circulation.
a Schematic shows o.p.a. transplantation of particle-laden CD41+GFP+ MKs into c-mpl-/- mice for tracking platelet distribution. b Fluorescent images of lung sections from recipient c-mpl-/- mice show MKs (CD41+GFP+DAPI+) remain loaded with Cy5-PSs (red) 72 h after transplantation. c Immunofluorescent images of liver sections from recipient c-mpl-/- mice 72 h after MK transplantation. Donor MK-derived platelets (green) overlap with Cy5-PSs (red). d Ratio of platelet-bound and platelet-free particles in liver, spleen and kidney determined by RFI analysis of fluorescent images (n = 6 randomly captured images). e Fluorescent images show donor-derived platelet (green) in the liver is transported through blood vessel (Alexa Fluro 647-dextran+, circled out with white dotted line). f Immunofluorescent images of EVs (CD63+, yellow) and Cy5-PS (red) in liver sections from recipient c-mpl-/-mice 72 h after MK transplantation. CD41+GFP+ donor MK-derived platelets (green) with engulfed Cy5-PSs (red) are shown in the bottom as reference. g Fluorescent images of liver sections from WT and c-mpl-/- mice after exposure to Cy5-PSs for 72 h. Fewer particles are seen in c-mpl-/- mice. h ICP-OES analysis of SiO2 content in the lung, liver, kidney, spleen and peripheral blood of WT and c-mpl-/- mice treated with 2 mg/kg body weight SiO2 particles for 72 h (n = 8 mice per group). i Regression analysis of the particle content in the liver based on RFI quantification and ICP-OES method. Dotted lines represent 95% confidence intervals for the linear regression line. Each dot corresponds to one individual mouse sample. Statistical analysis was performed by linear regression analysis, two-side Pearson correlation test. j Counts per field of TUNEL positive cells in liver sections from WT and c-mpl-/- mice at 24 and 72 h after CB exposure (n = 3 mice per group). Box and whisker plots indicate median, interquartile range and 10th to 90th percentiles of the distribution (d, h). Data are presented as the mean values of 3 independent experiments ± SEM (d, h and j), P-value by one-way ANOVA (d, h and j). N.S, not significant. Figure 6a, created with BioRender.com, released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.
Given MK-platelet axis deficiency impaired the lungs’ ability to clear inhaled particles (Fig. 3), we examined the extrapulmonary distribution of particles in MK deficient c-mpl−/− mice and the model of CD42 Abs mediated depletion of lung MK14. We found 43% fewer fluorescent particles in the liver of c-mpl−/− mice when compared to WT animals (Fig. 6g and Supplementary Fig. 11e, P < 0.05). Similar results were also observed in anti-CD42b Ab-treated mice, which showed a 43% increase trapped particles in lungs, and consequent 40%, 36% and 48% decrease in particles in the liver, kidney and spleen, respectively, compared to isotype-IgG-pulsed mice (Supplementary Fig. 12). To analyze the contribution of Mks on neutrophil recruitment and particle clearance analogy to macrophages, MK-deficient and macrophage-deficient (81% depletion of CD11c+F4/80+ macrophages) mice were constructed. We found MPO+Ly6G+ neutrophils in the lung were reduced by 79% and 71% at 24 h, resulting in 202% and 290% increase of trapped particles after 72 h-exposure, respectively, in these 2 models relative to controls. Double-deficiency caused more severe dysfunction than either, suggesting that MKs would involve in immune cell recruitment and particle clearance independently (Supplementary Fig. 13a–c). To corroborate this finding, we used SiO2 as model particles and quantified the content of inhaled particles in each organ using inductively coupled plasma optical emission spectroscopy (ICP-OES)5. Consistent with the data in Supplementary Fig. 7g, h, more than 44% SiO2 particles were retained in the lungs of c-mpl−/− mice than those in WT mice (P < 0.05). Moreover, liver, kidney, spleen tissues and blood collected from c-mpl−/− mice 72 h after particle exposure respectively showed 34%, 38%, 35% and 36% less SiO2 than WT mice (Fig. 6h, P < 0.05). Interestingly, these decreases were nearly equal to the amount of SiO2 retained in the lungs of particle-treated c-mpl−/− mice. Thus, deletion of the MK-platelet axis hampered the transport of particles from the lung to extrapulmonary organs.
Regression analysis uncovered a strong correlation between the ICP-OES and RFI methods, supporting the reliability of our findings (Fig. 6i). Combining data from these 2 methods, the contribution of MK-platelet axis in extrapulmonary transport of particles was calculated by taking the difference between the total amount of extrapulmonary particles detected in specific organs of WT mice and c-mpl−/− mice, and dividing the difference by the total amount of extrapulmonary particles detected in specific organs in WT mice. Our calculations revealed that nearly 30% of particles in the distal organs (e.g., liver, kidney and spleen) were transported through the MK-platelet axis. To understand whether MK-mediated transport of inhaled particles incurred extrapulmonary injuries, we examined the liver sections of WT and c-mpl−/− mice exposed to CB particles for 24 and 72 h through TdT-mediated dUTP Nick-End Labeling (TUNEL) assay (Fig. 6j and Supplementary Fig. 13d). Reduced number of dead cells in liver of c-mpl−/− mice might be a result of combined action of reduced particle transport into liver and delayed inflammation (Supplementary Fig. 13e) Together, these results demonstrated that particle inhalation promoted platelet production from lung MKs. These activated platelets, in part, contributed to the transport of inhaled particles from to extrapulmonary organs (Fig. 7).
Discussion
The question of how inhaled PMs enter the circulation and further distribute around organs is of major scientific interest and is not clearly understood. As lung immune cells are primarily responsible for the reactions and clearance of inhaled airborne particle matters, addressing the current knowledge gaps on how lung resident immune cells recognize and respond to invaded particles is significant. In this study, we found the route of phagocyte-mediated phagocytosis and transport of particles across the air-blood barrier to achieve extrapulmonary transport. Our findings unraveled that, benefiting from the resident location in the interstitial and alveolar regions, lung MKs acted as critical responders to inhaled particles, as evidenced by quickly crawling towards and engulfment of particles. Consequently, activated MKs released activated platelets into the blood circulation through thrombopoiesis. Admittedly CB-increased cytokines in blood can also stimulate circulating platelets, resulting in a high number of activated platelets in circulation59, but we uncovered that the rapid increase of circulating activated platelets were more likely triggered by lung MKs. Lung MKs-derived platelets increased the risk of resembling fibrin aggregates in lung and systemic hypercoagulable state, due to their enhanced adhesion and clotting potentials compared to normal, resting platelets. Importantly, these platelets inherited engulfed particles from their parent MKs, accelerating the translocation of particles from the lung to extrapulmonary organs such as the liver for clearance.
To summarize, our study has uncovered that lung MKs have distinct functions in the clearance and biodistribution of inhaled PMs that might explain the particle-induced injuries found in distant organs. Lung MKs crawled along the alveolar wall to engulf inhaled particles, and promoted extrapulmonary transport of particles through releasing particle-laden platelets. This proof-of-concept for the cell delivery of particles supports the discovery of new subtypes of immune cells-responding to inhaled particles or the investigation of of resident/recruited immune cells-conducted contributions in crossing behavior of PMs through special biological barriers. More investigations on other paths of PMs entering the bloodstream and distributing throughout the body still need to be explored in the future.
Methods
Particle matter preparation and animal inhalation exposure
Airborne PM2.5 samples were collected by DL-6100 medium flow PM2.5 sampler (Dongliweiye Environmental Protection Equipment Co., Ltd., China) in Beijing, China from November, 2022 to March, 2023. PM2.5 specimens loaded onto the polytetrafluoroethylene filter membranes were eluted in ultrapure water by ultrasonic extraction and then freeze-dried. CB particles (20 nm, Macklin Inc, China), PSs (0.08 μm, 0.2 μm, 2 μm, Invitrogen, USA) and Cy5-PSs (0.2 μm, Invitrogen) were purchased.
All animal care and protocols were approved by the Animal Ethics Committee at the Research Center for Eco-Environmental Sciences (RCEES), Chinese Academy of Sciences (CAS). The approval number of animal experiment is AEWC-RCEES-2020001. C57/6J WT and BALB/c mice were purchased from the Vital River Laboratory Animal Technology Co. Ltd (Beijing, China). MK deficient mice (c-mpl−/−) were provided by GemPharmatech Co. Ltd (China). PF4-cre mice (strain: #008535) and mTmG mice (strain: #008535) were obtained from the Jackson Laboratory. PF4-mTmG mice were identified among the offspring resulting from the crossing of PF4-cre mice and mTmG mice, based on the specific expression of Cre recombinase, as described11. The housing and experimental procedures for the mice strictly adhered to the guidelines of the National Institutes of Health (NIH) and the Animal Management Committee of RCEES, CAS. Mice used for experiments were 7–8 weeks old, male and female, and were age-matched. These mice were bred, fed food (Xietong Pharmaceutical Bio-engineering Co., Ltd, Cat#1010002) and drank water in a specific pathogen-free environment, with a 12 h light/dark cycle. The temperature was maintained between 21 and 24 °C, while humidity was kept between 30 and 70%. Particle solution (2 mg/mL) was prepared in endotoxin-free PBS solvent. For o.p.a., 20 μL particle solution was injected into the trachea of anesthetized mice via a 20-gauge catheter. For inhalation exposure, mice were allowed to inhale suspended dry particles in an atmospheric chamber to simulate human breathing60. Suspended 160 μg/mL solution of particles was atomized into the atomizing chamber to expose the animals for 3 days (240 min per day). Control animals received vehicle solution PBS only. Blood routine parameters of each mouse was determined with a hematology analyzer (Nihon Kohden, Japan).
Cell culture
Human leukemia cell line-Dami (Cat#20190827-1) and Meg-01 cells (Cat #1101HUM-PUMC000521) were purchased from the Pricella (China) and National Infrastructure of Cell Line Resource (Beijing, China), respectively. These cells were cultured in DMEM medium (Gibico, USA), containing 10% fetal bovine serum (Gibico) and 1% penicillin-streptomycin double antibody26 (Gibico). Before conducting the experiments, Meg-01 and Dami cells were authenticated by identification of Short Tandem Repeat (STR) on ABI 3730xl DNA Analyzer platform (Applied Biosystems, Foster City, California). Dami and Meg-01 cells were treated with 1 μM phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich, USA) in DMEM medium (Gibico) for 2 days to induce matured MKs, respectively.
Detection of cell subpopulations in pulmonary alveoli, lung parenchyma and extrapulmonary tissue through flow cytometry
After particle exposure, mouse was anesthetized by intraperitoneal injection of 1% (0.01 g/mL) sodium pentobarbital, and BLAF was collected for the analysis of cell subtypes in pulmonary alveoli. To avoid infiltration of blood cells and platelets, the preparation process of single-cell suspension from BLAF was optimized. Platelets attached to the surface of red blood cells or adhered to white blood cells were cut off using a special wash-free cracking filter kit (Attune™ CytPix™ No-Wash No-Lyse Filter Kit, Invitrogen) on an Attune NxT flow cytometer platform (Thermo Fisher Scientific, USA). Intact and lysed erythrocytes were cut off based on hemoglobin absorption at 405 nm ultraviolet light. The single-cell suspensions of lung parenchyma were prepared by enzymatic digestion with DNaseI (Solarbio, China) and LiberaseTM (Roche, Switzerland). The single-cell suspensions of bone marrow and spleen were prepared. All cell samples were filtered with 70-μm cell strainer, with 1.0 × 106 cells per tissue sample were isolated for staining. Cell surface staining was performed with a series of fluorescent dye-conjugated Abs at 4 °C for 20 min protecting from light. Detailed information of Abs used in flow cytometry was described in Supplementary Table 3. All fluorescent dye-conjugated Abs were obtained from the eBiosicence (San Diego, CA, USA), including MKs (CD41+Hoechst+), alveolar macrophages (F4/80+CD11c+CD170+CD11b−), interstitial macrophages (F4/80+CD11c+CD11b+), PMNs (F4/80−CD11b+Gr-1hi), dendritic cells (CD11c+MHCII+CD103+/CD11b+), eosinophils (CD54+CD62L+CD11b+), neutrophils (Ly6G+CD11b+), basophils (CD45+CD49b+CD11b+), and MKp (Lin-Sca-1-c-kit+ CD45+CD150+CD41+).
Histological analysis, and Masson, TUNEL, immunofluorescence staining of tissue sections
Lung and lymph nodes collected from particle-treated mice were processed for 4% paraformaldehyde fixing and paraffin embedding according to standard immunohistochemistry procedures, as described24. Following these standard protocols, tissue specimens were stained using H&E or Masson trichrome kits. H&E or Masson-stained sections were examined by light microscopy.
Immunofluorescence analysis of tissue sections was performed following established procedure24. Lung sections were dual stained with anti-mouse MPO Abs (Proteintech, USA) combined with anti-mouse Ly6G Abs (Servicebio, China) to denote neutrophil, and anti-mouse CD41 Abs (Abcam, USA) combined with anti-mouse CD42b Abs (Servicebio) or anti-mouse PF4 Abs (Abcam) to confirm MKs. After primary Ab binding, these sections were incubated with fluorescein isothiocyanate isomer I (FITC)- and Cy5-conjugated secondary Abs. For vessel staining, lung sections were stained with anti-mouse CD31 Abs (Abcam), followed by Cy5-conjugated secondary Abs. Liver sections were examined by TUNEL kit (Beyotime, China) to examine cell death, and stained with anti-mouse CD45 Abs (Abcam) to detect immune cells, respectively. All slices were imaged using a panoramic scan (3DHISTECH, Budapest, Hungary). For fluorescent image analyses of tissue sections, under 10-40 x magnification, 3-5 fields of each view from individual mouse were quantified. These counts were then averaged to represent the value for each mouse.
To visualize blood vessels and analyze their integrity, Alexa Fluor 647-labeled dextran (2256832, Thermo Fisher Scientific) was administrated into mice at 50 mg/kg body weight through intravenous injection (i.v.) route11. Mice were sacrificed for organ collection 5 min after injection. These prepared frozen slices were then examined on fluorescence imaging system under epi-illumination using appropriate filter sets.
3D fluorescence imaging of whole lung through FDISCO method
To visualize the 3D location of MKs in whole lungs by LSFM, lungs from PF4-mTmG mice received advanced optical clearing treatment (known as FDISCO) to transform opaque tissues into transparent, light-permitting specimens21. After FDISCO clearing, the tissues were stored in DBE in airtight glass chambers at 4 °C in the dark. Prior to an optical clearing, the tissue was embedded the DRGs in PBS/1% Agarose gel columns in one plane to facilitate subsequent image acquisition. These tissues were then immersed in an ascending series of precooled (4 °C) tetrahydrofuran (Cat# 186562, Sigma-Aldrich) double distilled water solutions (50%, 75%, 3 × 100%) for dehydration and delipidation. Triethylamine (Cat# 471283, Sigma-Aldrich) was used to adjust the pH of the solutions to 9.0 and all steps were performed in the dark at 4 °C on an orbital shaker. The specimens were further immersed in ethanol/dibenzyl ether (Cat# 108014, Sigma-Aldrich) solutions overnight at 4 °C in the dark, with 2 times changes of the solution, to match the refractive index of the tissue with the microscope objective. To optimize the imaging solution for glycerol/CLARITY (clear lipid-exchanged acrylamide-hybridized rigid imaging/immunostaining/in situ-hybridization-compatible tissue-hydrogel) objectives, the best trade-off between transparency-dependent imaging depth and spherical aberration in ascending ethanol/dibenzyl ether solutions for each tissue was optimized. The transparent lung tissue was imaged by LSFM (Ultramicroscope, LaVision BioTec, Germany) workflow as described21.
Lung intravital imaging by 2PIVM
To observe real-time movement of MKs in the lung, PF4-mTmG mice were pretreated with 2 mg/kg body weight Cy5-PSs before imaging. Mice were then anaesthetized with ketamine and secured to the microscope stage. For anesthesia maintain, the fixed mice were intubated via the trachea with a tracheal cannula, continuously delivering isoflurane (2% isoflurane/98% O2) through the mouse ventilator (MiniVent 845; Hugo Sachs Elektronik). A small surgical incision was positioned between third and fourth ribs to expose the lung lobe, and microscope objective was then adjusted close to the lobe surface. Using channels of eGFP (excitation 488 nm) and tdTomato (excitation 561 nm), random fields (738 μm x 738 μm area) in the lung were captured with a two-photon confocal laser scanning microscope (Leica TCS SP8, Germany) every 5 min for 30 min, along with z-depths of 5 μm (total of 840 μm z-depth). Images were further analyzed with Leica Application Suite X (Leica).
Alv. MKs and Int. MKs by intra-alveolar staining
To ensure the Alv. and Int. MKs accurately isolated based location, an intra-alveolar microinjection method was used to stain only alveolar cells. Non-specific binding of Abs with Fc receptors on immune cell surface was first blocked by injecting the mice with 2 μg TruStain fcX TM (anti-mouse CD16/32 Abs) for 5 min. Thereafter, 2 μg PE-labeled anti-mouse CD41 Abs or isotype control (eBRG1, eBioscience) was administered into the alveoli through o.p.a. route. Alv. MKs and Int. MKs were defined as FITC-CD41+PE-CD41+Hoechst+ and FITC-CD41+PE-CD41−Hoechst+cells, respectively. Besides, double immunostaining (CD41 and CD42 Abs) was performed on sorted cells for observation with a confocal microscope (Olympus, Japan).
Isolation and Giemsa-Wright staining of lung MKs and monocyte/macrophages
Alv. and Int. MKs were isolated and enriched through magnetic bead separation from the single-cell suspension of BALF and lung parenchymal tissues, respectively. After being blocked with TruStain fcX TM and incubated with CD41-Biontin Abs (MWReg30, eBioscience, USA), CD41+ cells were isolated with MagniSort™ Streptavidin Positive Selection Beads (MSPB-6003-71, Invitrogen), according to manufacturer’s instruction61. Analysis of MK purity was performed using flow cytometry, where these isolated cells were incubated with PE-labeled CD41 Abs and subjected to positive cell detection. The isolated MKs were cultured in DMEM medium containing 25 ng/mL TPO (BioLegend, USA), 25 ng/mL SCF (BioLegend) and 10 ng/mL IL-6 (BioLegend) for maturation14. After being incubated with CD170-Biontin Abs (S17007L, Biolegend, USA), CD170+ cells were further isolated with MagniSort™ Streptavidin Positive Selection Beads (MSPB-6003-71, Invitrogen), according to manufacturer’s instruction. To distinguish MKs from alveolar monocyte/macrophages, isolated CD170 (Siglec F)+ cells and Alv. MKs were both subjected to cell smears and stained with Wright-Giemsa dye (Solarbio) for morphological characterization.
Assessment of MK phagocytosis of particles
To recognize the engulfment of particles by MKs in vivo, the frozen lung sections of particle-treated PF4-mTmG mice were processed into slices by a cryostat, and imaged using a panoramic scan (3DHISTECH) after cell nuclei staining with DAPI (10 μg/mL, Beyotime). More than 15 random fields in the image were manually quantified using Image J software (NIH, USA) to count the number of particle positive MKs.
To determine engulfed particles in MKs at the single-cell level, primary MKs from Cy5-PS-treated mice were subjected to flow imaging analysis, as described14. After being labeled with FITC-conjugated anti-CD41 Abs, 5000 MKs cells were collected on Imaging Flow Cytometer (Amnis ImageStream MKII, USA) for data analysis using IDEAS software (Amnis, USA). The content of engulfed particles per cell was quantified based on the fluorescence intensity of intracellular Cy5-PSs.
To visualize the intracellular distribution of engulfed particles in MKs, sorted lung MKs, Dami- and Meg-01- derived MKs received 200 ng/mL Cy5-PS treatment for 12 h, were subjected to cell membrane staining with 5 μM fluorescein isothiocyante-carbocyanine dyes (Beyotime), and cell cytoskeleton staining with 10 ng/μL Alexa 488-phalloidin (Beyotime), respectively. And these cells were subjected to observation under Fluoview FX1000 confocal microscope62 (Olympus) with 60-100 × magnification. Besides, using a high-resolution TEM (Hitachi, Tokyo, Japan), the phagocytosis in CD41+ MKs was examined after being dehydrated, embedded, ultrathin-sectioned and stained with uranyl acetate and lead citrate (Solarbio).
Determination of cytokine content and gene expression
The concentrations of cytokines, including IL-1β and IL-6, were determined in mouse sera and cell medium using ELISA kits according to the manufacturer’s instructions (Mouse IL-6 ELISA kit (NEOBIOSCIENCE, China)), IL-1β ELISA kit (NEOBIOSCIENCE). Primers used for Reverse transcription-PCR (RT-PCR) are as follows: Caspase-1 (F: ACAAGGCACGGGACCTATG, R: TCCCAGTCAGTCCTGGAAATG), Nalp3 (F: ATTACCCGCCCGAGAAAGG, R: TCGCAGCAAAGATCCACACAG), ASC (F: TGCCAGAGCTGATTGACATTC, R: GGCATACCAGAAGGTGGTGAG), IL-1β (F: ATGATGGCTTATTACAGTGGCAA, R: GTCGGAGATTCGTAGCTGGA). RT-PCR was used to quantify gene expression level, with β-actin as an internal reference gene.
Western blotting was performed by SDS–polyacrylamide gel electrophoresis (SDS–PAGE)63. PMA-pulsed Dami cells were exposed to CB particles with different doses (0, 0.02, 0.2, 2 and 20 μg/mL) for 24 h. Cells were lysed and analyzed by Western blotting, using the anti-mouse Capsase-1 (1:1000 dilution, Abcam, USA) and anti-mouse IL-1β (1:1000 dilution, Abcam) primary Abs for detection. Expression levels were normalized with an internal reference (β-actin).
Macrophage depletion and CD42 mediated depletion of MKs
For macrophage depletion, clodronate liposomes (YEASEN Inc, Shanghai, China) were injected into WT (C57/6 J) and c-mpl−/− mice at 48 h before particle administration through i.v. and o.p.a. routes to clear interstitial and alveolar macrophages25, respectively. In brief, 5 mg/kg body weight clodronate liposome (from Vrije University Amsterdam) with the volume of 150 μL and 30 μL was administered into those mice. The same amounts of PBS-laden liposomes (YEASEN Inc) were administered for control mice. For the depletion of lung MKs, WT (C57/6 J) mice were injected with 2 µg/g of anti-CD42 Abs or Isotype IgG (BioLegend) via i.v. route, according to the previous study14.
Evaluation of platelet activation and coagulation function
To access the activation of platelets, the expression of surface marker CD62p in isolated platelets was examined. Briefly, using sodium citrate anticoagulation tube (3.8% wt/vol, Sigma-Aldrich), blood of exposed mice was diluted with PBS at 1:4000 in volume, and stained with PE-conjugated anti-mouse CD41 and APC-conjugated anti-mouse CD62p Abs to detect the number of activated platelets within 1.0 × 106 platelets through flow cytometry.
For ex vivo adhesion assay, PRP was obtained from CB-exposed mice. Then, 200 μL volume PRP was stimulated with 2 U thrombin (Merck, USA) for 5 min before being incubated with collagen for 20 min64. After washing with D-Hank solution (Solarbio) to remove non-adhered platelets, platelets on collagen were imaged by an optical microscope (Olympus), and >15 random fields of view (40×) were analyzed using Image J software to count adhered platelets.
For analysis of coagulation function of platelets collected from medium of CB-treated Dami-MKs. Platelets normalizing to 1.0 × 106 per sample were stimulated with thrombin (Merck) before being incubated with collagen. More than 15 random fields of view (40×) in each group were imaged and analyzed. Whole blood from mice with or without particle treatment was measured with an automated coagulation analyzer (Lepu, China) for clotting time. Mouse tails were cut 5 mm from the tip and immersed in saline at 37 °C, and bleeding time was defined as time from the start of the bleeding until the end of bleeding.
The morphology and phagocytosis of platelets by Scanning electron microscope (SEM) and TEM
PRP collected from CB-exposed mice was first fixed in 2.5% glutaraldehyde overnight at 4 °C, then rinsed with phosphoric acid solution, and further fixed with 1% osmic acid solution for 2 h. Platelets were orderly dehydrated with graded ethanol solution (30-100%; v/v), a mixture containing alcohol, isoamyl acetate and pure isoamyl acetate. Sections of platelets were prepared and examined using a high-resolution SEM (JEOL JSM-6510, Japan), as described65.
For phagocytosis assay, 1.0 × 106 platelets in PRP were incubated with 60 μg/mL PSs (0.08, 0.2 and 2 μm diameter) at 37 °C for 30 min, and then washed 3 times with D-Hank solution to remove surface-adhered PSs. Platelets were subsequently pelleted by centrifugation (1.5 × 103 g, 10 min), followed by cell fixation and staining before TEM examination.
In vivo labeling and visualization of platelet production by live-cell imaging and flow cytometry
Isolated MKs were pretreated with Cy5-PSs and further cultured for 24-48 h. Before imaging, MKs were subjected to cell membrane staining. Super-resolution Olympus IX-83ZDC live-cell imaging system (Olympus) was used for dynamic imaging of cells following nuclei staining with 0.1% Hoechst 3334 (Invitrogen). To capture the detailed cellular process of thrombocytopoiesis, images of MKs cultured in microscope incubation (Olympus) at 37 °C and 5% CO2 were taken every 3 min for 30 min. Pulse-labeling targeting exiting circulating platelets was performed by injection of PE-labeled anti-CD41 Abs (0.1 μg/g, 100 μL endotoxin-free and sterile saline) into C57/6 J mice through i.v. route. Platelets negative for PE signal within 24 h were considered as newborn platelets.
MK transplant and biodistribution of MK-derived platelets
Donor MKs were isolated from the lung of PF4-mTmG mice, and then treated with 1 μg/mL Cy5-PSs (0.2 μm) for 12 h. After washed with PBS gently for 3 times, 1.0 × 105 lung MKs in 50 μL volume of PBS were delivered into the lung of c-mpl−/− recipient mice via a 20-gauge catheter14. In MK replenishment experiment, 2.0 × 106 isolated MKs were transferred into c-mpl−/− mice by o.p.a. 2 days before exposure with 2 mg/kg body weight CB particles. To investigate whether platelets could transport particles, platelets (CD41+GFP+DAPI−) from the peripheral blood of PF4-mTmG mice were collected. Next, 1.0 ×107 platelets (equivalent to the number of platelets produced by 1.0 × 105 MKs) and 50 ng Cy5-PSs (equivalent to the content of PSs engulfed by 1.0 ×105 MKs) were mixed and transplanted into the lung cavity of c-mpl−/− mice. Lung, liver, spleen and kidney in above experiments were harvested, and then were sliced into sections for fluorescent imaging following an established method66. To confirm whether Cy5-PSs could be delivered from the lung to extrapulmonary tissue through EVs, EVs were detected by immunofluorescence staining with anti-mouse CD63 Abs (1:100 dilution, Abcam) and Cy3-conjugated secondary Abs.
Assessment of particle content by ICP-OES and fluorescence intensity analysis
After 72 h of SiO2 exposure, the lung, liver, kidney, spleen and peripheral blood from mice were collected and digested with acid liquid (HNO3, H2O2 and HF at a volume ratio of 3:1:1). The content of SiO2 in each organ was measured by ICP-OES, following a standard protocol5. For quantification of particle fluorescence, the frozen lungs from Cy5-PSs treated-mice were sectioned into 5 μm slices and imaged for entire lung lobes using 3DHISTECH. The fluorescent intensities of particles on Cy5 channels were quantified, with each data point representing the average of 10-15 random fields of view per animal.
RNA-seq analysis
Due to the small number of resident MKs, isolated 2.0 ×105 CD41+ MKs from each lung were pooled together with these cells from 3 lungs. Total RNAs were extracted from the collected MKs using RNeasy Mini Kit (Qiagen, German) designed for small numbers of cells. The quality and purity of total RNA were evaluated by Agilent 2100 Bioanalyzer system (Agilent Technologies, CA, USA). Then, 2 ng RNA was subjected to RNA-Seq analysis on the DNBSEQ platform (Shenzhen, China)67. Each sample produced an average of 6.67 grams of data. The average comparison rate of the genome was 93.36% and the average comparison rate of the gene set was 75.60%, suggesting high sequencing quality and sequencing depth sufficient for transcriptome coverage. The typical MK markers were referenced in CellMarker 2.0 (http://bio bigdata.hrbmu.edu.cn/ CellMarker or http://117.50.127.228/CellMarker/). For differentially expressed genes in MKs, data were mapped to the known mRNA library and were further analyzed by using Metascape (http://metascape.org/) and DAVID 6.8 (https://david.ncifcrf.gov/).
Statistics and reproducibility
Experiments were repeated 3 times independently with similar results. Comparisons of experimental data were analyzed using SPSS statistical 17.0 software (SPSS, Chicago, USA). Two group comparisons were performed by two size student’s-test, while three or more groups were performed by one-way ANOVA. The statistical significance of enrichment analysis was determined by using one-side Fisher’s exact test with Benjamini–Hochberg correction. Two-tailed Wilcoxon rank-sum test was used to calculate P-value in Volcano plot. For regression analysis, these 2 parameters from ICP-OES and RFI quantification were performed using logistic regression analysis to calculate regression coefficients and P-value. For fluorescent image analyses, 3–6 randomly fields of each view from one individual mouse were quantified and averaged to represent the value for that mouse. Data are presented as the mean values of three independent experiments ±SEM. Statistical significance was set at P < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
RNA-seq data have been submitted to the Sequence Read Archive: SRX18463902, SRX18463901, SRX18463900, SRX18463899, SRX18436229, SRX18436228, SRX18436227, SRX18436226, SRX18436225 and SRX18436224. All other data supporting the findings of this study are available within the article and its supplementary information files. Source data are provided with this paper.
Code availability
No code or mathematical algorithm was used. The paper does not report the original code.
References
Weichenthal, S. et al. Association of sulfur, transition metals, and the oxidative potential of outdoor PM2.5 with acute cardiovascular events: a case-crossover study of Canadian adults. Environ. Health Perspect. 129, 107005 (2021).
Shah, A. S. et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet 382, 1039–1048 (2013).
Li, D. et al. Fluorescent reconstitution on deposition of PM(2.5) in lung and extrapulmonary organs. Proc. Natl Acad. Sci. USA 116, 2488–2493 (2019).
Choi, H. S. et al. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat. Biotechnol. 28, 1300–1303 (2010).
Liu, X. et al. Serum apolipoprotein A-I depletion is causative to silica nanoparticles-induced cardiovascular damage. Proc. Natl Acad. Sci. USA 118, e2108131118 (2021).
Qi, Y. et al. Passage of exogeneous fine particles from the lung into the brain in humans and animals. Proc. Natl Acad. Sci. USA 119, e2117083119 (2022).
Lehnert, B. E. Pulmonary and thoracic macrophage subpopulations and clearance of particles from the lung. Environ. Health Perspect. 97, 17–46 (1992).
Dehaini, D. et al. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv. Mater. 29, 10.1002 (2017).
Li, R. et al. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 19, 124–134 (2019).
Han, C. Z. et al. Macrophages redirect phagocytosis by non-professional phagocytes and influence inflammation. Nature 539, 570–574 (2016).
Lefrancais, E. et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544, 105–109 (2017).
Sun, S. et al. Single-cell analysis of ploidy and the transcriptome reveals functional and spatial divergency in murine megakaryopoiesis. Blood 138, 1211–1224 (2021).
Maratheftis, C. I., Andreakos, E., Moutsopoulos, H. M. & Voulgarelis, M. Toll-like receptor-4 is up-regulated in hematopoietic progenitor cells and contributes to increased apoptosis in myelodysplastic syndromes. Clin. Cancer Res. 13, 1154–1160 (2007).
Pariser, D. N. et al. Lung megakaryocytes are immune modulatory cells. J. Clin. Invest 131, e137377 (2021).
Jin, X. et al. Airborne particulate matters induce thrombopoiesis from megakaryocytes through regulating mitochondrial oxidative phosphorylation. Part Fibre Toxicol. 18, 19 (2021).
Ma, J. et al. From the lung to the knee joint: Toxicity evaluation of carbon black nanoparticles on macrophages and chondrocytes. J. Hazard Mater. 353, 329–339 (2018).
Cheng, Y. et al. Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China. Sci. Adv. 2, e1601530 (2016).
Paunescu, A. C. et al. Associations of black carbon with lung function and airway inflammation in schoolchildren. Environ. Int 131, 104984 (2019).
Arora, S., Dev, K., Agarwal, B., Das, P. & Syed, M. A. Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology 223, 383–396 (2018).
Zeng, Z. et al. Platelet-derived miR-223 promotes a phenotypic switch in arterial injury repair. J. Clin. Invest 129, 1372–1386 (2019).
Qi, Y. et al. FDISCO: Advanced solvent-based clearing method for imaging whole organs. Sci. Adv. 5, eaau8355 (2019).
Yeung, A. K., Villacorta-Martin, C., Hon, S., Rock, J. R. & Murphy, G. J. Lung megakaryocytes display distinct transcriptional and phenotypic properties. Blood Adv. 4, 6204–6217 (2020).
Swieringa, F. et al. Platelet control of fibrin distribution and microelasticity in thrombus formation under flow. Arterioscler Thromb. Vasc. Biol. 36, 692–699 (2016).
Dong, Z. et al. Airborne fine particles drive H1N1 viruses deep into the lower respiratory tract and distant organs. Sci. Adv. 9, eadf2165 (2023).
Neupane, A. S. et al. Patrolling Alveolar Macrophages Conceal Bacteria from the Immune System to Maintain Homeostasis. Cell 183, 110–125.e111 (2020).
Dai, M., Han, J., El-Amouri, S. S., Brady, R. O. & Pan, D. Platelets are efficient and protective depots for storage, distribution, and delivery of lysosomal enzyme in mice with Hurler Syndrome. Proc. Natl Acad. Sci. 111, 2680–2685 (2014).
Montenont, E. et al. Platelet WDR1 suppresses platelet activity and is associated with cardiovascular disease. Blood 128, 2033–2042 (2016).
Tanaka, T. et al. LAMP3 inhibits autophagy and contributes to cell death by lysosomal membrane permeabilization. Autophagy 18, 1629–1647 (2022).
Witte, A. et al. The chemokine CXCL14 mediates platelet function and migration via direct interaction with CXCR4. Cardiovasc Res. 117, 903–917 (2021).
Reddy, K. B., Smith, D. M. & Plow, E. F. Analysis of Fyn function in hemostasis and alphaIIbbeta3-integrin signaling. J. Cell Sci. 121, 1641–1648 (2008).
Rosa, J. P., Raslova, H. & Bryckaert, M. Filamin A: key actor in platelet biology. Blood 134, 1279–1288 (2019).
Severin, S., Ghevaert, C. & Mazharian, A. The mitogen-activated protein kinase signaling pathways: role in megakaryocyte differentiation. J. Thromb. Haemost. 8, 17–26 (2010).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Behrens, K. et al. Runx1 downregulates stem cell and megakaryocytic transcription programs that support niche interactions. Blood 127, 3369–3381 (2016).
Elgueta, R. et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229, 152–172 (2009).
Winneberger, J. et al. Platelet endothelial cell adhesion molecule-1 is a gatekeeper of neutrophil transendothelial migration in ischemic stroke. Brain Behav. Immun. 93, 277–287 (2021).
Lin, Y. F. & Haynes, C. M. Metabolism and the UPR(mt). Mol. Cell 61, 677–682 (2016).
Jiang, S. et al. Cytokine production by primary bone marrow megakaryocytes. Blood 84, 4151–4156 (1994).
Warnatsch, A., Ioannou, M., Wang, Q. & Papayannopoulos, V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349, 316–320 (2015).
Dostert, C., Pétrilli, V., Van Bruggen, R., Steele, C. & Mossman, B. T. Tschopp Jr. innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Mrass, P., Petravic, J., Davenport, M. P. & Weninger, W. Cell-autonomous and environmental contributions to the interstitial migration of T cells. Semin Immunopathol. 32, 257–274 (2010).
Hellen, N. et al. P-selectin mobility undergoes a sol-gel transition as it diffuses from exocytosis sites into the cell membrane. Nat. Commun. 13, 3031 (2022).
Gurney, A. L., Carver-Moore, K., de Sauvage, F. J. & Moore, M. W. Thrombocytopenia in c-mpl-deficient mice. Science 265, 1445–1447 (1994).
Bruns, I. et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat. Med 20, 1315–1320 (2014).
Alexander, W. S., Roberts, A. W., Nicola, N. A., Li, R. & Metcalf, D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood 87, 2162–2170 (1996).
Rossaint, J. et al. Platelets orchestrate the resolution of pulmonary inflammation in mice by T reg cell repositioning and macrophage education. J. Exp. Med 218, e20201353 (2021).
Franck, G. et al. Flow perturbation mediates neutrophil recruitment and potentiates endothelial injury via TLR2 in mice: implications for superficial erosion. Circ. Res. 121, 31–42 (2017).
Rich, D. Q. et al. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA 307, 2068–2078 (2012).
Machlus, K. R. et al. Synthesis and dephosphorylation of MARCKS in the late stages of megakaryocyte maturation drive proplatelet formation. Blood 127, 1468–1480 (2016).
Mezger, M. et al. Platelets and immune responses during thromboinflammation. Front Immunol. 10, 1731 (2019).
Valet, C. et al. Sepsis promotes splenic production of a protective platelet pool with high CD40 ligand expression. J. Clin. Invest 132, e153920 (2022).
Delehanty, L. L. et al. Stromal inhibition of megakaryocytic differentiation is associated with blockade of sustained Rap1 activation. Blood 101, 1744–1751 (2003).
Suraneni, P. K. & Crispino, J. D. The Hippo-p53 pathway in megakaryopoiesis. Haematologica 101, 1446–1448 (2016).
Cortin, V. et al. Efficient in vitro megakaryocyte maturation using cytokine cocktails optimized by statistical experimental design. Exp. Hematol. 33, 1182–1191 (2005).
Patel-Hett, S. et al. The spectrin-based membrane skeleton stabilizes mouse megakaryocyte membrane systems and is essential for proplatelet and platelet formation. Blood 118, 1641–1652 (2011).
Gieré, R., Blackford, M. & Smith, K. TEM study of PM2.5 emitted from coal and tire combustion in a thermal power station. Environ. Sci. Technol. 40, 6235–6240 (2006).
Zhang, W. et al. Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. Elife 5, e21407 (2016).
Karimi, N., Dalirfardouei, R., Dias, T., Lotvall, J. & Lasser, C. Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma - Contributions of platelet extracellular vesicles in plasma samples. J. Extracell. Vesicles 11, e12213 (2022).
Schafer, A. I. Thrombocytosis. N. Engl. J. Med 350, 1211–1219 (2004).
Qi, Y. et al. Co-Exposure of Ambient Particulate Matter and Airborne Transmission Pathogens: The Impairment of the Upper Respiratory Systems. Environ. Sci. Technol. 56, 15892–15901 (2022).
Maroney, S. A. et al. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood 109, 1931–1937 (2007).
Zufferey, A. et al. Mature murine megakaryocytes present antigen-MHC class I molecules to T cells and transfer them to platelets. Blood Adv. 1, 1773–1785 (2017).
Ma, J. et al. A designed alpha-GalCer analog promotes considerable Th1 cytokine response by activating the CD1d-iNKT axis and CD11b-positive monocytes/macrophages. Adv. Sci. 7, 2000609 (2020).
Bezman, N. A. et al. Requirements of SLP76 tyrosines in ITAM and integrin receptor signaling and in platelet function in vivo. J. Exp. Med 205, 1775–1788 (2008).
Ma, Q. et al. Mitochondrial PIP3-binding protein FUNDC2 supports platelet survival via AKT signaling pathway. Cell Death Differ. 26, 321–331 (2018).
Ma, J. et al. Computer-aided discovery of potent broad-spectrum vaccine adjuvants. Angew. Chem. Int Ed. Engl. 62, e202301059 (2023).
Patterson, J. et al. Impact of sequencing depth and technology on de novo RNA-Seq assembly. BMC Genomics 20, 604 (2019).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22150006 to S.J.L. and No. 22076210 to J.M.), the Youth Innovation Promotion Association of Chinese Academy of Science (No. 2022042 to J.M.), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB0750000 to J.M., S.J.L. and G.J.), and the National Key Research and Development Program of China (No. 2018YFA0901101 and 2021YFE0101500 to S.J.L.).
Author information
Authors and Affiliations
Contributions
J.Q. performed the experiments and collected the data with assistance from J.M. (2PIVM and 3D-imaging), Z.D. (2PIVM and animal experiments), Q.R. (animal experiments and RNA-Seq), Q.S. (flow cytometry and solution preparation), J.L. (2PIVM and data analysis), M.G. (cell experiment and RNA-Seq), G.L. (histopathology and cell transplant), S.Z. (histopathology and data analysis). J.M. conceptualized the project, obtained fundings, trained the students, designed, and set up the experiments, analyzed the data, and wrote the manuscript with help from S.L. (training, consultation, funding, resources and writing), G.Q. (writing and consultation), G.J. (consultation and resources).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Cha-Xiang guan, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qiu, J., Ma, J., Dong, Z. et al. Lung megakaryocytes engulf inhaled airborne particles to promote intrapulmonary inflammation and extrapulmonary distribution. Nat Commun 15, 7396 (2024). https://doi.org/10.1038/s41467-024-51686-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51686-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.