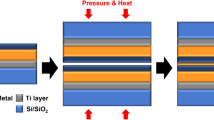
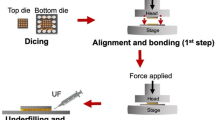
Abstract
Direct copper-to-copper (Cu-Cu) bonding is a promising technology for advanced electronic packaging. Nanocrystalline (NC) Cu receives increasing attention due to its unique ability to promote grain growth across the bonding interface. However, achieving sufficient grain growth still requires a high thermal budget. This study explores how reducing grain size and controlling impurity concentration in NC Cu leads to substantial grain growth at low temperatures. The fabricated NC Cu has a uniform nanograin size of around 50 nm and a low impurity level of 300 ppm. To prevent ungrown NC and void formation caused by impurity aggregation, we propose a double-layer (DL) structure comprising a normal coarse-grained (CG) layer underneath the NC layer. The CG layer, with a grain size of 1 μm and an impurity level of 3 ppm, acts as a sink, facilitating impurity diffusion from the NC layer to the CG layer. Thanks to sufficient grain growth throughout the entire NC layer, cross-interface Cu-Cu bonding becomes possible under a low thermal budget, either at 100 °C for 60 min or at 200 °C for only 5 min.
Similar content being viewed by others
Introduction
3D ICs have appeared to be a promising solution for the physical limit of Moore’s law1,2,3,4. Silicon chips are stacked vertically, where microbumps and through-silicon-via (TSV) are used for the vertical interconnects between chips. The current bonding method relied on Cu bumps or pillars capped with solder materials, but the solder material requires space as it may be squeezed out to cause electrical shorts after bonding. Alternatively, using a microbump with less solder in a finer-pitch package will cause another process and reliability issues that may become problematic at 20 μm pitch1. Recently, direct Cu-Cu bonding has attracted attention because of its potential to replace solder joints using tiny direct Cu-Cu connections, thereby providing higher interconnect density to enable advanced packaging technology with pitches of 10 μm and below. Two technologies are usually used for direct Cu-Cu bonding, including thermo-compression bonding (TCB) and hybrid bonding (HB).
Currently, by using a surface-activated approach5,6,7,8, direct Cu-Cu bonding can be obtained. For a normal CG Cu, the temperature required for complete Cu bonding is usually above 300 °C for 60 min9. The temperature is higher than the melting temperature of eutectic SnPb and Pb-free SnAgCu solders, where the typical reflow temperature is below 250 °C in minutes10,11,12,13,14,15,16. Such high operating temperature and prolonged Cu-Cu bonding may damage the finished devices, particularly temperature-sensitive devices (e.g., DRAM), and cause issues of wafer warpage, misalignment, and thermal stress due to different thermal expansion coefficients of various materials. Therefore, it is desirable to reduce the thermal budget for direct Cu-Cu bonding at temperatures below 200 °C within minutes to meet industrial production requirements for a wide range of applications.
The community has been under active research to conduct direct Cu-Cu bonding at a low thermal budget. For example, an ultra-high vacuum (UHV) combined with an ion-surface-activation technique, known as surface-activated bonding (SAB), has been applied to produce high-quality and low-temperature bonding8,17,18. Yet, the strict requirement of the production environment (10−8 torr vacuum) has substantially raised the cost, making it less attractive for industrial production. Moreover, surface passivation using plasma6, organic self-assembly monolayer19, or noble metal deposition20 has been attempted to prevent surface oxidation; however, the complicated pre-bonding surface treatment is time-consuming and economically inefficient. Therefore, while direct Cu-Cu bonding has long been proposed using SAB at room temperature, recent advancements have placed greater emphasis on achieving low temperature and rapid bonding without the requirement of a vacuum environment or complex pre-treatment process. Recently, an approach of grain engineering has been focused on using (111)-oriented nanotwinned (NT) Cu for Cu-Cu bonding at the temperature of around 200 oC21,22,23,24,25,26,27,28. The surface diffusion coefficient of (111) plane is 3–4 orders of magnitude faster than other surfaces, so the bonding temperature can be lowered. Note that a complete bonding using NT Cu at low temperatures still takes about 1 h. In addition to the long processing time, most of the above works still present a visible sharp interface after bonding. Eliminating the bonded interface is advantageous, particularly in scenarios involving impact conditions such as electrical current, heat, or mechanical loading. Accordingly, NC Cu was proposed for Cu-Cu bonding due to its thermal instability and rapid grain growth, which allows for grain growth across the bonding interface. However, currently, NC Cu still requires a high thermal budget at 250 °C for 1 h to accomplish Cu-Cu bonding29. Note that NC Cu is usually fabricated by adding or overdosing additives to the electroplating bath, resulting in a higher co-deposited impurity level ranging up to a few thousand ppm. Hence, impurity aggregation and enrichment may lead to void formation and ungrown NC grains, raising reliability concerns such as electromigration30.
This work focuses on the study of NC grain growth mechanism and proposes a DL structure to enhance the bonding performance. First, we deepen the understanding of NC Cu by exploring the influence of temperature and grain boundary (GB) energy as driving forces31, as well as impurity density as an inhibitory force. The Cu material with a uniform NC size ≈ 50 nm was fabricated to enhance the driving force while maintaining low impurity levels in the range of a few hundred ppm to mitigate inhibitory forces. At high temperatures, both the diffusivity and mobility of GB increase, overshadowing the inhibitory effects. At lower temperatures, the inhibitory effects become more significant. Therefore, to achieve Cu-Cu bonding at low temperatures, it is crucial to investigate the influence of both annealing temperature and impurities on grain growth in NC Cu. Second, a DL structure comprising NC Cu on top of CG Cu was proposed for direct Cu-Cu bonding, enabling substantial grain growth and achieving cross-interface Cu bonding at low temperatures ranging from 100 to 200 °C. The NC layer, with a thickness of 300 nm, serves as a glue layer, promoting cross-interface grain growth. The CG layer, with a thickness of 3 μm, a grain size ≈ 1 μm, and impurity levels of around 3 ppm, serves as a sink to facilitate impurity diffusion from the NC layer. This prevents impurity aggregation and enrichment, ensuring the sufficient grain growth of NC layer and preventing void formation near the Cu seed layer.
Results
Material properties of NC Cu
Scanning transmission electron microscopy (STEM) in dark-field (DF) mode was employed to examine the distribution of grain sizes. NC Cu films were synthesized using high- or low-impurity electrolytes with adjusted additive ratios under current densities of 40 and 60 mA cm−2. The processes are denoted as NC-4H, NC-6H, and NC-6L accordingly (4 and 6 represent the current densities, and H and L represent high- and low-impurity conditions). The grain size distributions shown in Figs. 1a–c demonstrate similar grain sizes ≈ 50 nm for all three samples. The diffraction rings in the insets of Figs. 1a–c indicate randomly distributed crystals in the out-of-plane direction. Nanosized grains with a large number of GBs would enhance the driving force for grain coarsening. Moreover, decreasing the grain size typically results in a decrease in the coarsening temperature. However, a reverse phenomenon has been reported where a drop in GB energy occurs when grain sizes decrease below a critical size within the range of 50 to 100 nm, leading to a reduced driving force for recrystallisation and an increased coarsening temperature32,33. Accordingly, in our fabrication process, it is important to achieve an average grain size that is small enough to ensure a low coarsening temperature, but not excessively small to avoid falling below the critical size. Figure 1d–e depicts the X-ray diffraction (XRD) measurement using the 'Chi scan' technique, with predefined holder angles of 0° and 15° (Suppl. Fig. 1a). The results show that the Cu seed layer exhibited a prominent (111) peak at 'Chi = 0°', while it was undetectable at 'Chi = 15°'; a similar pattern was observed in the NT-Cu (Suppl. Figs. 1b, c). This indicates that the majority of crystal orientations for the Cu seed layer and NT-Cu are (111) in the out-of-plane direction. In contrast, NC-4H, NC-6H, and NC-6L exhibited (111) peaks at two angles, with a stronger intensity at 'Chi = 0°' and a reduced intensity at 'Chi = 15°'. It should be noted that at 'Chi = 0°', the intensity of the Cu-(111) peak in the NC film would be intensified due to the signal from the Cu-(111) seed layer. Accordingly, along with the results of diffraction rings obtained from TEM, we conclude that the crystal orientation in NC-4H, NC-6H, and NC-6L is randomly distributed. Other than grain size and orientation, the kinetics of grain coarsening would be suppressed by pinning GBs with second-phase drag, solute or impurity drag, or chemical ordering. The electroplated Cu inevitably contains co-deposited impurities, which exert a pinning effect on the GBs, leading to a decrease in GB energy, and consequently reducing the driving force for grain growth. Additionally, due to a substantial reduction in GBs as grains grow larger and that impurities tend to concentrate at GBs, there is an increase in impurity density at GBs, which gradually amplifies the pinning effect as grain growth34. The impurity concentration, with a particular focus on the three major impurities (carbon, sulphur, and chlorine), measured by secondary-ion mass spectrometry (SIMS), is shown in Fig. 1f: NC-4H ( ≈ 1200 ppm), NC-6H ( ≈ 1200 ppm), and NC-6L ( ≈ 300 ppm). Assuming that impurities like carbon, sulphur, and chlorine segregate in GB due to their limited solubility in Cu, and they don’t diffuse out of the film during annealing, we can accordingly calculate the impurity density in GB. Figure 1g–h summarises the impurity density (in atom m-2) based on the different values of GB area per unit volume. The calculated impurity density in the annealed sample is about 40 times the value of the as-prepared sample (e.g., NC-6L from ≈ 0.47 × 1018 to ≈ 19 × 1018 atom m-2), which provides numerical proof that the pinning effect becomes significant as the grain grows34. Hence, it is necessary to control the impurity level to guarantee sufficient grain growth in direct Cu-Cu bonding, particularly at low temperatures.
DF-STEM images and TEM diffraction patterns of as-prepared samples and corresponding distribution of grain size: (a) NC-4H (yellow), (b) NC-6H (cyan-blue), and (c) NC-6L (magenta-pink). XRD patterns of the as-prepared NC Cu (Colours refer to the characteristics listed in (a)) and Cu seed layer (dark grey) at (d) 'Chi = 0°' and (e) 'Chi = 15°'. f Impurity (carbon (grey), sulphur (orange), and chlorine (green)) concentration examined by SIMS. Comparison of impurity density between: (g) as-prepared and (h) annealed NC Cu, corresponding to the grain size of 50 nm and 2 μm respectively. Source data are provided as a source data file.
To achieve direct Cu-Cu bonding at low thermal budgets, it is crucial to understand the interplay between the driving force induced by temperature and the pinning effect induced by co-deposited impurities, not only for ensuring proper growth of the grains over the bonding interface but also for achieving sufficient grain growth throughout the whole NC layer. In Fig. 2a, the resistance change is plotted as the measured resistance per initial resistance versus time at room temperature. This change is attributed to grain growth with GB reduction, exhibiting a trend of self-annealing rate that follows NC-6L > NC-6H > NC-4H. Here, in the absence of a heating effect, the excess GB energy is the primary factor driving grain growth in NC Cu, while impurities act as the inhibitory force. Note that NC-4H and NC-6H have comparable grain sizes and impurity concentrations, but NC-6H exhibits a faster self-annealing rate compared to NC-4H. Figure 2b illustrates the resistance change over time when the samples were annealed at temperatures ranging from 100 to 250 °C. At a lower temperature of 100 °C, NC-6H displays a faster drop in resistance than NC-4H, similar to the trend observed during self-annealing at room temperature. However, when the temperature is raised to 250 °C, NC-4H and NC-6H exhibit similar trends of resistance drop, which will be further explained by the analysis of differential scanning calorimetry (DSC). Compared to NC-6H and NC-4H, NC-6L shows a considerably higher rate of resistance drop across all temperature ranges, which is believed to be a result of its unique combination of nanosized grains and low-impurity levels. Note that the resistance drop of NC-6L annealed above 200 °C was too fast to be recorded so that the case at 150 °C is shown instead. Here, t50, 50% of the overall resistance drop, is 410 s for 100 °C and 37 s for 150 °C.
Resistance drop of the NC-4H (yellow), NC-6H (cyan-blue), and NC-6L (magenta-pink) annealed at (a) room temperature (≈ 22 °C) and (b) temperatures larger than 100 °C. The graph represents the results as means ± s.d. of five samples. c DSC analysis with a heating rate of 10 °C min-1, the first scan (cycle 1) and the repeated cycle (cycle 2) were indicated by the solid and the dashed line respectively. d BF-STEM images showing the existence of dislocation indicated by the yellow arrows in as-prepared NC-6H in comparison to NC-4H. e Activation energy of grain growth calculated by the first exothermic peak in DSC results and comparison with the values from other studies (grey for ref. 38 and diagonal lines fill for ref. 39). Note that all colours refer to the characteristics listed in (a). Source data are provided as a source data file.
In general, the driving force for grain growth in NC is the stored energy in the form of dislocations and GBs. As shown in Fig. 2c, DSC was measured to investigate the excess GB energy stored in NC Cu. During heating, exothermic peaks were observed, indicating the elimination of GB and grain coarsening. Both NC-4H and NC-6H exhibited two exothermic peaks; the first large exothermic peak occurred in the temperature range of 100 to 160 °C, followed by a second small exothermic peak ranging from 180 to 250 °C. NC-6H released more heat (2.0488 J g−1) in the first peak than NC-4H (1.6443 J g−1), suggesting that more NC in NC-6H underwent growth. Upon heating above 300 °C, the grains grew to a size of several μm, and no NC was found in the sample (Suppl. Figs. 2a–c). Figure 2d presents high-magnification bright-field (BF) images of the as-prepared NC-4H and NC-6H obtained from STEM. In NC-6H, a certain number of dislocations or dislocation loops can be observed (indicated by yellow arrows), whereas only a few of them are visible in NC-4H. Despite having similar average grain sizes and impurity levels, the presence of these defects in NC-6H can accelerate the grain growth rate35. The DSC analysis and the presence of more defects in NC-6H provide an explanation for the faster resistance drop observed in NC-6H compared to NC-4H at room temperature and temperatures below 100 °C. Furthermore, the second exothermic peaks of NC-4H and NC-6H occurred before 250 °C, which corresponds to the resistance measurement where similar rates were observed for both NC-4H and NC-6H annealed at a high temperature of 250 °C. In contrast, NC-6L exhibited only a single exothermic peak (5.1057 J g−1), indicating a simpler heat-releasing mechanism and consistent with its significantly faster rate of resistance drop compared to NC-4H and NC-6H.
From a thermodynamic perspective, based on the DSC data and calculation of excess surface area of GB, the excess GB energy (γGB) for NC-4H, 6H, and 6L are 0.57, 0.59, and 0.90 J m−2, respectively. A higher γGB indicates a higher driving force for grain growth in the NC material36. From a kinetic perspective, GBs with high energy are more likely to exhibit larger diffusion coefficients37. Fig. 2e illustrates the calculated activation energy of grain growth (\(E\)G) derived from the first peak of the DSC analysis (Suppl. Fig. 2d). The \(E\)G for NC-4H, NC-6H, and NC-6L are 0.89, 0.79, and 0.63 eV atom-1, respectively. Both NC-4H and NC-6H exhibit activation energy values comparable to those reported in other studies, ranging from 0.75 to 0.92 eV atom-138,39. In contrast, NC-6L exhibits a relatively lower EG of 0.63 eV atom-1. Basically, EG can be related to the activation energy of GB diffusion (EGBD), comprising the effective vacancy formation enthalpy (Hf), and the effective vacancy migration enthalpy (Hm) (Suppl. Note 1)39,40,41. In NC Cu, a higher γGB correlates with a lower Hf. Impurities would pin vacancies and hinder GB migration through the drag effect. Thus, a high impurity level leads to an increase in Hm42,43. For instance, sulphur atoms tend to form strong chemical bonds with Cu atoms in the form of a two-dimensional phase39,44, with a favourable formation energy of about −0.39 eV atom-145. Consequently, the high γGB combined with a low impurity level in NC-6L contributes to a lower EG and EGBD, leading to an accelerated grain growth rate. All the calculated values can be found in Suppl. Table 1.
DL structure for low-temperature bonding
Figure 3a–d and Suppl. Fig. 3 illustrate the problems arising from impurities when utilizing a single NC-6L layer. DF-STEM image of Fig. 3a reveals the presence of an interfacial layer between the Cu seed layer and the as-prepared NC-6L. This could be attributed to the abundant adsorption of additives at the initial nucleation stage during electroplating46. In Fig. 3b, scanning ion microscopy (SIM) shows a thin layer of ungrown NC Cu (Region α, yellow dashed line) on top of the Cu seed layer after annealing NC-6L at 100 °C for 30 min. The inserted zoom-in BF-STEM image in Fig. 3b and the zoom-out image in Suppl. Fig. 3a provides additional support for this phenomenon of ungrown NC grains above the Cu seed layer. As mentioned above, the grain growth process is known to reduce GBs, leading to increased impurity densities in the GBs. This, in turn, hinders the outward diffusion of impurities from the bottom NC, especially when GBs already contain a high concentration of impurities after a certain period of annealing, as the diffusion paths primarily depend on GBs. Figure 3c represents the SIMS investigation of the annealed NC-6L, confirming the higher concentration of impurities near the interface between NC-6L and the Cu seed layer; this is attributed to the aforementioned higher adsorption of additives at the initial stage and the presence of ungrown NC Cu in region α after annealing. This phenomenon raises concerns for reliability, as the remained NC layer with a large number of GBs can promote atomic displacement and void formation under electrical current, mechanical stress, and temperature. Moreover, even with extended annealing times (100 °C/3 h), it remains challenging to initiate the growth of the remained NC (Suppl. Fig. 3b). Therefore, using solely NC Cu for low-temperature direct Cu-Cu bonding is not suitable. Here, it is important to note that the presence of the observed ungrown NC Cu in this study is a result of low-temperature annealing at 100 °C. In contrast, higher temperatures (e.g., 200 °C) can promote sufficient grain growth (Suppl. Figs. 3c–f); however, under high temperatures, the impurities and voids present near the Cu seed layer would undergo movement, coalescence, and aggregation, resulting in the formation of sizable voids, as shown in Fig. 3d observed by scanning electron microscope (SEM). Therefore, even using NC alone for bonding can lead to a good NC-NC bonding interface, but issues arise at the interface between NC and the Cu seed layer, including incomplete grain growth at low temperatures and void formation at high temperatures.
Single NC layer: (a) DF-STEM image of the interface (indicated by the white arrows) between Cu seed layer and as-prepared NC-6L. b SIM and corresponding inserted BF-STEM images of NC-6L after annealing at 100 °C for 30 min showing the abnormal grain growth in region α indicated by the yellow dotted line; the boundary of region α is marked by the white dashed lines. c SIMS analysis of the region α indicated in (b) showing a sharp increase in impurity content (Cu: orange, C: dark grey, S: yellow, Cl: cyan-green, and Si: cyan-blue); the dashed-line refers to the white dashed lines in (b). d SEM observation of a single NC-6L layer after annealing at 200 °C for 5 min showing a large number of defects near the Cu seed layer. NC-CG DL structure: (e) SIM image of the as-prepared structure of DL (CG + NC-6L); the original boundary between CG and NC-6L is marked by the white dashed line. f SIM image showing the crystal protrusion (yellow dotted line) from the original boundary (white dashed line) in DL after annealing at 100 °C for 10 min. SIMS analysis of as-prepared (solid lines) and annealed (dashed lines) DL: (g) Cl and (h) C and S. Colours refer to the characteristics listed in (c) and the grey dashed lines indicate the original boundary between CG and NC-6L. i SEM observation of the first CG layer after annealing at 200 °C for 5 min. Note that the white dashed lines indicate the boundary between CG and Cu seed layer in (e), (f), and (i). Source data are provided as a source data file.
To address this problem, a DL structure was proposed as a solution, using an NC-6L layer as a “glue layer” electroplated on top of the CG layer (Fig. 3e). By employing this structure, when the sample was annealed at 100 °C for 10 min, the NC layer readily grew and merged with the CG grains at the interface between the NC and CG layers, as shown in Fig. 3f (the crystal protrusion, denoted by the yellow dotted line). In Fig. 3g, h, SIMS was used to understand the change of impurity distribution for the as-prepared DL structure (NC-6L ≈ 2 μm on CG layer ≈ 3 μm) and the sample after annealing at 100 °C. At a depth of around 2 μm in the as-prepared sample, a significant difference in impurity distribution is observed at the interface between the NC-6L and CG layers. This substantial disparity in impurity distribution diminishes after annealing the sample under a low thermal budget at 100 °C for 10 min, as indicated by the dashed lines. A noticeable decrease in impurity content is observed near the interface between the NC and CG layers. The impurity diffusion from NC-6L to CG under heat is driven by the large concentration gradient; the low-impurity CG layer (≈ 3 ppm) can act as an impurity sink that facilitates the downward diffusion of impurities from the high-impurity NC layer and promotes the grain growth process. Furthermore, in Fig. 3i, when employing CG in contact with the bottom Cu seed layer, no visible voids were observed at the interface near the Cu seed layer after annealing at a higher temperature of 200 °C.
Evaluation of bonding performance
With the advantages of lower impurity concentration, lower activation energy for grain growth, and higher excess GB energy in NC-6L, it becomes possible to achieve direct Cu-Cu bonding with a reduced thermal budget. First of all, it is crucial to remove oxides and contamination from the bonding surfaces beforehand. Subsequently, for direct Cu-Cu bonding, the process involves two stages: (1) the initial contact of Cu surfaces to eliminate gaps and achieve void closure through plastic deformation of the contact area due to surface roughness, (2) the formation of GB and ripening of interfacial voids driven by the Gibbs-Thomson effect47,48,49. This process leads to the densification of the bonded interface, as illustrated in the schematic diagram shown in Fig. 4a. GB diffusion, as the rate-determining step in stage 2, plays a crucial role by transporting atoms from the deformed region to interfacial voids through a stress-driven process. Note that the NC structure is believed to contribute not only to the low activation energy of GB diffusion but also to the faster creep rate during the bonding process50. According to Coble creep behaviour, the creep rate and atomic flux are accelerated when the grain size is reduced (Suppl. Note 2). Additionally, the randomly distributed orientation of the NC layer also aids in enhancing bonding performance due to the presence of different crystal orientations on both sides, which promotes GB diffusion51. Previous studies have reported that bonding interfaces with identical crystalline orientations densify at a slower rate, while bonding interfaces with mismatched orientations densify significantly faster51. The diffusion of atoms at GBs with different crystalline orientations exhibits notable atom diffusion on the X-Y plane towards the voids, whereas atom diffusion on the void surfaces, known as surface diffusion, occurs in a relatively minimal amount.
a Schematic diagram of direct Cu-Cu bonding using DL structure. IPF-Z information of the bonding interface using DL structure under: (b) 100 °C/20 MPa/30 min, (c) 100 °C/20 MPa/60 min, (d) 150 °C/20 MPa/10 min, and (e) 200 °C/10 MPa/5 min. Colour legend and reference direction are indicated in the figure. f DF-STEM image of the cross-interface bonding region (200 °C/10 MPa/5 min) and corresponding EDS mapping of Cu. g SEM image shows the pattern designed for the micro-tensile sample. The boundary between CG and NC-6L are marked by the white dashed lines. h As-prepared tensile sample and the bonding interface is indicated by the white dashed line. i The gripper used for the micro-tensile tests. The white dashed line refers to the bonding interface in (h). j SEM image of the fracture morphology of the control sample (CG-300 °C/10 MPa/30 min) where the bonding interface is highlighted by the white dashed line. SEM image of the fracture morphology of DL structure: (k), (l) 200 °C/10 MPa/5 min, (m) 100 °C/20 MPa/60 min, and (n) 100 °C/20 MPa/30 min. o Typical ‘stress-strain’ curves obtained from the micro-tensile tests: DL-100 °C/30 min (yellow), DL-100 °C/60 min (cyan-blue), DL-200 °C/5 min (cyan-green), and CG-300 °C/30 min (magenta-pink). p Statistical results of the bonding strength. Colours refer to the characteristics listed in (o). The average value is marked by the open circle and the maximum and the minimum values are indicated by the upper and lower boundary of the box respectively. q Comparison of several kinds of direct Cu-Cu bonding in recent studies. Source data are provided as a source data file.
The microstructure of the bonding interfaces was characterised by electron backscatter diffraction (EBSD) and shown by inverse pole figures in Z direction (IPF-Z): 100 °C/20 MPa/30 min (Fig. 4b), 100 °C/20 MPa/60 min (Fig. 4c), 150 °C/20 MPa/10 min (Fig. 4d), and 200 °C/10 MPa/5 min (Fig. 4e). Their corresponding SIM images can be found in Suppl. Fig. 4a–d. All the bonding experiments demonstrate complete bonding, with noticeable zig-zag cross-interfaces. In contrast, the control group, using only the CG layer for bonding under a high thermal budget of 300 °C/10 MPa/30 min, exhibited a straight-line interface (Suppl. Fig. 4e). Note that in Fig. 4b and Suppl. Fig. 4a, when the Cu-Cu bonding is subjected to an insufficient thermal budget of 100 °C/20 MPa/30 min, although a satisfactory bonding interface can be achieved, a few regions of ungrown NC Cu (black areas that cannot be analysed by EBSD) can be observed at the interface between the NC and CG layers, aligning well with our previous observations. By extending the annealing time to 60 min at 100 °C, sufficient grain growth can be achieved (Fig. 4c and Suppl. Fig. 4b). The bonding region in Fig. 4e was further examined using BF-STEM and energy-dispersive X-ray spectroscopy (EDS), as shown in Fig. 4f, which clearly reveals the presence of a wavy GB and submicron-sized voids. According to our observations, we have identified three types of cross-interface grain growth conditions once the NC layers come into contact (Suppl. Note 3). Firstly, the grain growth can initiate within the NC layer itself (Suppl. Fig. 5a). Secondly, it may involve one side growing and subsequently merging with the other side, extending near the boundary of the CG layer (Suppl. Fig. 5b). Thirdly, it can simultaneously grow from both sides of the NC layers and progress towards the bonding interface (Suppl. Fig. 5c). As these growth conditions occur concurrently and exclusively in the NC-NC bonding region, they result in the formation of a zig-zag profile within this region. Moreover, to validate the potential of using the DL structure in a real manufacturing process, we also examined its application on a line array with micron-scale dimensions (Suppl. Figs. 6a–c).
To evaluate the bonding strength, a series of micro-tensile tests were conducted via direct-pull technique52. Figs. 4g–i showcases the samples specifically prepared for these tests. The region of cross-interface bonding can be determined during the microfabrication process for sample preparation in the FIB system, as depicted in Fig. 4g. The final shape of the tensile sample and the gauge can be controlled and prepared accordingly, as shown in Fig. 4h. Figure 4i showcases the diamond tensile gripper utilized for conducting the micro-tensile test. The fracture morphology of the control group and DL groups were observed by SEM and the bonding interfaces are highlighted by the white dashed line. In the control group (Fig. 4j), the failure always occurs at the bonding interface, indicating a relatively weak bonding. Figs. 4k–l shows the fracture morphology of DL-200 °C/10 MPa/5 min, where Fig. 4k indicates the failure occurred on the bonding interface while Fig. 4l shows the failure above the bonding interface. Figure 4m and Fig. 4n demonstrate similar failure modes for DL-100 °C /20 MPa/60 min and DL-100 °C/20 MPa/30 min, respectively. In both cases, fractures occur either above or below the bonding interface, indicating that the bonding interface can sometimes be stronger than the bulk Cu. A typical ductile failure mode was shown in DL-200 °C/10 MPa/5 min in Suppl. Movie 1. The corresponding stress-strain curves are presented in Fig. 4o, where DL groups exhibit superior mechanical performance compared to the control group using CG. DL groups have superior mechanical strength but show smaller strains (30%-50%) compared to the control group (≈ 80%). The statistical results based on at least three samples for each condition are shown in Fig. 4p. It is noteworthy that all the samples utilizing the DL structure exhibit satisfactory quality, with an average tensile strength ≈ 560 MPa. The maximum strength recorded was even over 600 MPa. In contrast, the tensile strength of the control sample achieved an average value ≈ 455 MPa. DL-array also show good mechanical performance (≈ 500 MPa) and cross-interface grain growth. (Suppl. Note 4 and Suppl. Fig. 6d–i) Note that the sudden drop of the stress (or loading force) in the micro-tensile test is caused by the strain burst during the plastic deformation (Suppl. Note 5 and Suppl. Fig. 7). Figure 4q summarises recent research regarding direct Cu-Cu bonding. The thermal budget of Cu bonding using various microstructures was plotted, such as NT-Cu (squares), NC-Cu (stars), and electron-beam deposited or physical vapor deposited (circles) Cu. A trend of thermal budget can be found in the Fig. 4q, that is, the shorter the bonding time the higher the bonding temperature. By unleashing the potential of NC Cu and taking advantage of the DL structure, this work achieves a large reduction in thermal budget while attaining good bonding quality.
Discussion
This work demonstrates the application of NC-Cu and DL structure for direct Cu-Cu bonding under low thermal budget. By controlling current density and additives, we fabricate NC Cu with uniform nanograin sizes and low impurity levels, enabling fast grain growth and facilitating direct Cu-Cu bonding at low thermal budget. The investigation focuses on crucial factors including grain size, impurities, excess GB energy, and activation energy of grain growth. We highlight that direct Cu-Cu bonding using single NC Cu is not suitable, leading us to propose a DL structure with an NC Cu layer on top of a CG layer. In this structure, the CG layer acts as a sink for impurity diffusion from the NC layer, promoting sufficient grain growth of NC Cu at low temperatures and effectively eliminating the adverse effects of void or impurity aggregation near the Cu seed layer. By leveraging the DL structure using NC Cu, we achieve direct Cu-Cu bonding under low thermal budgets of either 200 °C/5 min or 100 °C/60 min. This bonding methodology demonstrates good mechanical performance, with a tensile strength exceeding 600 MPa. This combination of a low thermal budget and superior bonding quality offers a promising strategy for advanced electronic packaging applications, enabling reduced operating temperatures and increased throughput for industrial mass production.
Methods
Materials
The electroplating bath, SS303 (0.48 mol L-1 CuSO4·5H2O, 1.90 mol L−1 sulphuric acid, 52 ppm chloride ion, and several additives), supplied by Doctech Ltd. (Hong Kong, China), was used to fabricate different kinds of Cu films via direct current (DC) electroplating. By tuning the additives inside the bath and the current density, three kinds of NC Cu films were fabricated: 40 and 60 mA cm-2 with a high level of impurity content (NC-4H and NC-6H), 60 mA cm-2 with a low level of impurity content (NC-6L). A normal silicon wafer (with a total thickness ≈ 625 μm including a 100 nm Titanium (Ti) layer and 200 nm Cu seed layer) was used as the substrate for the electrodeposition of Cu film. The Ti and Cu seed layer was deposited by sputter deposition and it has an out-of-plane (111) structure. The deposition conditions are 13 mT working Chamber Pressure, Ti 2000 W / Cu 6000 W DC Power, 132 cm3 STP min-1 Argon. In addition, another kind of CG Cu film (total impurity ≈ 3 ppm) with uniform and fine grain size (≈ 1 μm) was fabricated by DC electroplating (40 mA cm-2) in an electroplating bath provided by Doctech Ltd. In DL structure, the CG layer was firstly electroplated on the Cu seed layer (≈ 3 μm) and then the NC layer with a thickness ≈ 1 μm was electroplated on the first CG layer as a Cu-glue for the direct Cu-Cu bonding.
Resistance measurement
The shape of the electroplated Cu film was carefully controlled as a square with a total area of 0.5 cm2 (≈ 0.71 × 0.71 cm2). The film thickness is ≈ 5 μm. Measurement of sheet resistance was carried out on the stage with a built-in 4-point probe where the probes were set onto the middle of the film. The annealing process was conducted in a tube furnace under vacuum condition.
Microstructure characterisation and impurity analysis
The average grain size of the electroplated Cu film was characterised by STEM (Thermo Scientific Talos F200X). The observation was conducted under an accelerating voltage of 200 kV and several modes were employed according to different requirements including DF and BF. STEM or TEM sample was prepared by using a twin-jet electropolishing device (Struers, TenuPol-5). An operated voltage of 5 V was applied to thin and polish the sample in an electropolishing bath (50 vol.% deionized water, 25 vol.% phosphoric acid, 25 vol.% acetic acid). The temperature was controlled at −5 to 0 °C during the thinning process. XRD (Rigaku SmartLab, Cu-Kα, Parallel Beam) was used to characterise the crystal structure of Cu film in θ/(2θ) scan mode. The tube voltage and current are 40 kV and 150 mA respectively. The step size was 0.01° and the scanning speed was 8° min−1. Two 'Chi' angles (0° and 15°) were predefined in the experiments. SEM/EDS (LEO 1530) was used to observe the bonding interface and conduct element analysis. The accelerating voltage and current were 20 kV and 0.4 nA respectively. EBSD (Oxford Instruments NordlysNano) performed in SEM (15 kV, 13 nA, step size: 200 nm) was employed to investigate the IPF-Z of the Cu film and evaluate the bonding interface. The cross-section of the Cu film was investigated by dual-beam focused-ion beam (FIB, Thermo Scientific Helios 5 CX). SIM and SEM can be realised in this system. SIMS was applied to analyse the impurity level in the Cu films. 2 keV Cs beam with a current of 96 nA was used, and the sputtering area was 260 × 260 μm2 with a dose density of 4.34 × 1018 ions cm-2.
Thermokinetic analysis
DSC (TA Instruments DSC250) was applied to analyse the thermokinetic characteristics of the electroplated Cu films. The software TRIOS in the DSC operation system can derive the enthalpy. The Cu film was peeled off from the silicon substrate and then the Ti layer was gently removed by abrasive paper (SiC, #2000) to rule out the heat flow caused by other materials. The Cu film was cleaned with DI water and purged with N2 gas. The total mass of the Cu was controlled at 5 to 6 mg for each DSC experiment. The typical heat-releasing process was characterised by using a heating rate of 10 °C min−1 and each sample was scanned twice to quantitatively reveal the total heat flow in the heat-up process. The heating rate was set as 5, 10, 15, 20, 25, 30 °C min−1 to calculate the activation energy of grain growth. The temperature scanning range was from 30 to 300 °C.
Activation energy of grain growth
The thermokinetic analysis was conducted according to the first exothermic peak in DSC experiments. The process of grain growth in NC metals can be regarded as a thermally activated phenomenon and it conforms to the Kissinger equation53,54:
where HR is the heating rate, T0 the onset temperature, EG the activation energy of grain growth, and \({{{\rm{R}}}}\) the gas constant. C is a constant introduced by the integral. By plotting the relationship between ln(HR/T02) vs. 1/T0, the value of EG can be derived from the slope of the plot as depicted in Suppl. Fig. 2d.
Impurity density calculation
The ‘intercept method’55,56 was conducted to define the value of GB area per unit volume (SV, in m2 g−1 or in m2 m−3) according to STEM observation (Figs. 1a–c) and EBSD results (Suppl. Fig. 2a–c). The value of as-prepared NC Cu (SV0) is ≈ 5.56 m2 g−1, corresponding to the average grain size of 50 nm. The value of annealed Cu (SVa) is ≈ 0.14 m2 g−1, corresponding to the average grain size of 2 μm. Impurity density describing the inhibitory force against the grain growth in NC Cu film was calculated according to the total impurity concentration (ci, in atom m-3) and GB area per unit volume. Then, the impurity density (ci*, in atom m-2) can be described as:
where VCu and MCu are the molar volume (7.114 cm3 mol-1) and molar mass (63.55 g mol-1) of Cu respectively.
Excess GB energy
In DSC experiments, each sample was scanned twice with a heating rate of 10 °C min-1, and the total heat flow in the heat-up process was quantitatively determined by the software TRIOS. Combined with the exothermal enthalpy (ΔH, in J g−1) measured from DSC experiments, the GB excess energy (γGB, in J m-2) can be estimated by57:
Where ΔSV = SV0 − SVa is the change of GB area per unit volume. The results are summarized in the Suppl. Table 1.
Direct Cu-Cu bonding
The wafer with the electroplated Cu film was cut into a small die (0.5 cm × 0.5 cm) and a large die (1 cm × 1 cm) respectively. The roughness of the Cu film was measured by atomic force microscopy (AFM, MultiMode 8-HR). The Cu films were polished by a chemical mechanical polishing (CMP) process with a root-mean-square surface roughness (RMS(Sq)) of 4.55 nm. The final thickness of the NC layer (NC-6L) was ≈ 300 nm. Then, these dies were successively cleaned with acetone, isopropanol, and acid solution (37 vol.% HCl:DI water = 1:1 v/v) in an ultrasonic cleaner. Finally, they were rinsed with DI water and dried with N2 gas. The bonding experiment was conducted after low-power (7 W) N2 plasma activation. These procedures are depicted in Suppl. Fig. 8. The bonding parameters were set as 100 °C /20 MPa/30 min, 100 °C /20 MPa/60 min, 150 °C /20 MPa/10 min, and 200 °C /10 MPa/5 min; 200 Pa vacuum. A control group was set in this work to evaluate the bonding quality compared to DL structure. Only a single CG layer was used in the control group and the bonding parameter was 300 °C /10 MPa/30 min.
Fabrication of DL-array
The micron-scale bonding pattern (or DL-array) was fabricated by photolithography with a line width of 25 μm. The total height of the photoresist (PR) was ≈ 3.5 μm. The 2D profile measured by profilometer (Bruker, DektakXT) is shown in Suppl. Fig. 6a. The DL structure was designed with an NC-6L layer (≈1 μm) on top of a CG layer (≈ 2 μm). The as-prepared Cu strips are shown in Suppl. Fig. 6b. The Cu seed and Ti layer insulated by PR were removed before the bonding. The bonding condition was set as 200 °C/10 MPa/5 min and the Cu strips on two silicon substrates were stacked together as shown in Suppl. Fig. 6c.
Bonding quality evaluation
Micro-tensile tests were used to characterise the mechanical strength of the bonding interface. The sample lamella was cut from the bonding die by the EasyLift nanomanipulator in the FIB system and mounted on a molybdenum (Mo) grid. The tensile sample with a gauge dimension of ≈ 400 nm × 400 nm was fabricated on the lamella by FIB system. Besides, the position of the bonding interface was controlled in the middle of the gauge (Figs. 4g–i). The final polishing step conducted on the lamella was 30 kV/30 pA to remove possible defects caused by the ion beam. Sample loading was performed under displacement control using a Hysitron Picoindenter (PIE-85) at nominal strain rates of 1 × 10−3 s−1. The sample was put into the gripper using the piezo stage, and sample loading was conducted under displacement control. The loading force and displacement can be recorded for further analysis of ‘stress and strain’.
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Source data are provided in this paper.
References
Tu, K. N. Reliability challenges in 3D IC packaging technology. Microelectron. Reliab. 51, 517–523 (2011).
Chen, C., Yu, D. & Chen, K.-N. Vertical interconnects of microbumps in 3D integration. MRS Bull 40, 257–262 (2015).
Chen, C. et al. Nanotwinning-assisted structurally stable copper for fine-pitch redistribution layer in 2.5D/3D IC. packaging. J. Mater. Res. Technol. 27, 4883–4890 (2023).
Zheng, Z. et al. Electrodeposition of (111)-oriented and nanotwin-doped nanocrystalline Cu with ultrahigh strength for 3D IC application. Nanotechnology 32, 225702 (2021).
Zhang, L. et al. Materials, processing and reliability of low temperature bonding in 3D chip stacking. J. Alloys. Compd. 750, 980–995 (2018).
Shigetou, A., Itoh, T., Sawada, K. & Suga, T. Bumpless interconnect of 6-μm-pitch Cu electrodes at room temperature. in IEEE Trans. Adv. Packag 31, 473–478 (2008).
Suga, T. et al. A new wafer-bonder of ultra-high precision using surface activated bonding (SAB) concept. in IEEE Electron. Compon. Technol. Conf. 1013–1018 (2001).
Shigetou, A. et al. Bumpless interconnect through ultrafine Cu electrodes by means of surface-activated bonding (SAB) method. in IEEE Trans. Adv. Packag. 29, 218–226 (2006).
Chen, K. N., Fan, A., Tan, C. S. & Reif, R. Temperature and duration effects on microstructure evolution during copper wafer bonding. J. Electron. Mater. 32, 1371–1374 (2003).
Tian, S. et al. Intermetallics-induced directional growth of Sn whiskers in Sn-3.5Ag coating on Al substrate. Appl. Surf. Sci. 539, 148135 (2021).
Lin, K. et al. Growth behavior and formation mechanism of porous Cu3Sn in Cu/Sn solder system. Mater. Charact. 178, 111271 (2021).
Wang, Y., Chu, D. T. & Tu, K. N. Porous Cu3Sn formation in Cu-Sn IMC-based micro-joints. in IEEE Electron. Compon. Technol. Conf. 439–446 (2016).
Yin, Z., Sun, F. & Guo, M. The fast formation of full Cu3Sn solder joints in Cu/Sn/Cu system by thermal gradient bonding. J. Mater. Sci.: Mater. Electron. 30, 2146–2153 (2019).
Huang, M. L., Zhao, J. F., Zhang, Z. J. & Zhao, N. Dominant effect of high anisotropy in β-Sn grain on electromigration-induced failure mechanism in Sn-3.0Ag-0.5Cu interconnect. J. Alloys. Compd. 678, 370–374 (2016).
Kim, S. H., Park, G. T., Park, J. J. & Park, Y. B. Effects of annealing, thermomigration, and electromigration on the intermetallic compounds growth kinetics of Cu/Sn-2.5Ag microbump. J. Nanosci. Nanotechnol. 15, 8593–8600 (2015).
Zheng, Z. et al. Study of grain size effect of Cu metallization on interfacial microstructures of solder joints. Microelectron. Reliab. 99, 44–51 (2019).
Shigetou, A., Hosoda, N., Itoh, T. & Suga, T. Room-temperature direct bonding of CMP-Cu film for bumpless interconnection. in IEEE Electron. Compon. Technol. Conf. 755–760 (2001).
Shigetou, A., Itoh, T. & Suga, T. Direct bonding of CMP-Cu films by surface activated bonding (SAB) method. J. Mater. Sci. 40, 3149–3154 (2005).
Peng, L. et al. High density bump-less Cu-Cu bonding with enhanced quality achieved by pre-bonding temporary passivation for 3D wafer stacking. in Int. Symp. VLSI Technol. Syst. Appl. 1–2 (2011).
Hsu, S. Y., Chen, H. Y. & Chen, K. N. Cosputtered Cu/Ti bonded interconnects with a self-formed adhesion layer for three-dimensional integration applications. IEEE Electron Device Lett 33, 1048–1050 (2012).
Ong, J. J. et al. Low-Temperature Cu/SiO2 hybrid bonding with low contact resistance using (111)-oriented Cu surfaces. Materials 15, 1888 (2022).
Ong, J. J., Tran, D. P., Yang, S. C., Shie, K. C. & Chen, C. Shearing characteristics of Cu-Cu joints fabricated by two-step process using highly <111>-oriented nanotwinned Cu. Metals 11, 2021 (1864).
Juang, J. Y. et al. Copper-to-copper direct bonding on highly (111)-oriented nanotwinned copper in no-vacuum ambient. Sci. Rep. 8, 13910 (2018).
Huang, J. W. et al. Copper-to-copper direct bonding using different (111) surface ratios of nanotwinned copper films. in IEEE International Conference on Electronics Packaging (ICEP) 52–55 (2019).
Lin, P. F., Tran, D. P., Liu, H. C., Li, Y. Y. & Chen, C. Interfacial characterization of low-temperature Cu-to-Cu direct bonding with chemical mechanical planarized nanotwinned Cu films. Materials 15, 937 (2022).
Chang, S. Y., Chu, Y. C., Tu, K. N. & Chen, C. Effect of anisotropic grain growth on improving the bonding strength of <111>-oriented nanotwinned copper films. Mater. Sci. Eng., A 804, 140754 (2021).
Juang, J. Y., Shie, K. C., Li, Y. J., Tu, K. N. & Chen, C. Low resistance and high electromigration lifetime of Cu-to-Cu joints using (111)-oriented nanotwinned copper. Adv. Mater. Lett. 12, 1–4 (2021).
Liu, C. M. et al. Low-temperature direct copper-to-copper bonding enabled by creep on (111) surfaces of nanotwinned Cu. Sci. Rep. 5, 9734 (2015).
Wang, Y., Huang, Y. T., Liu, Y. X., Feng, S. P. & Huang, M. X. Thermal instability of nanocrystalline Cu enables Cu-Cu direct bonding in interconnects at low temperature. Scr. Mater. 220, 114900 (2022).
Tu, K. N. Recent advances on electromigration in very-large-scale-integration of interconnects. J. Appl. Phys. 94, 5451–5473 (2003).
Rebhan, B. & Hingerl, K. Physical mechanisms of copper-copper wafer bonding. J. Appl. Phys. 118, 135301 (2015).
Zhou, X., Li, X. & Lu, K. Size dependence of grain boundary migration in metals under mechanical loading. Phys. Rev. Lett. 122, 126101 (2019).
Zhou, X., Li, X. Y. & Lu, K. Enhanced thermal stability of nanograined metals below a critical grain size. Science 360, 526–530 (2018).
Michels, A., Krill, C. E., Ehrhardt, H., Birringer, R. & Wu, D. T. Modelling the influence of grain-size-dependent solute drag on the kinetics of grain in nanocrystalline materials. Acta Metall 47, 2143–2152 (1999).
Lee, H., Nix, W. D. & Wong, S. S. Studies of the driving force for room-temperature microstructure evolution in electroplated copper films. J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.-Process., Meas., Phenom. 22, 2369–2374 (2004).
Mishra, A., Kad, B. K., Gregori, F. & Meyers, M. A. Microstructural evolution in copper subjected to severe plastic deformation: experiments and analysis. Acta Mater 55, 13–28 (2007).
Sørensen, M. R., Mishin, Y. & Voter, A. F. Diffusion mechanisms in Cu grain boundaries. Phys. Rev. B 62, 3658–3673 (2000).
Fu, H., Zhou, X., Xue, H., Li, X. & Lu, K. Breaking the purity-stability dilemma in pure Cu with grain boundary relaxation. Mater. Today 55, 66–73 (2022).
Surholt, T. & Herzig, C. H. R. Grain boundary self-diffusion in Cu polycrystals of different purity. Acta Mater 45, 3817–3823 (1997).
Balluffi, R. W. & Medalist, R. F. M. Grain boundary diffusion mechanisms in metals. Metall. Mater. Trans. B 13, 527–553 (1982).
Suzuki, A. & Mishin, Y. Diffusion mechanisms in grain boundaries. J. Metastable Nanocryst. Mater. 19, 1–24 (2004).
Yamakov, V., Moldovan, D., Rastogi, K. & Wolf, D. Relation between grain growth and grain-boundary diffusion in a pure material by molecular dynamics simulations. Acta Mater 54, 4053–4061 (2006).
Koju, R. K. & Mishin, Y. The role of grain boundary diffusion in the solute drag effect. Nanomaterials 11, 2348 (2021).
Meiners, T., Peng, Z., Gault, B., Liebscher, C. H. & Dehm, G. Sulfur-induced embrittlement in high-purity, polycrystalline copper. Acta Mater 156, 64–75 (2018).
Korzhavyi, P. A., Abrikosov, I. A. & Johansson, B. Theoretical investigation of sulfur solubility in pure copper and dilute copper-based alloys. Acta Mater 47, 1417–1424 (1999).
Moriyama, M., Konishi, S., Tsukimoto, S. & Murakami, M. Effect of organic additives on formation and growth behavior of micro-void in electroplating copper films. Mater. Trans. 45, 3172–3176 (2004).
Shie, K. C., Gusak, A. M., Tu, K. N. & Chen, C. A kinetic model of copper-to-copper direct bonding under thermal compression. J. Mater. Res. Technol. 15, 2332–2344 (2021).
Gondcharton, P., Imbert, B., Benaissa, L. & Verdier, M. Voiding phenomena in copper-copper bonded structures: role of creep. ECS J. Solid State Sci. Technol 4, 77–82 (2015).
Made, R. I. et al. Experimental characterization and modeling of the mechanical properties of Cu-Cu thermocompression bonds for three-dimensional integrated circuits. Acta Mater 60, 578–587 (2012).
Gollapudi, S. et al. Creep in nanocrystalline materials: role of stress assisted grain growth. Mater. Sci. Eng. A 527, 5773–5781 (2010).
Tatsumi, H., Kao, C. R. & Nishikawa, H. Impact of crystalline orientation on Cu–Cu solid-state bonding behavior by molecular dynamics simulations. Sci. Rep. 13, 23030 (2023).
Kiener, D. & Minor, A. M. Source truncation and exhaustion: insights from quantitative in situ TEM tensile testing. Nano Lett 11, 3816–3820 (2011).
Donthu, S., Vora, M., Lahiri, S., Thompson, C. & Yi, S. Activation energy determination for recrystallization in electroplated-copper films using differential scanning calorimetry. J. Electron. Mater. 32, 531–534 (2003).
Li, X. Y., Zhou, X. & Lu, K. Rapid heating induced ultrahigh stability of nanograined copper. Sci. Adv. 6, eaaz8003 (2020).
Suzuki, Y. et al. Effect of surface area of grain boundaries on stress relaxation behavior in pure copper over wide range of grain sizes. Mater. Sci. Eng., A 794, 139585 (2020).
Kusama, T. et al. Ultra-large single crystals by abnormal grain growth. Nat. Commun. 8, 354 (2017).
Terwllliger, C. D. & Chiang, Y. ‐M. Measurements of excess enthalpy in ultrafine-grained titanium dioxide. J. Am. Ceram. Soc. 78, 2045–2055 (1995).
Acknowledgements
S.-P.F. thanks for the support from the ITF Fund (ITS/104/22) from the Innovation and Technology Commission of Hong Kong, and TRS Grant (T46-705/23-R) from the Research Grants Council of Hong Kong. S.-P.F. also thanks the support from the startup grant of the City University of Hong Kong (9380143). C.H. thanks Dr. Z. Zheng for the instructions on the electroplating process.
Author information
Authors and Affiliations
Contributions
C.H. conceived the concept, conducted the experiments, analysed data, and wrote the manuscript. C.H. and J.Z. conducted the micro-tensile test and analysed the data. R.Z. provided help in microfabrication in FIB. C.H. and C.C. conducted the design of photoresist patterns and photolithography. S.J. helped to modify the CMP process and conduct the AFM experiment. C.H. and K.M. conducted the direct Cu-Cu bonding. Y.-T.H., C.-C.C., and S.-J.C. supplied the electroplating bath and provided suggestions for tunning the electrolyte. Y.L. provided the guide for the micro-tensile test. K.-N.T. and S.-P.F. reviewed and edited the manuscript. S.-P.F. conceived the concept, supervised the research, and wrote the draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Liang Zhang, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, C., Zhou, J., Zhou, R. et al. Nanocrystalline copper for direct copper-to-copper bonding with improved cross-interface formation at low thermal budget. Nat Commun 15, 7095 (2024). https://doi.org/10.1038/s41467-024-51510-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51510-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.