Abstract
Chiral amides are important structure in many natural products and pharmaceuticals, yet their efficient synthesis from simple amide feedstock remains challenge due to its weak Lewis basicity. Herein, we describe our study of the enantioselective synthesis of chiral amides by N-alkylation of primary amides taking advantage of an achiral rhodium and chiral squaramide co-catalyzed carbene N–H insertion reaction. This method features mild condition, rapid reaction rate (in all cases 1 min) and a wide substrate scope with high yield and excellent enantioselectivity. Further product transformations show the synthetic potential of this reaction. Mechanistic studies reveal that the non-covalent interactions between the catalyst and reaction intermediate play a critical role in enantiocontrol.
Similar content being viewed by others
Introduction
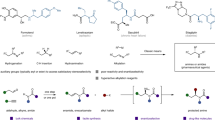
Chiral amides are common structural units in natural products, pharmaceuticals, and agrochemicals (Fig. 1a)1,2,3,4,5, therefore, their efficient synthesis is of great significance and has received increasing attention. Traditional synthesis of chiral amides is the condensation of carboxylic acids or their derivatives with chiral amines6, which has been extensively studied and relies highly on coupling agents to facilitate dehydration. As a complementary approach, the assemble of chiral amide through asymmetric N-alkylation of readily available primary amide feedstock provides an opportunity to create new chiral center in a catalytic manner (Fig. 1b). However, due to the weak nucleophilicity of amide caused by the delocalization of their N-lone pair into the carbonyl group as well as the difficulty in controlling stereochemistry during the C(sp3)–N bond formation step, practical method for highly enantioselective N-alkylation of amide remains rare with only a few successful examples using secondary alkyl halide7, benzylic C–H bond8,9, imine10,11, as alkylation agent. Thus, the development of alternative amide N-alkylation protocols that can efficiently produce chiral amide under mild condition is highly desired. In this context, the enantioselective carbene N–H bond insertion12,13,14, featuring high reactivity, mild reaction conditions and good functional group tolerance15,16, provides a suitable technique for N-alkylation of amide.
In recent years, asymmetric N–H bond insertion reactions have been applied successfully to various types of amines, such as aromatic amines17,18,19,20,21,22,23, carbamates24,25,26,27,28, aliphatic amines29,30, and ammonia31, with high yield and excellent enantioselectivity. However, to the best of our knowledge, highly enantioselective N–H insertion of amides remains a challenge, with only one example of enantioselective N–H insertion reaction between diazoesters and amides reported, with ee values up to 77%32. This may be a function of properties specific to amides. First, the low Lewis basicity of amides interferes with its bonding to metal-carbene species. Second, the ylide intermediate that forms from the nucleophilic addition of the amide to the metal carbene has a comparatively high acidity, favoring a tendency toward deprotonation rather than interaction with a chiral catalyst (Fig. 2a).
To overcome these challenges, we need to find a chiral organocatalyst that could efficiently mediate the proton transfer process of the reaction intermediates. Through a systematic screen of catalysts, we found that chiral squaramides with a quinine skeleton can effectively control the enantioselectivity of the amide N–H bond insertion reaction of carbenes generated from diazoketone decomposition. Herein, we describe our strategy for enantioselective N-alkylation of primary amide using carbene insertion into amide N–H bond (Fig. 2b). Under mild condition, low catalyst loading (1 mol% for both metal and organocatalyst) and fast reaction rate (1 min in all cases), the reaction efficiently installs a sp3-C chiral center to the amide feedstock. This method provides a powerful tool for the synthesis of structural divergent chiral amides and related useful molecules (see General Information in Supplementary Information for details).
Results
Optimization of reaction conditions
We began by exploring reaction conditions using 3-phenylpropionamide (1a) and 1-diazo-1-phenylpropan-2-one (2a) as model substrates (Table 1; see Reaction Optimization section in Supplementary Information for details). When copper catalysts such as Cu(MeCN)4PF6 and CuTp* (copper hydrotris(3,5-dimethylpyrazolyl)borate) were used to decompose the diazoketone, no desired product was detected (entries 1, 2). The use of dirhodium(II) acetate (Rh2(OAc)4) afforded the insertion product 3a with 80% yield (entry 3). We then used Rh2(OAc)4 in combination with organocatalysts to control the enantioselectivity of the reaction. Chiral spiro phosphoric acids and amides C1–C3, which have been demonstrated to be enantioselective proton transfer catalysts in the previously reported carbamate N–H insertion reactions33, gave only very low ee in this reaction ( < 10%) (entries 4–6). Organocatalysts C4–C7 of urea, thiourea, and squaramide with chiral amino groups showed low to moderate enantioselectivity (entry 7–10). To our delight, introduction of a hydroquinine or hydroquinidine skeleton to the squaramide catalyst (C8 and C9) significantly improved the enantioselectivity to 80% ee (entry 11, 12). Diastereomeric catalysts C8 and C9 yielded enantiomers of the insertion product with the same ee values, which offers a choice for obtaining chiral amides with different configurations. Further evaluation of dirhodium catalysts showed that substitution on the carboxylic acid ligand impacts the enantioselectivity of the reaction (entry 13–16), with triphenylacetatic acid being the best ligand (entry16, Rh2(TPA)4, 88% ee). Furthermore, the use of Rh2(TPA)4 significantly accelerated the reaction, which was completed within 1 min, and increased the enantioselectivity of the reaction to 90% ee (entry 17). Adding the diazoketone dropwise and lowering the reaction temperature to 0 °C further improved the yield and enantioselectivity (entries 18 and 19).
Substrate scope
Under the optimal reaction conditions, the scope of substrates was studied (Fig. 3; see Synthesis and Analytical Data in Supplementary Information for details). Surprisingly, reactions using substrates with all types of structure and functional groups were finished within 1 min after the setup, showing the efficiency of this method. First, a broad range of amides were investigated in the reaction with diazoketone 2a (Fig. 3a). Primary amides with different chain lengths underwent the reaction smoothly to afford the corresponding α-amido ketones (3a–3e) with high yield (96–99%) and excellent enantioselectivities (90–93% ee). Amides with bulkier alkyl group gave higher enantioselectivity but lower yield (3f and 3g vs 3d). Amides with cycloalkyl or saturated heterocycles afforded insertion products (3h–3o) in high yields (85–99%) and with high enantioselectivities (88–96% ee). In addition to aliphatic amides, aromatic amides bearing either an electron-donating substituent (3q), an electron-withdrawing substituent (3r), or a halogen (3s–3v) all gave desired products with high yields (93–99%) and high enantioselectivites (92–96% ee).The reaction using amides having naphthyl, furyl, thiophenyl, pyridyl, and indole groups also underwent well, affording the desired products (3w–3aa) in high yields with high enantioselectivities. It is worth noting that since the basicity of the quinuclidine group of catalyst C8 is much higher than that of pyridyl, the pyridyl group will not interfere with the reaction. Moreover, for 1H-indole-2-carboxamide, the insertion reaction selectively occurs at amide N–H rather than at indole N–H. Acrylamide and propynamide were also suitable substrates for the reaction, affording insertion products (3ab and 3ac) in satisfactory yield and ee. These results demonstrated that the amide insertion reaction has good functional group tolerance.
Next, the substrate scope with respect to the diazoketones was investigated in the amide N–H insertion reaction (Fig. 3b). Installation of electron-donating substituents (4a, 4b, 4m) or electron-withdrawing substituents (4c–4i, 4o–4q) and phenyl (4k) on the aryl ring of the diazoketones resulted in good yields and moderate to high enantioselectivities (74–93% ee). The halogen atoms in the products offer opportunities for subsequent synthetic transformations. The diazoketones containing a naphthyl group (4ij and 4n) also worked well and gave high enantioselectivities (94% ee and 91% ee, respectively). To our delight, the switch of methyl to ethyl of the diazoketone substrate had no significant effect on the enantioselectivity of the reaction (4l). We then used this protocol to investigate the derivatizations of various pharmaceutical agents and other bioactive molecules, such as indomethacin, rufinamide, oxaprozin, naproxen, levetiracetam, penicillin, glutamine, and asparagine (Fig. 3c). Under the standard conditions, these compounds smoothly underwent the asymmetric insertion reaction to afford the corresponding chiral N-alkylated amides (5a–5h) with high yields (82–99%) and good enantioselectivities (83–90% ee) or diasteroselectivities (86:14 to >99:1). Products 5f–5h show that N-alkylation occurs on primary amides but not on secondary amides and carbamates. These results demonstrate the applicability of this protocol for the synthesis of drug-like molecules.
Applications
To further demonstrate the application potential of the method in synthesis, we carried out scale-up experiments and a series of product transformations. At 1 mmol scale, 3l and 3p can be produced smoothly without obvious decrease of yield or ee (Fig. 4a). In the synthesis of chiral compounds, a good method should be able to selectively prepare each enantiomer. To our delight, under standard conditions, catalysts C8 and C9, which are diastereomers, produced the insertion product 3a and ent-3a, respectively, with the same yield and ee but with opposite configuration (Fig. 4b). Given the ubiquity of chiral amino alcohols34,35,36,37, especially tertiary alcohols38, in natural products, biologically active molecules, and chiral ligands and auxiliaries, we conducted Grignard reactions on the insertion product 3p, producing chiral 1,2-amino tertiary alcohols (Fig. 4c; see Determination of the Configuration of 7d in Supplementary Information for details). The chiral center adjacent to the ketone was not epimerized by the strongly basic Grignard reagent and effectively controlled the newly formed chiral center with excellent diastereoselectivities (dr >20:1 in all cases, 7a–7h). Wittig reaction of 3p gave allylic amide 8, and reduction afforded the 1,2-vicinal amino alcohol 9, which was further transformed into chiral phenyl oxazoline 10 through a MsCl mediated cyclization (Fig. 4d)39,40,41. In addition, the dehydrogenase inhibitor phosphoglycerate 1242 was synthesized in excellent 98% yield, 96% ee, and >20:1 diastereoselectivity by amide insertion followed by a reduction (Fig. 4e).
a Scale-up synthesis. b Enantio-divergent synthesis of 3a. c Scope of Grignard addition reaction of 3p (all in 0.05 mmol scale). d Transformation of 3p to chiral allylic amide, amido-alcohol and chiral oxazoline. e Enantio- and diastereo-selective synthesis of phosphoglycerate dehydrogenase inhibitor 12.
Mechanism studies
The mechanism of the amide N–H bond insertion reaction was presumed to be similiar to that of the hydrogen bonding donor (HBD) catalyzed carbene insertion reaction, which has been extensively studied in our group and others27,29,30,43,44. First, dirhodium(II) triphenylacetate I reacts with diazaoketone 2a to generate a highly reactive Rh-carbenoid intermediate II by releasing one equivalent N2 (Fig. 5a). The Rh-carbenoid species undergoes nucleophilic addition with amide 1c to form a metal associated ylide intermediate III, which is rapidly decomposed to give free enol IV. The free enol is captured by catalyst C8 to form a hydrogen bonding complex V, and the proton of squaramide is attracted by the electron-negative carbon of the enol to form the intermediate VI. Intermediate VI undergoes an enantioselective proton transfer to afford product 3c and releases the squaramide catalyst C8. A plot of log(er) values versus Hammett σ values of the insertion products generated from various substituted diazoketones shows a linear correlation between enantioselectivity and the electronic effect of the substituents (Fig. 5b), which shows that the carbon linked to the substituted phenyl group has a certain amount of charge in the transition state. The relatively small slope (ρ = –0.69) indicates that there is no obvious charge separation in the enantio-determining step and the proton transfer more likely proceeds in a concerted way through a cyclic TS45. Control experiments were also conducted to examine the role of the carbonyl group of the diazo substrate (Fig. 5c). The insertion reactions of α-diazoester and diazoamide with butanamide yielded the desired products with low ee. Removing the carbonyl group from the diazo compound led to messy results, and no insertion product was obtained. These results revealed that an electron-deficient carbonyl group in the diazo substrate is essential in increasing the electrophilicity of metal-carbene intermediate to gain reactivity with amide and efficiently bonding with the chiral catalyst.
To further understand the origin of the enantioselectivity of the reaction, we studied the proton transfer step considering the influence of rhodium complex using density functional theory calculations (Fig. 6). Previous works on copper(I) and HBD co-catalyzed insertion reaction suggest that the coordination of Cu with the sulfur atom of the thiourea or the oxygen atom of the squaramide plays important role in lowering the energy barrier of the proton transfer step29,31. However, the same effect was also found in the Rh(II) and squaramide cooperative catalytic system, and the coordination of dirhodium with hydroquinine nitrogen atom significantly lowered the energy barrier by 6.6 kcal/mol (see Computational Study in Supplementary Information and Computational Data in Supplementary Data 1 for details). Therefore, we analyzed the proton transfer step, including the participation of the dirhodium catalyst. The lowest-energy transition state structures for the major and minor enantiomers of the product, RhTPA-TSR and RhTPA-TSS, are presented in Fig. 6, in which the quinuclidine of catalyst abstracts the proton of the enol hydroxyl group and the squaramide donates its proton to the enol carbon. The energy difference between RhTPA-TSR and RhTPA-TSS is 2.2 kcal/mol, resulting in the major product with (R)-configuration, which agrees with the experimental observations. Both transition states have multiple hydrogen bonding interactions between the chiral squaramide catalyst and the enol intermediate. However, more hydrogen bonds and stronger π–π stacking interaction were found in RhTPA-TSR than in RhTPA-TSS by weak interaction analysis of the independent gradient model based on the Hirschfeld partition (IGMH)46,47,48,49, suggesting that the enantioselectivity was controlled mainly by non-covalent interactions.
Discussion
In conclusion, we have established an efficient and straightforward method for the enantioselective N-alkylation of primary amides through carbene insertion into amide N–H bond. This method features mild condition, rapid reaction rate and broad substrate scope. The success of this transformation relies on the combined properties of the achiral dirhodium catalyst and the chiral squaramide catalyst. DFT studies and IGMH analysis show that the non-covalent interactions between the catalyst and reaction intermediate are decisive for the enantiocontrol. Future efforts will focus on applying this strategy to the alkylation of amides using other alkylation agents.
Methods
General procedure for enantioselective N–H insertion of amide 1a with diazoketone 2a
The Rh2(TPA)4·DCM (2.9 mg, 0.002 mmol, 1 mol%), chiral squaramide catalyst (1.3 mg, 0.002 mmol, 1 mol%), and 3-phenylpropionamide 1a (30.0 mg, 0.2 mmol, 1.0 eq.) were introduced into an oven-dried Schlenk tube containing a stir bar in a argon-filled glove box. Then, the Schlenk tube was sealed with a rubber, moved out of the glove box and injected with 1.5 mL DCM. The tube was cooled down to 0 °C and 0.5 mL of DCM solution of 1-diazo-1-phenylpropan-2-on 2a (32.0 mg, 0.2 mmol; diazo compound may be thermally unstable and should be handled carefully with personal protective equipment) was added dropwise within 5 mins via a syringe while stirring. The resulting mixture was allowed to stir for another 1 min at 0 °C. Upon completion, organic volatiles were evaporated in vacuo, and the residue was purified by flash chromatography purification (PE/EA = 3:1, v/v). Product 3a was obtained as a withe solid (56.5 mg, 99% yield) and characterized by NMR and HPLC (see NMR Spectra and HPLC Spectra in Supplementary Information for details).
Data availability
All data regarding materials and methods, optimization studies, experimental procedures, DFT calculations, NMR spectra and HPLC spectra can be found in the Supplementary Information. The Cartesian coordinates of the optimized structures are included in the Supplementary Data. All other data are available from the corresponding authors upon request.
References
Brow, D. G. & Bostrom, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Pitzer, J. & Steiner, K. Amides in nature and biocatalysis. J. Biotechnol. 235, 32–46 (2016).
Wang, X. Challenges and outlook for catalytic direct amidation reactions. Nat. Catal. 2, 98–102 (2019).
Wu, F.-P., Holz, J., Yuan, Y. & Wu, X.-F. Copper-catalyzed carbonylative synthesis of β-boryl amides via boroamidation of alkenes. CCS Chem. 31, 2643–2654 (2021).
Lu, Y.-X. et al. Anodic electrosynthesis of amide from alcohol and ammonia. CCS Chem. https://doi.org/10.31635/ccschem.023.202302727 (2023).
Valeur, E. & Bradley, M. Amide bond formation: beyond the myth of coupling reagents. Chem. Soc. Rev. 38, 606–631 (2009).
Chen, C., Peters, J. C. & Fu, G. C. Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity. Nature 596, 250–256 (2021).
Chen, X.-M., Lian, Z. & Kramer, S. Enantioselective intermolecular radical amidation and amination of benzylic C–H bonds via dual copper and photocatalysis. Angew. Chem. Int. Ed. 62, e202217638 (2023).
Dai, L., Chen, Y.-Y., Xiao, L.-J. & Zhou, Q.-L. Intermolecular enantioselective benzylic C(sp3)−H Amination by cationic copper catalysis. Angew. Chem. Int. Ed. 62, e202304427 (2023).
Hatano, M., Ozaki, T., Sugiura, Y. & Ishihara, K. Enantioselective direct aminalization with primary carboxamides catalyzed by chiral ammonium 1,1′-binaphthyl-2,2′-disulfonates. Chem. Commun. 48, 4986–4988 (2012).
Kurihara, T. et al. Synthesis of 1,1′-spirobiindane-7,7′-disulfonic acid and disulfonimide: application for catalytic asymmetric aminalization. Chem. Asian J. 13, 2378–2381 (2018).
Gillingham, D. & Fei, N. Catalytic X–H insertion reactions based on carbenoids. Chem. Soc. Rev. 42, 4918–4931 (2013).
Su, Y.-X., Huang, M.-Y., Zhu, S.-F. & Catalytic, N. –H. Insertion reactions with α-diazoacetates: an efficient method for enantioselective amino acid synthesis. ChemCatChem 10, e202300539 (2023).
Zhu, S.-F. & Zhou, Q.-L. Transition-metal-catalyzed enantioselective heteroatom-hydrogen bond insertion reactions. Acc. Chem. Res. 45, 1365–1377 (2012).
Ford, A. et al. Modern organic synthesis with α-diazocarbonyl compounds. Chem. Rev. 115, 9981–10080 (2015).
Doyle, M. P., McKervey, M. A. & Ye, T. Modern Catalytic Methods For Organic Synthesis With Diazo Compounds. (Wiley, 1998).
Arredondo, V., Hiew, S. C., Gutman, E. S., Premachandra, I. & Van Vranken, D. L. Enantioselective palladium-catalyzed carbene insertion into the N–H bonds of aromatic heterocycles. Angew. Chem. Int. Ed. 56, 4156–4159 (2017).
Hou, Z.-R. et al. Highly enantioselective insertion of carbenoids into N–H bonds catalyzed by copper(I) complexes of binol derivatives. Angew. Chem. Int. Ed. 49, 4763–4766 (2010).
Liu, B., Zhu, S.-F., Zhang, W., Chen, C. & Zhou, Q.-L. Highly enantioselective insertion of carbenoids into N–H bonds catalyzed by copper complexes of chiral spiro bisoxazolines. J. Am. Chem. Soc. 129, 5834–5835 (2007).
Pan, J.-B. et al. A spiro phosphamide catalyzed enantioselective proton transfer of ylides in a free carbene insertion in o N–H bonds. Angew. Chem. Int. Ed. 6, e202300691 (2023).
Xu, X.-F., Zavalij, P. Y. & Doyle, M. P. Synthesis of tetrahydropyridazines by a metal-carbene-directed enantioselective vinylogous N–H insertion/lewis acid-catalyzed diastereoselective mannich addition. Angew. Chem. Int. Ed. 51, 9829–9833 (2012).
Zhu, S.-F., Xu, B., Wang, G.-P. & Zhou, Q.-L. Well-defined binuclear chiral spiro copper catalysts for enantioselective N–H insertion. J. Am. Chem. Soc. 134, 436–442 (2012).
Zhu, Y. et al. Asymmetric N–H insertion of secondary and primary anilines under the catalysis of palladium and chiral guanidine derivatives. Angew. Chem. Int. Ed. 53, 1636–1640 (2014).
Lee, E. C. & Fu, G. C. Copper-catalyzed asymmetric N–H insertion reactions: couplings of diazo compounds with carbamates to generate α-amino acids. J. Am. Chem. Soc. 129, 12066–12067 (2007).
Guo, J.-X., Zhou, T., Xu, B., Zhu, S.-F. & Zhou, Q.-L. Enantioselective synthesis of α-alkenyl α-amino acids via N-H insertion reactions. Chem. Sci. 7, 1104–1108 (2016).
Li, Y. et al. Enantioselective synthesis of unnatural carbamate-protected α-alkyl amino esters via N–H bond insertion reactions. ACS Catal. 12, 13143–13148 (2022).
Xu, B., Zhu, S.-F., Xie, X.-L., Shen, J.-J. & Zhou, Q.-L. Asymmetric N–H insertion reaction cooperatively catalyzed by rhodium and chiral spiro phosphoric acids. Angew. Chem. Int. Ed. 50, 11483–11486 (2011).
Xu, B., Zhu, S.-F., Zuo, X.-D., Zhang, Z.-C. & Zhou, Q.-L. Enantioselective N–H insertion reaction of α-aryl α-diazoketones: an efficient route to chiral α-aminoketones. Angew. Chem. Int. Ed. 53, 3913–3916 (2014).
Li, M.-L., Yu, J.-H., Li, Y.-H., Zhu, S.-F. & Zhou, Q.-L. Highly enantioselective carbene insertion into N–H bonds of aliphatic amines. Science 366, 990–994 (2019).
Yang, W. et al. Enantioselective formal vinylogous N–H insertion of secondary aliphatic amines catalyzed by a high-spin cobalt(II) complex. J. Am. Chem. Soc. 143, 9648–9656 (2021).
Li, M.-L., Pan, J.-B. & Zhou, Q.-L. Enantioselective synthesis of amino acids from ammonia. Nat. Catal. 5, 571–577 (2022).
Ni, Y., Guo, X., Hu, W.-H., Liu, S.-Y. & Asymmetric, N. –H. Insertion reaction of α-diazoesters and carbamates co-catalyzed by dirhodium acetate, sufonic acid and chiral sulfonamide urea. Chin. J. Org. Chem. 34, 107–111 (2014).
Ren, Y.-Y., Zhu, S.-F. & Zhou, Q.-L. Chiral proton-transfer shuttle catalysts for carbene insertion reactions. Org. Biomol. Chem. 16, 3087–3094 (2018).
Ager, D. J., Prakash, I. & Schaad, D. R. 1,2-Amino alcohols and their heterocyclic derivatives as chiral auxiliaries in asymmetric synthesis. Chem. Rev. 96, 835–876 (1996).
Fache, F., Schulz, E., Tommasino, M. L. & Lemaire, M. Nitrogen-containing ligands for asymmetric homogeneous and heterogeneous catalysis. Chem. Rev. 100, 2159–2231 (2000).
Gupta, P. & Mahajan, N. Biocatalytic approaches towards the stereoselective synthesis of vicinal amino alcohols. N. J. Chem. 42, 12296–12327 (2018).
Li, S.-F., Du, H.-W., Davies, P. W. & Shu, W. Synthesis of unprotected α-tertiary amines and 1,2-amino alcohols from vinyl azides by light induced denitrogenative alkylarylation/dialkylation. CCS Chem. https://doi.org/10.31635/ccschem.023.202302999 (2023).
Shibasaki, M. & Kanai, M. Asymmetric synthesis of tertiary alcohols and α-tertiary amines via Cu-catalyzed C–C bond formation to ketones and ketimines. Chem. Rev. 108, 2853–2873 (2008).
Klein, S. I. et al. Identification and initial structure-activity relationships of a novel class of nonpeptide inhibitors of blood coagulation factor Xa. J. Med. Chem. 41, 437–450 (1998).
Skoda, E. M., Davis, G. C. & Wipf, P. Allylic amines as key building blocks in the synthesis of (E)-alkene peptide isosteres. Org. Process Res. Dev. 16, 26–34 (2012).
Connon, R., Roche, B., Rokade, B. V. & Guiry, P. J. Further developments and applications of oxazoline-containingligands in asymmetric catalysis. Chem. Rev. 121, 6373–6521 (2021).
Weinstabl, H. et al. Intracellular trapping of the selective phosphoglycerate dehydrogenase (PHGDH) inhibitor BI-4924 disrupts serine biosynthesis. J. Med. Chem. 62, 7976–7997 (2019).
Furniel, L. G., Echemendía, R. & Burtoloso, A. C. B. Cooperative copper-squaramide catalysis for the enantioselective N–H insertion reaction with sulfoxonium ylides. Chem. Sci. 12, 7453–7459 (2021).
Gu, X. et al. Catalytic asymmetric P–H insertion reactions. J. Am. Chem. Soc. 145, 20031–20040 (2023).
Wang, J.-B. Reaction mechanism and synthetic application of α-diazo carbonyl compounds. Chin. J. Org. Chem. 21, 980–985 (2001).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Lefebvre, C. et al. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 19, 17928–17936 (2017).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Lu, T. & Chen, Q. Independent gradient model based on Hirshfeld partition: a new method for visual study of interactions in chemical systems. J. Comput. Chem. 43, 539–555 (2022).
Acknowledgements
The authors thank the National Key R&D Program of China (2022YFA1504302, Q.-L.Z.), the National Natural Science Foundation of China (22188101, Q.-L.Z., 91956000, Q.-L.Z., 92256301, Q.-L.Z.), the Fundamental Research Funds for the Central Universities, and the Haihe Laboratory of Sustainable Chemical Transformations for financial support.
Author information
Authors and Affiliations
Contributions
Q.-L.Z. conceived the study; X.-G.Z. and Q.-L.Z. designed the experiments and analyzed the data; X.-G.Z., Z.-C.Y., J.-B.P. and X.-H.L. synthesized substrates and catalysts. X.-G.Z. performed the reactions and mechanistic study. X.-G.Z. and Q.-L.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, XG., Yang, ZC., Pan, JB. et al. Enantioselective synthesis of chiral amides by carbene insertion into amide N–H bond. Nat Commun 15, 4793 (2024). https://doi.org/10.1038/s41467-024-48266-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-48266-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.