Abstract
The maintenance of hematopoietic stem cell (HSC) functional integrity is essential for effective hematopoietic regeneration when suffering from injuries. Studies have shown that the innate immune pathways play crucial roles in the stress response of HSCs, whereas how to precisely modulate these pathways is not well characterized. Here, we identify the E3 ubiquitin ligase tripartite motif-containing 47 (Trim47) as a negative regulator of the mitochondrial antiviral-signaling protein (MAVS)-mediated innate immune pathway in HSCs. We find that Trim47 is predominantly enriched in HSCs, and its deficiency impairs the function and survival of HSCs after exposure to 5-flurouracil (5-FU) and irradiation (IR). Mechanistically, Trim47 impedes the excessive activation of the innate immune signaling and inflammatory response via K48-linked ubiquitination and degradation of MAVS. Collectively, our findings demonstrate a role of Trim47 in preventing stress-induced hematopoietic failure and thus provide a promising avenue for treatment of related diseases in the clinic.
Similar content being viewed by others
Introduction
Hematopoietic stem cells (HSCs) are a rare population resided in a specialized bone marrow (BM) niche, where they are endowed with the ability of self-renewal and multilineage differentiation to continuously generate various kinds of blood cells1,2. Under steady-state conditions, HSCs are mainly remained in a quiescent state which offers protection against a series of injuries3,4. During hematopoietic stress caused by chemotherapy drugs, infections, irradiation (IR) or BM transplantation, HSCs can exit dormant state to repopulate the damaged hematopoietic system5,6. However, the excessive activation of cell cycle may impair HSC self-renewal capacity and result in hematopoietic failure7,8. Therefore, a deeper insight into the molecular networks underlying the balance between HSC quiescence and activation may be beneficial to effectively facilitate hematopoietic regeneration and prevent HSC depletion to the greatest extent.
Tripartite motif (Trim) family includes dozens of members, most of which are post-translational modification factors with E3 ubiquitin ligase activity9. As reported, Trims are involved in various biological processes, including cell proliferation, differentiation, metabolism, and apoptosis, by promoting target protein degradation via the ubiquitin-proteasome pathway10. Notably, several Trim proteins, such as Trim4, Trim25, Trim31, and Trim44, function as key innate immunity mediators by modulating pattern recognition receptors (PRRs) signals or the production of inflammatory cytokines11,12,13,14. Studies have shown that HSCs are sensitive to immune-related signaling, whereas whether they participate in HSC biology is not fully understood15,16.
Trim47 is a member of the Trim family and found to be implicated in multiple pathological and physiological processes9,17. It was reported that Trim47 upregulation accelerates the growth of glioma through ubiquitination and degradation of Foxo118. Meanwhile, Trim47 can bind to and degrade p53 protein to promote the progression of renal cell carcinoma19. Moreover, Trim47 overexpression leads to severe cerebral ischemia-reperfusion injury, which may be attribute to its role in the regulation of apoptosis and inflammation20. Previous studies revealed that E3 ubiquitin ligases play an essential role in the hematopoietic regulation, such as MAEA, Itch, and Cbl-b21,22,23. In particular, a recent study reports that Trim31 deficiency increases HSC proliferation and induces leukemia initiation because of the elevated levels of CDK8 protein24.
Here, we show that Trim47 is predominantly enriched in HSCs with robust self-renewal capacity. Although Trim47 is dispensable for homeostatic hematopoiesis, its loss decreases HSC pool and impairs HSC long-term reconstitution ability after hematopoietic stress due to reduced quiescence and increased apoptosis. Mechanistically, Trim47 inhibits the hyperactivation of the innate immune response by directly targeting mitochondrial antiviral-signaling protein (MAVS) to promote its ubiquitin-mediated degradation, which contributes to HSC maintenance under stress. In summary, our findings disclose the critical role of Trim47 in preventing stress-induced HSC exhaustion, thus providing a target for the treatment of hematopoietic injury.
Results
Trim47 is predominantly enriched in the primitive HSCs
Previous studies have demonstrated that E3 ubiquitin ligase of Trim family proteins are involved in regulating the fate of several types of stem cells25,26, while their roles in adult HSCs is largely unknown. By analyzing single-cell RNA sequencing (scRNA-seq) data from murine hematopoietic stem/progenitor cells (HSPCs), we found that Trim47 was primarily enriched in the C1 cluster (Fig. 1A–C), which was considered to be the most primitive HSC population27. Similar results were obtained from other public databases (Supplementary Fig. 1A–C). Consistently, quantitative PCR (qPCR) and immunofluorescence staining showed that Trim47 was highly expressed in LT-HSCs, but was less expressed in hematopoietic progenitor cells and lineage-positive (Lin+) cells (Fig. 1D; Supplementary Fig. 1D–G). To further determine this finding, we generated transgenic mice (Trim47+/eGFP) in which a 2A-eGFP (enhanced green fluorescent protein) cassette was knocked into the Trim47 locus by homologous recombination (Fig. 1E). Flow cytometric analysis displayed that a small group of eGFP+ cells existed in the BM (Fig. 1F), which specially expressed endogenous Trim47 (Fig. 1G). In addition, the expression of Trim47 or the number and function of HSCs were comparable between Trim47+/+ and Trim47+/eGFP mice (Fig. 1H; Supplementary Fig. 1H–K). These results suggest that Trim47+/eGFP mice can be regarded as a veritably tool for tracking Trim47 levels in vivo. Likewise, the highest expression of Trim47 was observed in LT-HSCs from Trim47+/eGFP mice (Fig. 1I). Alternatively, eGFP+ BM cells were more enriched for HSPCs identified by CD34 and Flk2 (CD135) or signaling lymphocytic activation molecule (SLAM; CD150 and CD48) compared to eGFP- BM cells (Fig. 1J). These results indicate that Trim47 may play an important role in regulating HSC biology.
A Twelve distinct cell clusters were identified in HSPCs based on UMAP analysis from the GEO database (accession number: GSE90742). C1-C3, unprimed HSPC clusters (C1: the most primitive population); Mk megakaryocyte-primed progenitors, Er erythrocyte-primed progenitors, Mo1 type I macrophage-primed progenitors, Mo2 type I macrophage-primed progenitors, Ba basophilia-primed progenitors, Neu neutrophile-primed progenitors, DC dendritic cell-primed progenitors, B B cell-primed progenitors, T T cell-primed progenitors. B Violin plots showing the transcriptional levels of Trim family genes in the 12 different cell clusters. C UMAP plots showing the transcriptional levels of Trim47 in the 12 different cell clusters. D qPCR analysis of Trim47 mRNA expression in long-term HSCs (LT-HSCs, Lin- Sca1+ c-Kit+ CD34- Flk2-), short-term HSCs (ST-HSCs, Lin- Sca1+ c-Kit+ CD34+ Flk2-), multipotent progenitors (MPPs, Lin- Sca1+ c-Kit+ CD34+ Flk2+), common lymphoid progenitors (CLPs, Lin- CD127+ Sca1med c-Kitmed), megakaryocyte erythroid progenitors (MEPs, Lin- Sca1- c-Kit+ CD16/32- CD34-), granulocyte monocyte progenitors (GMPs, Lin- Sca1- c-Kit+ CD16/32+ CD34+), common myeloid progenitors (CMPs, Lin- Sca1- c-Kit+ CD16/32- CD34+) and Lineage+ cells (Lin+) sorted from the BM of normal WT mice (n = 3). E The strategy for construction of the Trim47+/eGFP mouse model. F Representative flow cytometric plots showing eGFP- and eGFP+ frequency in the BM cells from Trim47+/eGFP mice. Trim47+/+ mice were used as negative controls. G qPCR analysis of Trim47 mRNA expression in sorted eGFP- or eGFP+ cells from the BM of Trim47+/eGFP mice (n = 3). H qPCR analysis of Trim47 mRNA expression in the BM of Trim47+/+ and Trim47+/eGFP mice (n = 3). I Flow cytometric analysis of Trim47-eGFP expression in the indicated hematopoietic cells from the BM of Trim47+/eGFP mice (n = 5). MFI, mean fluorescence intensity. J Flow cytometric analysis of the percentage of the indicated HSPC populations in eGFP- and eGFP+ BM cells from Trim47+/eGFP mice (n = 5). Data are shown as the mean ± SD. Statistical analyses are determined by unpaired two-sided Student’s t test (G–H, J) and One-way ANOVA, followed by Tukey’s test (D, I). Exact P values are given in the Source Data file (***P < 0.001). Source Data are provided as a Source Data file.
Trim47 expression represents HSCs with enhanced quiescence and self-renewal capacity
Considering the finding that Trim47 was unevenly expressed in LT-HSCs, we then obtained the top 30% (eGFPhi), middle 40% (eGFPmid) and bottom 30% (eGFPlo) of Trim47 expression so as to discriminate different subsets of HSCs (Fig. 2A). Indeed, cell cycle analysis showed that with the upregulation of Trim47 expression, the percentage of G0 phase in LT-HSCs was evidently increased, while the percentages of G1 and S/G2/M phases were decreased (Fig. 2B). These findings were confirmed by the bromodeoxyuridine (BrdU) incorporation assay, suggesting that high Trim47 expression is closely associated with HSC quiescence (Fig. 2C). However, the apoptosis rate was comparable between HSC populations with different expression of Trim47 (Supplementary Fig. 2A). To determine whether Trim47 expression can identify functionally distinct HSC populations, eGFPlo and eGFPhi LT-HSCs were purified and planted in medium. It was noticed that eGFPhi LT-HSCs generated more progeny cells in culture, accompanied with increased colony-forming ability (Fig. 2D–F; Supplementary Fig. 2B). Subsequently, we conducted a HSC transplantation assay and found that eGFPhi LT-HSCs exhibited elevated chimerism levels in recipient mice (Fig. 2G; Supplementary Fig. 2C–E). Furthermore, bulk RNA-sequencing (RNA-seq) analysis showed that 1480 genes were upregulated in eGFPhi relative to eGFPlo LT-HSCs, while 1157 genes were downregulated (Fig. 2H). Consistent with our aforementioned results, gene set enrichment analysis (GSEA) revealed that HSC signature, long-term hematopoiesis, and stemness signature were enriched in eGFPhi LT-HSCs, whereas lineage signature, cell cycle checkpoint and mobilized HSC signature were enriched in eGFPlo LT-HSCs (Fig. 2I). Collectively, our data disclose that high expression of Trim47 may be described as a credible marker for HSCs with robust self-renewal capacity.
A The gating strategy of flow cytometry to discriminate the eGFPlo (bottom 30%), eGFPmid (middle 40%), and eGFPhi (top 30%) LT-HSCs from the BM of Trim47+/eGFP mice. Trim47+/+ mice served as negative controls. B Cell cycle analysis of eGFPlo, eGFPmid, and eGFPhi LT-HSCs from the BM of Trim47+/eGFP mice (n = 5). C The proportion of BrdU+ cells in eGFPlo, eGFPmid, and eGFPhi LT-HSCs from the BM of Trim47+/eGFP mice (n = 6). D The percentage of LSKs after 9 days culture of eGFPhi or eGFPlo LT-HSCs (1 × 103) sorted from the BM of Trim47+/eGFP mice (n = 5). E Colony formation analysis of 3 × 102 eGFPlo and eGFPhi LT-HSCs sorted from the BM of Trim47+/eGFP mice (n = 4). F Colony formation analysis of single eGFPlo and eGFPhi LT-HSCs sorted from the BM of Trim47+/eGFP mice (n = 3). G eGFPlo and eGFPhi LT-HSCs (5 × 102) sorted from the BM of Trim47+/eGFP mice were transplanted into lethally irradiated CD45.1 recipients, along with CD45.1 BM cells (5 × 105). At the indicated time points after transplantation, the percentage of donor-derived cells in the PB of recipient mice was analyzed by flow cytometry (n = 6). Representative flow cytometric plots at 16 weeks after transplantation are shown. (H, I) RNA-seq analysis of eGFPlo and eGFPhi LT-HSCs sorted from the BM of Trim47+/eGFP mice (Fold change > 2 and p < 0.05). H The scatter plots of differentially expressed genes (DEGs) between eGFPlo and eGFPhi LT-HSCs. Representative DEGs are displayed. I GSEA plots showing the enrichments of the indicated gene sets between eGFPlo and eGFPhi LT-HSCs. Data are shown as the mean ± SD. Statistical analyses are determined by unpaired two-sided Student’s t test (D–E, G; F-first/third/fourth panels), Mann-Whitney U test (F-second panel), R package edgeR (H) and One-way ANOVA, followed by Tukey’s test (B, C). Exact P values are given in the Source Data file (*P < 0.05, **P < 0.01, ***P < 0.001). Source Data are provided as a Source Data file.
Trim47 deletion reduces HSC pools following stress
To investigate the function of Trim47 in adult hematopoiesis, we generated Trim47−/− mice (Supplementary Fig. 3A). The deletion of Trim47 did not alter the peripheral blood (PB) counts and the total BM cellularity (Supplementary Fig. 3B, C). The frequencies and absolute numbers of HSPCs from Trim47−/− mice were also comparable to those in wild type (WT) mice (Supplementary Fig. 3D, E). In line with these findings, no obvious difference was found between WT and Trim47−/− HSCs in limiting dilution experiment and non-competitive transplantation (Supplementary Fig. 3F, G). These results hint that Trim47 is not involved in hematopoietic regulation under steady-state conditions.
We next want to know whether Trim47 deficiency affects HSC biology during hematopoietic stress, such as 5-flurouracil (5-FU) and IR challenge. Previous studies reported that c-Kit expression was transiently reduced and then restored to nearly normal level at least 8 days after 5-FU stress28,29. In view of this, we used an alternative HSC marker EPCR for flow cytometric analysis (Supplementary Fig. 4A). Interestingly, we found that Trim47 expression was sharply decreased and then was gradually increased in HSCs during the critical stage of hematopoietic recovery after 5-FU treatment (Fig. 3A), suggesting that its ablation may influences hematopoiesis during stress. Indeed, white blood cell (WBC), platelet (PLT) and red blood cell (RBC) counts were more seriously reduced in Trim47−/− mice compared with WT controls after a single 5-FU administration (Fig. 3B; Supplementary Fig. 4B). Consistently, mice reconstructed with Trim47−/− BM cells died earlier when subjected to sequential 5-FU injections (Fig. 3C). Furthermore, we analyzed HSPC phenotype at day 10 after 5-FU stress using conventional markers, when c-Kit expression had largely recovered29. The results revealed that the frequencies and absolute numbers of HSPC populations were significantly decreased in the BM from Trim47−/− mice (Fig. 3D; Supplementary Fig. 4C). To further substantiate these data, we performed a limiting dilution assay. A significantly reduced frequency of functional HSCs was observed in Trim47−/− mice following 5-FU treatment (Supplementary Fig. 4D). Notably, the defective phenotype of Trim47−/− HSCs induced by 5-FU was transplantable (Supplementary Fig. 4E). On the other hand, we used sublethal IR-induced non-pharmacological injury as another stress model, and generally similar hematopoietic changes were obtained in Trim47−/− mice at day 15 post-5.0 Gy IR (Fig. 3E–H; Supplementary Fig. 4F, G). Altogether, these results suggest that Trim47 may be recognized as a crucial factor to modulate hematopoiesis under short-term stress.
A The expression of Trim47-eGFP in Lin- EPCR+ CD150+ CD48- cells (referred to as ESLAM-HSCs) from the BM of Trim47+/eGFP mice at the indicated time points after 5-FU injection (n = 4). B The counts of WBC and PLT in the PB of WT and Trim47−/− mice at the indicated time points after 5-FU injection (n = 8). C BM cells (1 × 106) from WT and Trim47−/− mice were transplanted into lethally irradiated normal WT mice. Twelve weeks later, the survival rates of recipients were monitored after treatment with 5-FU weekly for 3 consecutive weeks (n = 10). D The percentages and numbers of the indicated HSPC populations in the BM of WT and Trim47−/− mice at day 10 after 5-FU injection (n = 6). E The expression of Trim47-eGFP in ESLAM-HSCs from the BM of Trim47+/eGFP mice at the indicated time points following 5.0 Gy IR (n = 4). F The counts of WBC and PLT in the PB of WT and Trim47−/− mice at the indicated time points following 5.0 Gy IR (n = 8). G BM cells (1 × 106) from WT and Trim47−/− mice were transplanted into lethally irradiated normal WT mice. Twelve weeks later, the survival rates of recipients were monitored after 7.5 Gy IR (n = 10). H The percentages and numbers of the indicated HSPC populations in the BM of WT and Trim47−/− mice at day 15 following 5.0 Gy IR (n = 6). Data are shown as the mean ± SD. Statistical analyses are determined by Mann-Whitney U test (B-PLT-24 day), unpaired two-sided Student’s t test (D, F, H; B-PLT-the other days), and log-rank test (C, G). Exact P values are given in the Source Data file (*P < 0.05, **P < 0.01, ***P < 0.001). Source Data are provided as a Source Data file.
Trim47 maintains the long-term repopulating capacity of HSCs
Then, we conducted HSC transplantation assays to analyze the effects of Trim47 deletion on HSC long-term reconstitution ability (Fig. 4A). The Trim47-deficient HSCs yielded lower levels of PB chimerism related to WT controls and the differences were gradually amplified from 4 to 16 weeks both in the primary and secondary transplantation (Fig. 4B). However, lineage distribution of HSCs was unaltered after Trim47 ablation (Fig. 4C). Meanwhile, a similar decrease in BM reconstitution was observed in the absence of Trim47 (Fig. 4D). To determine whether stress will enlarge the disparities of engraftment ability, HSC transplantation was performed after 5-FU treatment (Fig. 4E). As expected, the reconstruction function of Trim47-null HSCs was further declined when suffering from stress, although the lineage distribution was still unchanged (Fig. 4F–H). Moreover, we found that the frequency of EPCR+ cells in the SLAM-HSCs was comparable between WT and Trim47−/− mice after 5-FU and IR treatment (Supplementary Fig. 5A, B), which ruled out the possibility of the change of EPCR- non-stem populations in two groups under stress conditions. Importantly, EPCR+ SLAM-HSCs from Trim47-deficient mice displayed a severely reduced colony-forming ability relative to those from WT mice under stress conditions (Fig. 4I; Supplementary Fig. 5C), further reinforcing our findings. On the other hand, reciprocal BM transplantation and homing assays displayed that the compromised hematopoietic reconstitution capacity of Trim47−/− HSCs was not due to the deficiencies in microenvironment and HSC homing ability (Supplementary Fig. 5D–G). These results indicate an essential role of Trim47 in promoting HSC maintenance during stress in a cell-intrinsic manner.
A–D LT-HSCs (5 × 102) sorted from the BM of WT or Trim47−/− mice were transplanted into lethally irradiated CD45.1 recipients, along with CD45.1 BM cells (5 × 105). A The strategy of HSC transplantation. B The percentage of donor-derived cells in the PB of recipient mice at the indicated time points after primary and secondary transplantation (n = 6). Representative flow cytometric plots at 16 weeks after transplantation are shown. C The lineage distribution of donor-derived cells in the PB of recipient mice at 16 weeks after primary and secondary transplantation (n = 6). D The percentage of donor-derived BM cells, Lin-, LSKs, and LT-HSCs in the BM of recipient mice at 16 weeks after primary and secondary transplantation (n = 6). Representative flow cytometric plots of LSK chimerism are shown. (E-H) LT-HSCs (5 × 102) sorted from the BM of WT or Trim47−/− mice at day 10 after 5-FU injection were transplanted into lethally irradiated CD45.1 recipients, along with CD45.1 BM cells (5 × 105). E The strategy of 5-FU injection and HSC transplantation. F The percentage of donor-derived cells in the PB of recipient mice at the indicated time points after transplantation (n = 6). Representative flow cytometric plots at 16 weeks after transplantation are shown. G The lineage distribution of donor cells in the PB of recipient mice at 16 weeks after transplantation (n = 6). H The percentage of donor-derived BM cells, Lin-, LSKs, and LT-HSCs in the BM of recipient mice at 16 weeks after transplantation (n = 6). Representative flow cytometric plots of LSK chimerism are shown. I Serial replating analysis of 100 EPCR+ SLAM-HSCs sorted from the BM of WT and Trim47−/− mice at day 10 after 5-FU treatment. Colonies were counted at 7 days after each replating (n = 4). Data are shown as the mean ± SD. Statistical analyses are determined by unpaired two-sided Student’s t test (B–D, F–I). Exact P values are given in the Source Data file (**P < 0.01, ***P < 0.001). Source Data are provided as a Source Data file.
Trim47 promotes the quiescence and survival of HSCs after stress
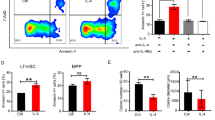
Given the deficit of hematopoietic reconstitution capacity following Trim47 deletion, we speculated that this may be relevant to decreased HSC quiescence. Compared to WT controls, the percentage of G0 phase was slightly reduced in LT-HSCs but not LSKs of Trim47−/− mice under homeostasis conditions (Fig. 5A). Intriguingly, the quiescence of HSCs was noticeably decreased in Trim47−/− HSCs in response to 5-FU stress (Fig. 5B). These results were further substantiated by the in vivo BrdU incorporation assay (Supplementary Fig. 6A, B). The above observations that Trim47 deletion accelerated HSC proliferation but slowed hematopoietic recovery in mice prompted us to assess whether Trim47 deficiency affects HSC survival. As anticipated, Trim47−/− HSCs exhibited a remarkable increase in apoptosis rate following 5-FU challenge, although this was largely unchanged at steady state (Fig. 5C, D). Besides, relative differences in quiescence, proliferation, and apoptosis were still significant between 5-FU-treated WT and Trim47−/− HSCs, after normalized to steady-state data (Supplementary Fig. 6C–E). Similar results were detected in Trim47-null HSCs upon IR exposure (Supplementary Fig. 6F–K).
Cell cycle analysis of LSKs and LT-HSCs in the BM of WT or Trim47−/− mice (A) at steady state or (B) at day 10 after 5-FU injection (n = 6). Apoptosis analysis of LSKs and LT-HSCs in the BM of WT or Trim47−/− mice (C) at steady state or (D) at day 10 after 5-FU injection (n = 6). E–G RNA-seq analysis of LT-HSCs sorted from WT and Trim47−/− mice at steady state or at day 10 after 5-FU injection (Fold change > 2 and p < 0.05). E The heatmap plots of upregulated genes or downregulated genes between WT and Trim47−/− LT-HSCs. Representative DEGs are displayed. F The scatter plots of DEGs between WT and Trim47−/− LT-HSCs. Representative DEGs are displayed. G GSEA plots showing the enrichments of the indicated gene sets between WT and Trim47−/− LT-HSCs with 5-FU treatment. H qPCR analysis of the mRNA expression of the indicated genes in LT-HSCs from the BM of WT and Trim47−/− mice at steady state or at day 10 after 5-FU injection (n = 3). Data are shown as the mean ± SD. Statistical analyses are determined by Mann-Whitney U test (A-first/sixth panels, B-fourth panel) and unpaired two-sided Student’s t test (C, D, H; A, B the other panels). Exact P values are given in the Source Data file (*P < 0.05, **P < 0.01, ***P < 0.001). Source Data are provided as a Source Data file.
Subsequently, we performed RNA-seq analysis and found that only 355 genes were changed between WT and Trim47−/− HSCs at steady state, whereas a total of 817 genes were exhibited to be differentially expressed after 5-FU treatment (Fig. 5E, F). Furthermore, GSEA revealed that the gene sets correlated with HSC maintenance were downregulated and gene sets related with HSC proliferation were upregulated in Trim47−/− HSCs when treated with 5-FU (Fig. 5G). Consistent with these data, the expression of Cyclins (Ccne1, Ccnd1, and Ccnd2) was increased and the expression of Cyclin dependent kinase inhibitor (Cdkn1c and Cdkn1b) was decreased in Trim47−/− HSCs after 5-FU injection (Fig. 5H). In contrast, only Ccnd1 and Cdkn1c appeared mildly altered in untreated groups (Fig. 5H). These data imply that Trim47 preserves the quiescence and survival of HSCs under stress conditions.
Trim47 deficiency causes the hyperactivation of innate immune signal in HSCs during stress
We next sought to explore the underlying mechanism by which Trim47 regulates HSC function. A recent study showed that 5-FU markedly induces the generation of endogenous double-stranded RNA (dsRNA), which can trigger the melanoma differentiation-associated protein 5 (MDA5)-MAVS signaling axis, a critical innate immune pathway29. Subsequently, MAVS stimulates downstream inflammatory pathways including TBK1-IRF3/7 and IKKα/β-NF-κB, and promotes the production of inflammatory cytokines, therefore modulating HSC proliferation via autocrine15,29. Interestingly, GSEA of RNA-seq data showed that the innate immune response and inflammatory signaling were more significantly activated in Trim47−/− HSCs after 5-FU stress (Fig. 6A; Supplementary Fig. 7A). However, the mRNA levels of MDA5 and MAVS were not significantly altered in HSCs with Trim47 deficiency both under untreated and 5-FU stress conditions (Supplementary Fig. 7B). Notably, MAVS protein expression was upregulated in Trim47−/− HSCs regardless of 5-FU treatment, although MDA5 protein levels remained unchanged (Fig. 6B; Supplementary Fig. 7C). However, the phosphorylation of TBK1, IRF3, IKKα/β, and p65, as well as p65 nuclear translocation, were only slightly but not statistically significantly increased in Trim47−/− HSCs at steady state (Fig. 6C–G; Supplementary Fig. 7D), which is reasonable because the levels of endogenous dsRNA were very low under normal conditions, as reported previously29,30. Importantly, the above inflammatory signals were more activated in Trim47−/− HSCs after 5-FU stress, compared with WT HSCs (Fig. 6C–G). Moreover, Trim47 deficiency obviously increased the production of inflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor (TNF), and interferon beta (IFN-β), in the BM supernatant of mice after 5-FU treatment (Fig. 6H; Supplementary Fig. 7E).
A GSEA plots showing the enrichments of innate immune response and inflammatory signaling-related gene sets between WT and Trim47−/− LT-HSCs with 5-FU treatment. Flow cytometric analysis of the protein expression of (B) MAVS, (C) p-TBK1, (D) p-IRF3, (E) p-IKKα/β and (F) p-p65 in LT-HSCs from the BM of WT and Trim47−/− mice at steady state or at day 10 after 5-FU injection (n = 5). G Immunofluorescence analysis showing p65 nuclear translocation in LT-HSCs sorted from the BM of WT and Trim47−/− mice at day 10 after 5-FU injection. The histograms on the right represent the ratio of nuclear p65 fluorescence intensity/cytoplasmic p65 fluorescence intensity (n = 35 for WT group, n = 27 for Trim47−/− group). Scale bar = 2 μm. The images shown are representative of three independent experiments. H ELISA analysis of the indicated inflammatory cytokine levels in the BM supernatant of WT and Trim47−/− mice at steady state or at day 10 after 5-FU injection (n = 5). I qPCR analysis of Noxa mRNA expression in LT-HSCs from the BM of WT and Trim47−/− mice at steady state or at day 10 after 5-FU injection (n = 3). J Flow cytometric analysis of Noxa protein expression in LT-HSCs from the BM of WT and Trim47−/− mice at steady state or at day 10 after 5-FU injection (n = 5). K LSKs sorted from the BM of WT or Trim47−/− mice at day 10 after 5-FU injection were infected with lentivirus carrying control (Ctrl) or shNoxa-2. The apoptosis of ZsGreen+ LT-HSCs was detected by flow cytometry at 48 h after infection (n = 5). Flow cytometric analysis of the (L) cell number, (M) cell cycle, and (N) apoptosis in LSKs and/or LT-HSCs from the BM of WT or Trim47−/− mice 24 h after pIpC injection (n = 6). Data are shown as the mean ± SD. Statistical analyses are determined by unpaired two-sided Student’s t test (B–F, H–J, L–N), Mann-Whitney U test (G) and One-way ANOVA, followed by Tukey’s test (K). Exact P values are given in the Source Data file (**P < 0.01, ***P < 0.001). Source Data are provided as a Source Data file.
On the other hand, accumulating evidence shows that MAVS can induce cell apoptosis by facilitating the expression of pro-apoptotic molecule Noxa31,32. Indeed, Noxa mRNA and protein levels were much higher in Trim47-deleted HSCs when challenged with 5-FU (Fig. 6I, J). Importantly, Noxa knockdown largely rescued the apoptosis of Trim47-null HSCs following 5-FU treatment (Fig. 6K; Supplementary Fig. 7F). The similar observations were obtained in Trim47−/− mice after exposed to IR (Supplementary Fig. 7G–S). To further substantiate our findings, we treated Trim47-deficient HSCs with polyinosinic-polycytidylic acid (pIpC), a dsRNA mimetic that has been reported to trigger MAVS activation29. As with 5-FU or IR stress, the detects were also found in Trim47−/− HSCs with pIpC treatment (Fig. 6L–N). In line with these findings, eGFPlo HSCs displayed higher activation of the innate immune signaling than eGFPhi HSCs in the BM of Trim47+/eGFP mice following stress (Supplementary Fig. 8A–L), suggesting that Trim47 expression level determines HSC response to stress. Hence, these data indicate that Trim47 deficiency leads to the excessive activation of MAVS-mediated inflammatory response, which may be responsible for the reduced quiescence and increased apoptosis of HSCs under stress.
Trim47 directly targets MAVS to promote its ubiquitin-mediated degradation in HSCs
As an E3 ubiquitin ligase, Trim47 is able to degrade specific target proteins through the ubiquitin-proteasome process18,19. Given that the mRNA levels of MAVS did not change but its protein expression evidently was increased after Trim47 depletion, combined with the finding from UbiBrowser 2.0 database that MAVS was a candidate substrate protein of Trim47, we speculated that Trim47 may promote the degradation of MAVS protein via ubiquitination (Supplementary Table 1). Expectedly, overexpressing Flag-tagged Trim47 enhanced K48-linked ubiquitination level of overexpressed Myc-tagged MAVS in HEK293T cells (Fig. 7A). Consistently, Trim47 knockout decreased the K48-linked ubiquitination level of endogenous MAVS in Lin- BM cells from mice after 5-FU stress (Fig. 7B). To determine whether the defects of Trim47−/− HSCs was attributable to the excessive activation of MDA5-MAVS pathway, we knocked MAVS down by lentivirus carrying short hairpin RNA against MAVS (shMAVS). It is clearly that shMAVS-3 was an optimal shRNA to inhibit the expression of MAVS and then was used in further tests (Supplementary Fig. 9A). Knockdown of MAVS significantly suppressed the activation of TBK1-IRF3 and IKKα/β-NF-κB pathways, as well as Noxa expression, in Trim47−/− HSCs (Fig. 7C–G). More importantly, MAVS knockdown partly rescued the cell cycle status, apoptosis, and hematopoietic reconstitution capacity of HSCs with Trim47 deficiency (Fig. 7H–J). Likewise, knocking down MAVS significantly rescued the WBC recovery of mice reconstructed with Trim47−/− HSPCs (Supplementary Fig. 9B). In conclusion, these results demonstrate that Trim47 may promote HSC maintenance during hematopoietic stress mainly through regulating the ubiquitinated degradation of MAVS (Fig. 7K).
A Coimmunoprecipitation analysis of MAVS K48-linked ubiquitination in HEK293T cells transfected with Myc-MAVS and together with control, HA-K48-linked ubiquitin or Flag-TRIM47 plasmids. Input as the loading control. The images shown are representative of three independent experiments. B Co-immunoprecipitation analysis of the K48-linked ubiquitination of endogenous MAVS in Lin- cells from the BM of WT or Trim47−/− mice at day 10 after 5-FU injection. Input as the loading control. The images shown are representative of three independent experiments. C–I WT or Trim47−/− LSKs were infected with lentivirus carrying Ctrl or shMAVS-3, and then the ZsGreen+ cells (5 × 103) were sorted and transplanted into lethally irradiated CD45.1 recipients, along with CD45.1 BM cells (5 × 105). At 16 weeks after transplantation, recipient mice were treated with 5-FU. Ten days later, these mice were used for subsequent analysis. Flow cytometric analysis of the protein expression of (C) p-TBK1, (D) p-IRF3, (E) p-IKKα/β, (F) p-65, and (G) Noxa in CD45.2+ LT-HSCs from the BM of recipient mice after 5-FU treatment (n = 5). H Cell cycle analysis of CD45.2+ LT-HSCs in the BM of recipient mice after 5-FU treatment (n = 5). I Apoptosis analysis CD45.2+ LT-HSCs in the BM of recipient mice after 5-FU treatment (n = 5). J LSKs sorted from the BM of WT or Trim47−/− mice at day 10 after 5-FU injection were infected with lentivirus carrying Ctrl or shMAVS-3, and then the ZsGreen+ cells (5 × 103) were sorted and transplanted into lethally irradiated CD45.1 recipients, along with CD45.1 BM cells (5 × 105). At 16 weeks after transplantation, the percentage of donor-derived cells in the PB of recipient mice was analyzed by flow cytometry (n = 5). K Schematic diagram demonstrating the role and underlying mechanism of Trim47 in regulating hematopoiesis under stress. Data are shown as the mean ± SD. Statistical analyses are determined by One-way ANOVA, followed by Tukey’s test (C–J). Exact P values are given in the Source Data file (**P < 0.01, ***P < 0.001). Source Data are provided as a Source Data file.
Discussion
HSCs are responsible for the maintenance of hematopoietic homeostasis and the hematopoietic regeneration under stress conditions, which are tightly modulated by many intrinsic factors and extrinsic signals33,34. It was well established that the insufficient ability of HSCs to return back to quiescence upon stress will cause their rapid exhaustion35,36,37. Although numerous studies have reported the regulation of HSC behavior under stress, the underlying mechanism remains to be elucidated. In this study, we demonstrate that Trim47 ensures the HSC survival and function under hematopoietic stress by inhibiting the overactivation of the innate immune pathway via ubiquitination of MAVS.
As a member of the Trim protein family, Trim47 is mainly located in the cytosol and nucleus17,20. Previous studies focus more on the role of Trim47 in tumorigenesis and progression due to its overexpression in multiple tumor cells, including breast cancer, glioma, and pancreatic cancer, while its normal physiological function is poorly understood17,18,38. Recently, Trim47 is found to be abundantly expressed in the several specific tissues such as brain astrocytoma, lung, heart, and kidney39,40,41. It was reported that Trim47 is required to modulate excitatory synapse development39. In addition, Trim47 plays a critical role in regulating lipopolysaccharide-induced acute lung injury40. Here, we observed that Trim47 was enriched in primitive HSCs, which was verified by scRNA-seq, qPCR, immunofluorescence and Trim47-eGFP reporter mouse model. Unfortunately, our data showed that Trim47 deletion did not affect homeostatic hematopoiesis. Interestingly, Trim47 was sharply downregulated in response to 5-FU and IR stress and then was significantly upregulated in HSCs during hematopoietic recovery stage, implying that Trim47 is sensitive to exogenous stimulation. Consistent with this notion, Trim47 can be induced by multiple stimuli in vascular endothelial cells42. Consequently, Trim47 deficiency decreased HSC pool when exposed to 5-FU and IR, eventually resulting in attenuated hematopoietic recovery. These findings reveal that Trim47 may be a potential hematopoietic regulatory factor, which functions primarily under stress conditions. Therefore, a stable or overexpression of Trim47 during days 3–9 after 5-FU or IR stress may be an effective way to prevent the exhaustion of HSCs, while further experiments are still need.
Under normal circumstances, most HSCs are retained in a dormant state in order to maintain their regenerative potential1,5. When subjected to various stress injuries, HSC will rapidly proliferate and differentiate to promote hematopoietic recovery, but this comes at the expense of their self-renewal capacity8,37,43. As reported, the excessive use of hematopoietic growth factors may impair HSC long-term reconstitution ability, although the acute myelosuppression induced by stress is effectively alleviated6. In this study, we found that Trim47−/− HSCs displayed slightly reduced quiescence at steady state. Of note, Trim47 deficiency led to significantly enhanced HSC proliferation but did not accelerate hematopoietic recovery, which may be because of remarkably increased apoptosis of HSCs after Trim47 deletion. Consistent with these data, transplantation assays showed that HSC long-term reconstitution ability was significantly compromised following Trim47 ablation, especially after 5-FU stress. However, studies have reported that Trim47 contributes to the proliferation of various cancer cells17,38. This completely opposite regulatory effect between HSCs and tumor cells is familiar with previously identified stem cell regulators, including Egr1, c-Myc, Tet2, Pbx1, Nkx2-3, etc44,45,46,47,48. Therefore, we demonstrate that Trim47 is essential for the HSC maintenance under stress conditions.
Substantial studies have shown that the innate immune pathways and inflammatory response play critical roles in HSC biology30,49. Innate immune system is the first line of defense against viral infection, depending on nucleic acid sensors to recognize foreign RNA or DNA from pathogens15,50. RIG-I-Like receptors (RLRs) consist of RIG-I, MDA5 and laboratory of genetics and physiology 2 (LGP2), preferring to recognize cytoplasmic dsRNA51,52. In addition to RNA virus, intracellular dsRNA is also derived from the transcripts of transcription of transposable elements (TEs) that can be induced by some stress factors53. It was reported that 5-FU treatment causes increased transcription of TE RNAs, which can be sensed by MDA5 and then trigger MAVS-mediated inflammatory signaling29. The activation of inflammatory response plays a crucial role in driving the rapid proliferation of HSCs in the context of stress15,29. However, how to precisely regulate the activity of the innate immune pathway in HSCs is still obscure. In the present study, we discovered that Trim47−/− HSCs exhibited more activated innate immune response and downstream inflammatory signaling following 5-FU and IR stress. Intriguingly, the protein, but not mRNA, levels of MAVS rather than MDA5 was significantly upregulated in Trim47−/− HSCs. On the other hand, the activation of MAVS may induce expression of Noxa, thereby contributing to the increased apoptosis in Trim47-deleted HSCs after 5-FU and IR challenge. Altogether, our data indicate that Trim47 promotes HSC maintenance probably through inhibiting the excessive activation of MAVS-mediated inflammatory response upon hematopoietic stress.
Trim47 was initially defined as a substrate-specific E3 ubiquitin ligase, which is responsible for the ubiquitination of intracellular proteins18,42. It is known that K48-lined ubiquitination mainly facilitates proteasomal degradation of target proteins, whereas proteins modified with K63-linked ubiquitination predominantly mediates cellular signaling activation40. At present, several target proteins interacting with Trim47 have been identified in other types of tissues, such as Foxo1, PPM1A, NF90, and TRAF218,40,54,55. In this work, we determined that Trim47 directly interacted with MAVS protein and promoted its K48-lined ubiquitination and degradation in HSCs. Importantly, knockdown of MAVS inhibited the excessive activation of the innate immune response, thereby significantly rescuing the phenotype and function of Trim47-null HSCs after stress. Nonetheless, we cannot exclude the possibility that other targets of Trim47 may be involved in the observed phenotype of HSCs. Substantial experiments are needed to be done to validate more targets of Trim47 in our follow-up study. It is thus reasonable to propose that Trim47 regulates HSC function upon hematopoietic stress mainly by ubiquitinating MAVS to promote its degradation. Combined this work with previously published studies, we believe that moderate activation of the innate immune signaling maybe beneficial to effective hematopoietic regeneration after stress.
In conclusion, we elucidate that Trim47 promotes the maintenance of HSCs under hematopoietic stress through modulating MAVS-mediated innate immune signaling. Our findings not only uncover an important role of Trim47 in preventing stress-induced HSC exhaustion but also provide a promising avenue for hematopoietic regeneration when subjecting to various stresses.
Methods
Animals
Trim47+/eGFP and Trim47−/− mice were constructed at the Model Organisms Center (Shanghai, China), and WT littermate mice were used as controls. Congenic CD45.1 mice were kindly given by Prof. Jinyong Wang (Institutes of Biomedicine and Health, Guangzhou, China). All mice were male (8–10 weeks old) and had a C57BL/6J background. The animal experiments were supported by the Animal Ethics Committee of the Third Military Medical University (Chongqing, China), and were performed in accordance with the guidelines by the Committee.
5-FU, IR or pIpC treatment
Mice were intraperitoneally injected with a single dose of 5-FU (150 mg/kg; #F6627, Sigma). For IR stress, mice were subjected to 60Co γ-ray at Irradiation Center of the Third Military Medical University. For pIpC administration, mice were intraperitoneally injected with a single dose of pIpC (10 mg/kg; #P1530, Sigma).
Flow cytometry
BM single-cell suspensions were prepared, stained and analyzed as we previously described47,56. In brief, hematopoietic cell phenotype was identified by the antibodies against lineage marker, Sca-1, c-Kit, CD34, Flk2, EPCR (CD201), CD150, CD48, CD16/32, CD127, Gr-1, CD11b, B220, CD3e, CD45.1 and CD45.2. To further detect the cell cycle, apoptosis, BrdU incorporation or intracellular protein expression, the antibodies were used as follows: anti-Ki67, anti-BrdU, anti-Annexin V, anti-Phospho-NF-κB p65 (Ser536), anti-MAVS, anti-MDA5, anti-Phospho-TBK1/NAK (Ser172), anti-Phospho-IRF3 (Ser396), anti-Phospho-IKKα/β (Ser176/180), anti-Noxa. Flow cytometric detection was performed by ID7000 (Sony Biotechnology) and the data were analyzed by the FlowJo v.10.0 software (BD Biosciences). Cell sorting was operated on a FACSAria III sorter (BD Biosciences). The details of these antibodies are shown in Supplementary Table 2.
qPCR analysis
The qPCR assay was carried out as we previously described57,58. The primer sequences are listed in Supplementary Table 3.
Transplantation assays
For HSC transplantation assay, eGFPhi and eGFPlo LT-HSCs (5 × 102) sorted from the BM of the Trim47+/eGFP or LT-HSCs (5 × 102) isolated from WT and Trim47−/− mice (CD45.2), along with CD45.1 BM cells (5 × 105), were intravenously transplanted into lethally irradiated (10.0 Gy) CD45.1 recipients. For the second transplantation, BM cells (1 × 106) derived from the primary recipients were transplanted into a second set of irradiated CD45.1 mice. For non-competitive transplantation, BM cells (1 × 106) from WT or Trim47−/− mice at steady state or 5-FU conditions were transplanted into lethally irradiated (10.0 Gy) CD45.1 mice. At 16 weeks after transplantation, the percentages and numbers of HSPCs in recipients were detected by flow cytometry. For reciprocal BM transplantation, CD45.1 BM cells (1 × 106) were transplanted into lethally irradiated (10.0 Gy) WT or Trim47−/− recipients. For homing assay, LSKs (1 × 105) sorted from WT and Trim47−/− mice were labeled by CFSE and then transplanted into lethally irradiated (10.0 Gy) WT recipient mice. At the indicated time after transplantation, the PB or BM obtained from recipients were analyzed by flow cytometry.
Limiting dilution assay
Briefly, BM cells (5 × 103, 1 × 104, 2.5 × 104, 5 × 104 or 1 × 105) sorted from WT or Trim47−/− mice with or without 5-FU treatment, along with CD45.1 BM cells (5 × 105), were intravenously transplanted into lethally irradiated CD45.1 recipient mice. The detailed procedures refer to the published work59.
HSC culture and colony-formation assays
The detailed procedures were described in our published work60. Briefly, eGFPhi or eGFPlo LT-HSCs (1 × 103) were sorted from Trim47+/eGFP mice and cultured in SFEM medium (#09650, Stem Cell Technologies). After 9 days culture, the cells were detected by flow cytometry. For colony-formation assays, single or bulk eGFPhi or eGFPlo LT-HSCs were seeded into methylcellulose medium (M3434; #03434, Stem Cell Technologies) and the colonies were viewed with a microscope at day 12 after culture.
HSC serial replating assay
In brief, 100 EPCR+ SLAM-HSCs were sorted from the BM of WT and Trim47−/− mice at day 10 after 5-FU treatment or at day 15 following 5.0 Gy IR, and then seeded into M3434 medium. After 7 days culture, colonies were counted, and then 5 × 104 cells were collected and reseeded into new dishes. A similar protocol was used for the 3rd and 4th rounds of replating. The number of colonies in culture were analyzed after each replating.
Enzyme linked immunosorbent assay (ELISA) assay
BM supernatant of WT and Trim47−/− mice were collected after 5-FU or IR treatment. Next, IL-6, TNF and IFN-β levels were analyzed by mouse IL-6 ELISA Kit (#PI326, Beyotime), TNF ELISA Kit (#JL10484, Jianglai Bio) or IFN-β ELISA Kit (#EK1510, Signalway Antibody) according to the manufacturer’s instructions, respectively.
scRNA-seq
The mouse LSK scRNA-seq data was obtained from the Gene Expression Omnibus (GEO) database (no. GSE90742) and reanalyzed using Seurat v4.0.1. Low quality data were excluded based on the following principles: (1) genes detected in less than 3 cells; (2) cells with fewer than 200 genes; and (3) cells with no less than 10% mitochondrial genes. Subsequently, the remaining 4785 cells were normalized and the influence of cell cycle-associated genes were eliminated. Data dimension reduction was performed via principal component analysis (PCA). Uniform manifold approximation and projection (UMAP) was applied for data visualization with 13 initial principal components. All cells were grouped into 12 clusters and cell types were identified using the previously reported surface markers27,60.
Bulk RNA-seq
Total RNA was isolated from eGFPhi and eGFPlo LT-HSCs or WT and Trim47−/− LT-HSCs, respectively. Then, RNA samples were used for RNA-seq analysis at Sinotech Genomics Co., Ltd (Shanghai, China). Fold change > 2 and p < 0.05 were considered as the criteria to screen the differential genes. The specific methods of heatmaps, scatter plots, and GSEA refer to our previous articles58,61. GSEA 4.2.1 software (http://www.gsea-msigdb.org/gsea) was used for GSEA analysis and the gene sets were derived from MSigDB (http://www.gsea-msigdb.org/gsea/msigdb). All the data have been uploaded to GEO database (no. GSE236768).
Lentiviral infection
All lentivirus were produced by Hanbio Co. Ltd (Shanghai, China), and shRNA sequences are displayed in Supplementary Tables 4 and 5. WT or Trim47−/− LSKs or Lin- cells were infected by lentivirus according to our previous studies56. After that, the ZsGreen+ cells (5 × 103) were sorted and then were used for rescued transplantation.
Ubiquitination assay
For analysis of the K48-linked ubiquitination of MAVS in HEK293T cells, HEK293T cells were transfected with plasmids expressing Myc-MAVS, HA-K48-linked ubiquitin or Flag-Trim47, and then whole-cell extracts boiled 2 min in IP buffer containing 1.0% (vol/vol) SDS, 50 mM Tris-HCl, pH 7.4, 50 mM EDTA, 150 mM NaCl, and a protease inhibitor cocktail (#04693132001, Merck) were immunoprecipitated with the Myc-specific antibody and analyzed by immunoblot with anti-HA. For analysis of the K48-linked ubiquitination of MAVS in Lin- cells from the BM of mice, the whole-cell extracts treated as above were immunoprecipitated with anti-MAVS and analyzed by immunoblot with anti-K48-linked ubiquitin. The details of these antibodies are shown in Supplementary Table 2.
Immunofluorescence
LT-HSCs were freshly sorted and evenly smeared onto slides. Subsequently, cells were fixed, permeabilized, and blocked as described in our previous studies56,58. The samples were further stained with anti-Trim47 or anti-p65, followed by incubated with secondary antibody. Images were obtained using a confocal microscope (Zeiss LSM800). The details of these antibodies are shown in Supplementary Table 2.
Statistical analysis
All data were analyzed by GraphPad Prism 8.0.2 (La Jolla, CA, USA) and SPSS 20.0 (IBM, Chicago, USA). Normality was assessed by the Shapiro-Wilk test. For data meeting the assumption of normality, comparison between two groups was assessed by unpaired Student’s t test, and comparisons among multiple groups were determined by One-way analysis of variance (ANOVA), followed by Tukey’s test. For data violating the assumption of normality, difference between two groups was analyzed using the Mann-Whitney U test. The differences in the survival rate of mice were analyzed using Log-rank test. The results were independently performed at least three times and presented as mean ± standard deviation (SD). P < 0.05, P < 0.01 and P < 0.001 were considered to be statistically significant differences. The detailed statistical analysis method and result of each figure is provided in the source data files.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the data supporting this study are available within the manuscript and Supplementary Information files or from the corresponding author upon request. RNA-seq data are available in GEO database with the accession GSE236768. Source data are provided with this paper.
References
Pinho, S. & Frenette, P. S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 20, 303–320 (2019).
Hurwitz, S. N., Jung, S. K. & Kurre, P. Hematopoietic stem and progenitor cell signaling in the niche. Leukemia 34, 3136–3148 (2020).
Kataoka, K. et al. Evi1 is essential for hematopoietic stem cell self-renewal, and its expression marks hematopoietic cells with long-term multilineage repopulating activity. J. Exp. Med. 208, 2403–2416 (2011).
Guo, P. et al. Histone variant H3.3 maintains adult haematopoietic stem cell homeostasis by enforcing chromatin adaptability. Nat. Cell Biol. 24, 99–111 (2022).
Borges, L., Oliveira, V. K. P., Baik, J., Bendall, S. C. & Perlingeiro, R. C. R. Serial transplantation reveals a critical role for endoglin in hematopoietic stem cell quiescence. Blood 133, 688–696 (2019).
Shao, L., Luo, Y. & Zhou, D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid. Redox Signal. 20, 1447–1462 (2014).
Hua, W. K. et al. HDAC8 regulates long-term hematopoietic stem-cell maintenance under stress by modulating p53 activity. Blood 130, 2619–2630 (2017).
Velardi, E. et al. Suppression of luteinizing hormone enhances HSC recovery after hematopoietic injury. Nat. Med. 24, 239–246 (2018).
Hatakeyama, S. TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem. Sci. 42, 297–311 (2017).
van Gent, M., Sparrer, K. M. J. & Gack, M. U. TRIM proteins and their roles in antiviral host defenses. Annu. Rev. Virol. 5, 385–405 (2018).
Okamoto, M., Kouwaki, T., Fukushima, Y. & Oshiumi, H. Regulation of RIG-I activation by K63-linked polyubiquitination. Front. Immunol. 8, 1942 (2017).
De Falco, F. et al. Bovine Delta papillomavirus E5 oncoprotein interacts with TRIM25 and hampers antiviral innate immune response mediated by RIG-I-like receptors. Front. Immunol. 12, 658762 (2021).
Liu, B. et al. The ubiquitin E3 ligase TRIM31 promotes aggregation and activation of the signaling adaptor MAVS through Lys63-linked polyubiquitination. Nat. Immunol. 18, 214–224 (2017).
Yang, B. et al. Novel function of Trim44 promotes an antiviral response by stabilizing VISA. J. Immunol. 190, 3613–3619 (2013).
Bowman, T. V. & Trompouki, E. Sensing stemness. Curr. Stem Cell Rep. 7, 219–228 (2021).
Khan, N. et al. M. tuberculosis Reprograms hematopoietic stem cells to limit myelopoiesis and impair trained immunity. Cell 183, 752–770.e722 (2020).
Azuma, K. et al. TRIM47 activates NF-kappaB signaling via PKC-epsilon/PKD3 stabilization and contributes to endocrine therapy resistance in breast cancer. Proc. Natl Acad. Sci. USA 118, e2100784118 (2021).
Wei, H., Ding, C., Zhuang, H. & Hu, W. TRIM47 promotes the development of glioma by ubiquitination and degradation of FOXO1. Onco Targets Ther. 13, 13401–13411 (2020).
Chen, J. X. et al. TRIM47 promotes malignant progression of renal cell carcinoma by degrading P53 through ubiquitination. Cancer Cell Int. 21, 129 (2021).
Hao, M. Q., Xie, L. J., Leng, W. & Xue, R. W. Trim47 is a critical regulator of cerebral ischemia-reperfusion injury through regulating apoptosis and inflammation. Biochem. Biophys. Res. Commun. 515, 651–657 (2019).
Wei, Q. et al. MAEA is an E3 ubiquitin ligase promoting autophagy and maintenance of haematopoietic stem cells. Nat. Commun. 12, 2522 (2021).
Rathinam, C., Matesic, L. E. & Flavell, R. A. The E3 ligase Itch is a negative regulator of the homeostasis and function of hematopoietic stem cells. Nat. Immunol. 12, 399–407 (2011).
Zuo, W. et al. c-Cbl-mediated neddylation antagonizes ubiquitination and degradation of the TGF-beta type II receptor. Mol. Cell 49, 499–510 (2013).
Zhang, K. et al. E3-ligase TRIM31 regulates hematopoietic stem cell homeostasis and MLL-AF9 leukemia. Haematologica 108, 2116–2129 (2023).
Jaworska, A. M., Wlodarczyk, N. A., Mackiewicz, A. & Czerwinska, P. The role of TRIM family proteins in the regulation of cancer stem cell self-renewal. Stem Cells 38, 165–173 (2020).
Nenasheva, V. V. & Tarantul, V. Z. Many faces of TRIM proteins on the road from pluripotency to neurogenesis. Stem Cells Dev. 29, 1–14 (2020).
Rodriguez-Fraticelli, A. E. et al. Clonal analysis of lineage fate in native haematopoiesis. Nature 553, 212–216 (2018).
Simonnet, A. J. et al. Phenotypic and functional changes induced in hematopoietic stem/progenitor cells after gamma-ray radiation exposure. Stem Cells 27, 1400–1409 (2009).
Clapes, T. et al. Chemotherapy-induced transposable elements activate MDA5 to enhance haematopoietic regeneration. Nat. Cell Biol. 23, 704–717 (2021).
Gao, Y. et al. m(6)A modification prevents formation of endogenous double-stranded RNAs and deleterious innate immune responses during hematopoietic development. Immunity 52, 1007–1021.e1008 (2020).
Boehmer, D. F. R. et al. OAS1/RNase L executes RIG-I ligand-dependent tumor cell apoptosis. Sci. Immunol. 6, eabe2550 (2021).
Qin, Y. et al. NLRP11 disrupts MAVS signalosome to inhibit type I interferon signaling and virus-induced apoptosis. EMBO Rep. 18, 2160–2171 (2017).
Mitchell, E. et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature 606, 343–350 (2022).
Yin, R. et al. Differential m(6)A RNA landscapes across hematopoiesis reveal a role for IGF2BP2 in preserving hematopoietic stem cell function. Cell Stem Cell 29, 149–159.e147 (2022).
Li, C. et al. Amino acid catabolism regulates hematopoietic stem cell proteostasis via a GCN2-eIF2alpha axis. Cell Stem Cell 29, 1119–1134.e1117 (2022).
Garcia-Prat, L. et al. TFEB-mediated endolysosomal activity controls human hematopoietic stem cell fate. Cell Stem Cell 28, 1838–1850.e1810 (2021).
Liu, Y., Chen, Y., Deng, X. & Zhou, J. ATF3 prevents stress-induced hematopoietic stem cell exhaustion. Front. Cell Dev. Biol. 8, 585771 (2020).
Li, L. et al. TRIM47 accelerates aerobic glycolysis and tumor progression through regulating ubiquitination of FBP1 in pancreatic cancer. Pharmacol. Res. 166, 105429 (2021).
Sharma, G. & Banerjee, S. Activity-regulated E3 ubiquitin ligase TRIM47 modulates excitatory synapse development. Front. Mol. Neurosci. 15, 943980 (2022).
Qian, Y. et al. TRIM47 is a novel endothelial activation factor that aggravates lipopolysaccharide-induced acute lung injury in mice via K63-linked ubiquitination of TRAF2. Signal Transduct. Target. Ther. 7, 148 (2022).
Mishra, A. et al. Gene-mapping study of extremes of cerebral small vessel disease reveals TRIM47 as a strong candidate. Brain 145, 1992–2007 (2022).
Wang, Z., Li, Z., Han, C., Cheng, Y. & Wang, K. TRIM47 promotes glioma angiogenesis by suppressing Smad4. Vitr. Cell. Dev. Biol. Anim. 58, 771–779 (2022).
Wang, N. et al. TWIST1 preserves hematopoietic stem cell function via the CACNA1B/Ca2+/mitochondria axis. Blood 137, 2907–2919 (2021).
Min, I. M. et al. The transcription factor EGR1 controls both the proliferation and localization of hematopoietic stem cells. Cell Stem Cell 2, 380–391 (2008).
Ficara, F., Murphy, M. J., Lin, M. & Cleary, M. L. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell 2, 484–496 (2008).
Nakajima, H. & Kunimoto, H. TET2 as an epigenetic master regulator for normal and malignant hematopoiesis. Cancer Sci. 105, 1093–1099 (2014).
Hu, M. et al. Transcription factor Nkx2-3 maintains the self-renewal of hematopoietic stem cells by regulating mitophagy. Leukemia 37, 1361–1374 (2023).
Sheng, Y. et al. Role of c-Myc haploinsufficiency in the maintenance of HSCs in mice. Blood 137, 610–623 (2021).
Lefkopoulos, S. et al. Repetitive elements trigger RIG-I-like receptor signaling that regulates the emergence of hematopoietic stem and progenitor cells. Immunity 53, 934–951.e939 (2020).
Liu, S. et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, aaa2630 (2015).
Hou, F. et al. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146, 448–461 (2011).
Rehwinkel, J. & Gack, M. U. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat. Rev. Immunol. 20, 537–551 (2020).
Barbieri, D. et al. Thrombopoietin protects hematopoietic stem cells from retrotransposon-mediated damage by promoting an antiviral response. J. Exp. Med. 215, 1463–1480 (2018).
Li, L. et al. Anti-fibrotic effect of melittin on TRIM47 expression in human embryonic lung fibroblast through regulating TRIM47 pathway. Life Sci. 256, 117893 (2020).
Dou, S. et al. Ubiquitination and degradation of NF90 by Tim-3 inhibits antiviral innate immunity. eLife 10, e66501 (2021).
Hu, M. et al. SRC-3 is involved in maintaining hematopoietic stem cell quiescence by regulation of mitochondrial metabolism in mice. Blood 132, 911–923 (2018).
Hu, M. et al. MicroRNA-21 maintains hematopoietic stem cell homeostasis through sustaining the NF-kappaB signaling pathway in mice. Haematologica 106, 412–423 (2021).
Lu, Y. et al. Tespa1 facilitates hematopoietic and leukemic stem cell maintenance by restricting c-Myc degradation. Leukemia 37, 1039–1047 (2023).
Chen, Z. et al. Nuclear DEK preserves hematopoietic stem cells potential via NCoR1/HDAC3-Akt1/2-mTOR axis. J. Exp. Med. 218, e20201974 (2021).
Hu, M. et al. CD63 acts as a functional marker in maintaining hematopoietic stem cell quiescence through supporting TGFbeta signaling in mice. Cell Death Differ. 29, 178–191 (2022).
Chen, N. et al. Melanocortin/MC5R axis regulates the proliferation of hematopoietic stem cells in mice after ionizing radiation injury. Blood Adv. 7, 3199–3212 (2023).
Acknowledgements
We thank Yang Liu for technical support in flow cytometry, and Liting Wang and Shaobo Wang for technical support in immunofluorescence microscopy. This work was supported by grants from the National Natural Science Foundation of China (No. 81930090, 82203974, U22A20279, 32271159), the Natural Science Foundation of Chongqing City (No. CSTB2024NSCQ-JQX0002, CSTB2023NSCQ-MSX0284), Science Foundation of State Key Laboratory of Trauma and Chemical Poisoning (No. 2024K004) and Postdoctoral Innovative Talent Support Program of China (No. BX20220398).
Author information
Authors and Affiliations
Contributions
F.C., Y. Lu., and Y.X. designed the study, performed experiments, analyzed data, and wrote the paper. N.C. and L.Y. performed animal experiments and analyzed data. X.Z. and H.Z. participated in some in vivo experiments. Y. Liu. and Z.C. participated in data analysis. Q.Z., S.C., J.C., and J.Z. participated in the initial experimental design and discussed the manuscript. J.W., M.H., and S.W. conceived and supervised the study, and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, F., Lu, Y., Xu, Y. et al. Trim47 prevents hematopoietic stem cell exhaustion during stress by regulating MAVS-mediated innate immune pathway. Nat Commun 15, 6787 (2024). https://doi.org/10.1038/s41467-024-51199-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51199-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.