Abstract
The maintenance of machinery requires its operational understanding and a toolbox for repair. The methods for the precision engineering of native proteins meet a similar requirement in biosystems. Its success hinges on the principles regulating chemical reactions with a protein. Here, we report a technology that delivers high-level control over reactivity, chemoselectivity, site-selectivity, modularity, dual-probe installation, and protein-selectivity. It utilizes cysteine-based chemoselective Linchpin-Directed site-selective Modification of lysine residue in a protein (LDMC-K). The efficiency of the end-user-friendly protocol is evident in quantitative conversions within an hour. A chemically orthogonal C-S bond-formation and bond-dissociation are essential among multiple regulatory attributes. The method offers protein selectivity by targeting a single lysine residue of a single protein in a complex biomolecular mixture. The protocol renders analytically pure single-site probe-engineered protein bioconjugate. Also, it provides access to homogeneous antibody conjugates (AFC and ADC). The LDMC-K-ADC exhibits highly selective anti-proliferative activity towards breast cancer cells.
Similar content being viewed by others
Introduction
The functional well-being of a living organism thrives on the seamless coordination of diverse biomolecules. Hence, molecular-level intervention becomes vital for rescue whenever a component malfunctions. For instance, the ongoing development of inhibitors1,2,3,4 and degraders5,6,7 provides access to an extended landscape of undruggable proteome. Assisted by chemoproteomics, the leads often emerge from the top-down screening of a ligand library against the proteome. Such activity-based protein profiling also provides vital insight into the off-target modifications responsible for multiple failures. Hence, controlling the precision while targeting a site in a complex protein-derived ecosystem needs urgent attention. Initially, it requires a profound knowledge of operating principles in the bioconjugation of model peptides8,9 or isolated proteins. Subsequently, we can translate the concepts while understanding the impact from additional layers of complexity. Alongside, such platforms are desired to probe biological systems10 and meet the requirements of protein-based healthcare tools and therapeutics11,12. For example, the challenge of heterogeneity and ambiguous conjugation sites has been the most significant roadblock to developing antibody-drug conjugates (ADCs) for directed cancer chemotherapeutics. A chemical platform that empowers the regulation of homogeneity, drug-to-antibody ratio, and site-of-drug-conjugation is of immense value in this perspective.
The biochemical single-site engineering of chemically orthogonal handles such as carbonyls creates an excellent opportunity for their precise conjugation13,14. However, controlling the selectivity in the chemical modification of a genetic manipulation-free protein faces a spectrum of challenges. The magnitude depends on whether the protein is isolated or accompanied by other biomolecules. For example, we and others demonstrated that the labeling of reactivity hotspots provides efficient tools for chemoselective and site-selective modification of isolated proteins15,16,17,18,19,20,21,22,23,24,25,26,27. However, such methods are not equipped to deal with the drastic increase in competing functional groups presented by a complex mixture of proteins. Besides redefining the chemoselectivity and site-selectivity, it also presents the question of protein-selectivity for achieving absolute precision. In this perspective, the non-covalent ligands bound to an electrophilic warhead have been able to offer an interesting case28,29. Besides, residue-specific labeling emerged as a robust tool for targeting N-Cys30,31,32,33 and N-Gly34. The impact could be multifold if a chemical technology can exhibit a combination of chemoselectivity, site-selectivity, modularity, and protein-selectivity to constitute a platform that can address the precision engineering of proteins in a complex mixture.
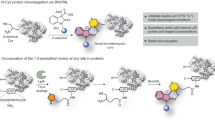
We recently demonstrated that a chemical method could deliver single-site protein modification irrespective of the residue’s reactivity order. Here, a lysine-derived linchpin-directed modification (LDM®) results in the labeling of a His (LDMK-H, LDMK-X), Lys (LDMK-K), and Asp (LDMK-X)35,36,37. The high-frequency Lys residues regulate the number of linchpins and direct the modification of the other residue. It is noteworthy that Lys-derived linchpin would offer excellent opportunities with an isolated protein (Fig. 1a). As we increase the number of proteins in the mixture, it becomes exceedingly challenging to identify the LDM reagent suitable for precise modification of a single protein. In this perspective, we hypothesized that a method could be valuable if it derives linchpin from a low-frequency residue to modify a high-frequency site (steps 1–4, Fig. 1b). Such an expedition would require the development of a chemoselective electrophile that could enable a rapid reaction with the identified residue at low micromolar concentration. We argued that cysteine could be an ideal residue to anchor the linchpins with FC if we can solve a combination of two non-trivial challenges. At first, the linchpin must be released under physiological conditions after the completion of the bioconjugation. Secondly, the linchpin-released functional group must render a chemically unique handle for late-stage modifications. We realized that the popular cysteine-selective electrophiles would not serve the purpose38. It is not surprising as they evolved to deliver enhanced stability of the thiol-adduct. On the contrary, there are only a few electrophilic reagents where reversibility was pursued in addition to chemoselectivity39. Hence, it became necessary to develop one that can meet the requirements of our hypothesis.
a Requirement: low-frequency residue-derived proximity regulator to create a unique targetable landscape and opportunity for simultaneous regulation of reactivity, chemoselectivity, site-selectivity, modularity, and protein-selectivity. b Hypothesis: Cys-linchpin-directed modification of Lys residue (LDMC-K). Step 1, linchpin formation: rapid, intermolecular, chemoselective reaction of FC with Cys; step 2, cyclization: intramolecular, irreversible, site-selective reaction of FK with Lys; step 3, FC to FX: sequential C-S and C-C bond dissociation; step 4, late-stage modification.
Here, we report a Cys-derived linchpin-directed single-site modification of lysine (LDMC-K) to meet the technological demands. The success of site-selective LDMC-K required an appropriate multifunctional reagent (FC-spacer-FK, Fig. 1b). At first, we developed nitroolefin (FC) as an electrophile capable of rendering a rapid chemoselective reaction with cysteine (>99% conversion). Subsequently, we identified an acylating group (FK) for a relatively slow, irreversible, and chemoselective reaction with lysine. However, the success of LDMC-K required us to develop another methodology for reverting the nitroolefin thio-Michael adduct. Also, such a C-S bond dissociation should render a functional group amenable to orthogonal late-stage modification. In this perspective, we developed a one-pot sequential protocol for retro-Michael/retro-Henry reaction that operates under mild conditions with remarkable efficiency over two steps. Importantly, it offers an aromatic aldehyde to enable the capture, enrichment, purification, and late-stage installation of probes. At first, we demonstrate that LDMC-K offers chemoselectivity and site-selectivity to render single-site modification of a protein. The simulation results gave insight into how the design, length, and adaptable rigidity of the spacer regulate the bioconjugation site. Subsequently, we demonstrate that the method translates efficiently to the protein mixture or cell lysate and addresses the question of protein selectivity. Besides, we present a user-friendly protocol for isolating analytically pure protein tagged with the probe of interest at a single site. In this modular platform, the reagent design enables modification of a lysine beyond protein-defined reactivity order. We demonstrate that LDMC-K could deliver the single-site installation of various biophysical probes. The chemical and functional space expansion for selective targeting has also opened gateways for dual-probe bioconjugates. We established it through LDMC-K-enabled installation of a fluorophore, affinity tag, or NMR probe coupled with chemoselective single-site installation of the second tag at the linchpin site to ensure proximal precision dual-labeling of the protein. Further, we demonstrate that the methodology enables the synthesis of homogeneous ADC capable of highly selective anti‐proliferative activity towards HER‐2 expressing SKBR‐3 breast cancer cells.
Results
Design and synthesis of LDMC-K reagent
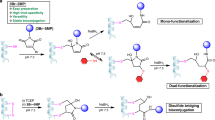
The design of the LDMC-K reagent involved three critical building blocks: FC, FK, and the spacer. We initiated by searching for an electrophile that can result in a rapid chemoselective reaction with cysteine (FC). Besides, it must offer the attribute of on-demand C-S bond dissociation to render an orthogonally maneuverable functional group. In this perspective, we selected a set of soft electrophiles (1a-1l) bearing polarized double bonds and diffused electron densities for screening with thiol-based reagent 2 (Fig. 2a and Supplementary Figs. 15–26, also see Supplementary Table 1). A few electrophiles (1a–c, 1f–i) were non-reactive under the reaction conditions. Interestingly, vinyl sulfone 1e resulted in 60% conversion (3e). While acrylonitrile (1j) has shown better reactivity (3j, 83% conversion), another vinyl sulfone derivative, 1d led to quantitative yields. Keeping the synthetic maneuverability constraints in mind, we furthered our screening. The maleimide (1k) also worked well to result in high conversions to the thio-Michael adduct. However, it is challenging to trigger retro-Michael addition or nucleophilic substitution in this case without interference from side reactions, particularly hydrolysis38. To our delight, nitroolefin (1l) resulted in quantitative conversions (Fig. 2a). Further, we mixed twenty amino acids with unprotected side-chain and found it to exhibit exclusive Cys-selectivity (Fig. 2b and Supplementary Figs. 27–28, also see Supplementary Table 2). Hence, we selected the thio-Michael adduct with nitroolefin (3l) to test it for the C-S bond dissociation reaction under mild aqueous conditions (Fig. 2c).
The C-S bond dissociation proceeded with moderate efficiency through retro-Michael pathway over time or in the presence of DABCO, TMG, and urea (Fig. 2c, also see Supplementary Table 3 and Supplementary Figs. 29–37). The regenerated nitroolefins can be used for late-stage installation of probes with nucleophilic thiol handles. However, such a system is more suited for applications involving reversibly installed tags but not for biologics such as ADCs. Hence, it was important for us to develop a method for the transformation of FC to another chemically orthogonal handle (FX) that can result in stable probe conjugates. The subsequent transformation of FC, such as retro-Henry pathway involving C-C bond dissociation, remained elusive in the previous cases. However, DBU, DIPEA, and TEA enabled the retro-Henry reaction to form aldehyde in moderate conversions (14–22% in 24 h, Supplementary Table 3). After a thorough screening, we were delighted to establish a one-pot sequence of reactions involving both C-S and C-C bond dissociation under mild conditions (55% within 12 h, bicarbonate buffer, pH 8.5, Supplementary Table 3). The chemoselectivity with thiol bioconjugation and chemically orthogonal dissociation rendering FX made nitroolefin the preferred group to be examined as the FC component.
The aryl group in these test studies offers the synthetic maneuverability for subsequent installation of FK and spacer. This feature is vital as the screening of the second electrophilic group, FK, needs to be performed in conjugation with the FC and spacer. This leap of faith was necessary to validate the chemoselectivity and site-selectivity along with the relative reactivity order of FC versus FK. In this perspective, we identified acylating groups to serve the latter’s purpose. Next, we synthesized LDMC-K reagents (9a–9d) with a range of leaving groups to establish the right combination of functional groups (Fig. 3a; for multistep synthesis of reagents, see Supplementary Figs. 1–5). We selected β-lactoglobulin A (BLGA, 8a, PDB ID: 3BLG, Supplementary Fig. 39) with one free Cys and fifteen Lys residues as the model protein to validate the hypothesis (Fig. 3a). The NHS-ester derivative (9a) was highly reactive and resulted in heterogeneous labeling of proteins (Supplementary Figs. 38 and 40). Upon replacing the leaving group to bring down the reactivity, the tribromophenoxide derivative (9b) resulted in mono-labeled BLGA (10b, 9% conv., Supplementary Fig. 41). Interestingly, substituting one bromine with a morpholine amide (9c) led to improved conversions (10c, 30%, Supplementary Fig. 42) while retaining the homogeneity. Finally, we were delighted to note quantitative yields (10d, >99%, Supplementary Fig. 43) with tetrafluorophenoxide (9d) as the leaving group. With desired FC and FK in hand, we synthesized a set of LDMC-K reagents for further evaluation (9e–9h, Fig. 3b and Supplementary Figs. 6–9).
Investigation with peptides
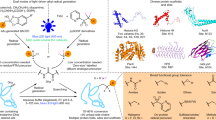
At this point, we designed a set of experiments with peptides to validate the reactivity and selectivity attributes of LDMC-K (Fig. 4 and Supplementary Table 4). The reaction time (1 h) was selected to keep conversions in a range where we can visualize the differences in efficiency. At first, we examined the reactivity of FC containing control reagent (1 l) with Cys in a peptide (BzLCGLLG, 12a) devoid of Lys. Here, a rapid C-S bond formation led to the formation of the Michael adduct (13a, 77% conv., Fig. 4a and Supplementary Fig. 44). Next, we designed and synthesized a peptide BzLCGLLK (12b) with Cys (i) and Lys (i + 4) in proximity. We treated this peptide with the LDMC-K reagent (9d). Gratifyingly, a clear sequence of C-S bond formation followed by cyclization resulted in excellent conversions (14b, 75% conv., Fig. 4b and Supplementary Fig. 48) within an hour. The MS and MS-MS confirmed the selective modification of proximal Lys (i + 4). To re-validate, we pre-installed a protecting group on Cys [BzLC(tBu)GLLK (12c)] and treated it with the same LDMC-K reagent (9d). With all the other reaction parameters retained, no conversions were observed in this case (14c, 0% conv., Fig. 4c and Supplementary Fig. 49). Finally, we treated the peptide BzLCGLLKGLLK (12d) with 9d to test for site-selectivity (14d, 33% conv., Fig. 4d and Supplementary Fig. 50). The MS and MS-MS confirmed the selective modification of proximal Lys (i + 4) while the other Lys (i + 8) remained unaffected. Next, we investigated the potential competition from selected residues from the amino acid pool examined earlier (Fig. 2b). For this purpose, we synthesized the single-site mutants for LCGLLK (12b), where K is replaced with Tyr (12e), Ser (12f), and His (12g). The linchpin formation could not render covalent bond formation with both Tyr and Ser (Figs. 4e and 4f). On the other hand, the His forms acyl imidazole that undergoes hydrolysis, rendering unmodified residue (Fig. 4g). These experiments confirmed that such competing residues would not leave traces after the C-S bond dissociation. Next, we established the efficiency of the LDMC-K workflow with a similar peptide equipped with two additional arginine residues for enhanced solubility (Fig. 4h). The rapid LDMC-K results in the cyclic peptide (14i) within 15 minutes. The subsequent C-S bond dissociation, thiol interception with maleimide, and retro-Henry reaction allow quantitative aldehyde generation (15b, >99%). Finally, the oxime formation proceeds in excellent conversions (>99%) to render the dual-labeled peptide 15c (Fig. 4h).
a Control reagent 1l with FC and Cys-containing peptide 12a results in efficient C-S bond formation. b LDMC-K reagent 9d mixed with peptide 12b having Cys and Lys at i and i + 4 positions result in C-S bond followed by C-N bond formation rendering the cyclic peptide 14b within 1 h. c LDMC-K reagent 9d with peptide 12c having thiol protected Cys and Lys at i and i + 4 positions result in no conversion within 1 h. d LDMC-K reagent 9d with peptide 12d having Cys at i and two Lys residues at i + 4/i + 8 positions result in C-S bond formation followed by site-selective C-N bond formation at i + 4 position. Control experiments to examine competition with e Tyr, f Ser, and g His. h Establishing the complete LDMC-K workflow. The selected MS for 14i, 15a, 15b, and 15c are given in the inset. Also see Supplementary Figs. 102–106 for detailed data, full XIC, and MS spectra.
Precision engineering of native proteins–simultaneous regulation of reactivity, chemoselectivity, site-selectivity, and modularity
After validating the hypothesis and a clear understanding of reactivity and selectivity parameters, we were all set to establish the capability of LDMC-K with a native protein. At first, we re-validated the chemoselectivity of the nitroolefin-based control reagent (1l) with BLGA (8a). The proteolytic digestion of 1l-labeled BLGA with α-chymotrypsin, peptide mapping (labeled VCQCL; residues 118–122; m/z 844.7 [M + H]+), and MS-MS confirmed the chemoselective labeling of C121 (Supplementary Fig. 52). In a control experiment, the proteins devoid of Cys did not result in any irreversible bioconjugation (Supplementary Table 5 and Supplementary Fig. 51). Now, we had to establish the efficiency of the C-S bond dissociation protocol (Fig. 5a). For this purpose, we selected the optimized example (Fig. 3) and synthesized mono-labeled BLGA (10d) with LDMC-K reagent (9d) in >99% conversion (step 1, Fig. 5a). In a control experiment, the BLGA (8a) pre-treated with maleimide does not result in bioconjugation with 9d (Supplementary Fig. 53). The non-reactivity of 9d with proteins devoid of free Cys revalidates the case (Supplementary Table 6 and Supplementary Figs. 58–64). Also, the reduced nitroolefin derivative of LDMC-K reagent 9d (11a) leads to heterogeneous modification of BLGA, potentially through Cys-based thioester (Supplementary Fig. 54). Further, it was interesting to note that our findings with the small molecule model system (Fig. 2c) translated for the C-S bond dissociation in BLGA bioconjugate (10d). We trapped the retro-Michael reaction-led nitroolefin (path I, Fig. 5a and Supplementary Fig. 55) immediately by a thiol to avoid the reversible reaction. We were delighted to note the efficient translation of one-pot C-S and C-C bond dissociation to proteins for transforming FC (nitroolefin) to FX (aldehyde) under operationally simple conditions (path II, Fig. 5a and Supplementary Fig. 56). To enable unambiguous protein sequencing, we further treated the BLGA bioconjugate (17) with hydroxylamine (18). The subsequent mono-labeled product (20d) was digested with α-chymotrypsin enabling the identification of labeled KKY in peptide mapping (residues 100–102, m/z 643.3 [M + H]+). The MS-MS confirmed the site of modification as K101 (Supplementary Fig. 66). Besides multiple other Lys residues, the other proximal Lys (K100) does not undergo any modification highlighting the method’s capability in distinguishing closely placed targets.
a Workflow for C-S bond formation, cyclization, C-S/C-C bond dissociation, and FC to FX transformation. b The chemoselective, site-selective, and modular single-site labeling of a native protein (β-lactoglobulin, BLGA, 8a). c The plot of % conversion versus time highlights the progress of Michael addition and cyclization with BLGA in the presence of LDMC-K reagent 9d. Data are presented as mean values (±SD), n = 3 independent experiments. d Adaptable rigidity of LDMC-K reagent: Probability distribution for the reagent (9d) and end-to-end distance between electrophilic C1 and C11 atoms in water with (red) or without (black) BLGA. The most probable conformations corresponding to peaks are shown. e Probability distribution of the distance between electrophilic C11 atom of the LDMC-K reagent 9d and nearby lysine residues (NZ atom) in BLGA. f Interaction of C11 in 9d with the nearby lysine residues. Inset highlights the interaction of Lys101 with the C11 (9d).
After establishing the concept unambiguously, we were equipped with all the tools of LDMC-K technology to test it further. We selected the LDMC-K reagents (9e and 9f) with ortho- and meta-disubstituted aromatic ring-based spacer in place of para-disubstituted aryl ring in 9d. Interestingly, eight-fold less bioconjugation reagent 9e was enough for six-fold improvement in reaction time to render mono-labeled BLGA within 1 h (Fig. 5b and Supplementary Fig. 67). Also, the LDMC-K reagent (9f) turned out to be remarkable and delivered mono-labeled BLGA (8a) within an hour (Fig. 5b and Supplementary Fig. 68). We released the linchpin for these two bioconjugates by C-S/C-C bond dissociation and the subsequently formed aldehyde was captured with benzyloxyamine (19). These adducts were then subjected to protease degradation followed by peptide mapping. Both 20e and 20f resulted in labeled KKY (residues 100–102, m/z 733.3 [M + H]+) that upon subsequent MS-MS confirmed the site-of-modification as K101 (Supplementary Figs. 67 and 68). To gain further insights into the sequence of steps, we performed a time-dependent investigation of LDMC-K-enabled bioconjugation (Fig. 5c and Supplementary Fig. 65). Here, mixing BLGA and LDMC-K reagent 9d, quenching it with dilution after ten seconds, and subjecting it immediately to MS confirmed a rapid C-S bond formation with FC. At this point, no acylation is observed. Within the next twenty seconds, the increase of linchpin formation by thio-Michael addition (Cys-FC) is accompanied by cyclization (Lys-FK), while the latter becomes more prominent after 2 minutes. The Michael adduct disappears gradually and renders >99% cyclic adduct within 6 h.
With these detailed studies in the background, the next step was to establish modular capabilities. The initial noteworthy result in this perspective came with >99% conversion to bioconjugate 10g within 3 h when we treated BLGA with the re-designed LDMC-K reagent 9g (Fig. 5b). Upon subsequent steps involving C-S/C-C bond dissociation followed by oxime formation, we subjected the mono-labeled product 20g to protein sequencing. The proteolytic digestion and peptide mapping led to the identification of labeled KGL (residues 8–10, m/z 550.4 [M + H]+). The MS-MS confirmed the labeling of site K8 and the modularity of LDMC-K technology (Supplementary Fig. 69). Interestingly, the closely placed K91 and K141 do not get labeled at all. We further challenged the method to label another unique Lys residue in the vicinity of Cys121. Gratifyingly, the LDMC-K reagent 9h rendered the chemoselective and site-selective modification of K135 (EKF, residues 134–136, m/z 806.4 [M + H]+). Again, the neighboring Lys residues (K138 and K141, Supplementary Fig. 70) do not compromise the bioconjugation selectivity. Overall, these results established that LDMC-K can simultaneously regulate reactivity, chemoselectivity, site-selectivity, and modularity with excellent control.
Molecular dynamics simulation: impact of linchpin formation on the adaptable conformational rigidity of LDMC-K reagent and downstream site-selection
We anticipated that the effectiveness and selectivity of the LDMC-K reagents would be primarily dependent on their geometry and conformation. It is tempting to speculate that these parameters would regulate the effective distance between FC and FK. Hence, the BLGA modification must happen if the distance between the linchpin (Cys121) and the target site (Lys) matches the spacer’s effective length. However, the dynamics of the protein and the reagent could make the linear correlation unreliable. Hence, we resorted to MD simulation to provide microscopic insights into the structure and dynamics of this complex molecular system and investigate our assumptions’ validity.
At first, we analyzed the conformational space of the LDMC-K reagent (capped) in an aqueous medium in the absence and presence of the protein. Figure 5d shows the distance distribution between the atoms C1 and C11, which forms covalent bonds with the S atom of Cys121 and side-chain N atom of target Lys, respectively. Hence, it would be an appropriate parameter to capture the conformational space explored by the LDMC-K reagent and check whether it is consistent with the linchpin-target distance in the protein. It shows the comparison of the distance distribution in both systems. In absence of protein (black line), there is a broad distribution with two predominant peaks around 0.85 nm and 1.0 nm. The representative structures of these conformations in water are highlighted (Fig. 5d). There is a minor peak above 1.0 nm, which is not analyzed in detail. This data clearly indicates that the LDMC-K reagent can’t be treated as a rigid entity. Instead, it exists in multiple possible conformations in an aqueous medium that is characterized by a significant difference in its effective length. Interestingly, this distribution landscape changes drastically in the presence of the protein (BLGA), and there is a major peak at around 0.8 nm (red line). Although there are signatures of other conformations, their population is substantially low. The shift in the conformational ensemble state is induced by the local environment and interactions with the protein. Consequently, a subset of this population is stabilized preferentially. This attribute of adaptable rigidity of otherwise flexible LDMC-K reagent enables it to adjust as per the surface landscape of the protein. These detailed investigations also confirm that the design principle of the bioconjugation reagent merely based on a static length of the extended configuration would not be successful in general.
With these insights, we turned our attention to the LDMC-K reagent in the presence of protein. Being anchored to Cys121, this large and flexible molecule has considerable degrees of freedom to access and interact with different parts of the protein. But the key question is whether it can come into close contact with any of the target Lys residues, particularly the Lys101 residue, which gets selectively modified by LDMC-K reagent 9d. Further, we investigated the distance distribution between the S atom of the Cys121 and N atom of various Lys residues in the neighborhood (Supplementary Fig. 91). The peaks for both Lys101 and Lys135 appear in proximity around 1 nm, making them tough competitors to be modified selectively by the linchpin.
In order to investigate the propensity of the linchpin to approach various Lys residues in a selective manner, we looked into the probability distribution of the distance between the C11 atom and the N atoms of multiple nearby Lys residues (Lys100, Lys101, and Lys135, Fig. 5e). The comparison of these distance distributions clearly establishes that the C11 atom has the highest propensity to approach the Lys101 residue compared to others. A representative structure showing the proximal interaction is shown in Fig. 5f. Results obtained from all three independent trajectories are consistent with this trend (Supplementary Fig. 92). Thus, our simulation results validate the experimental findings and provide a mechanistic basis of action of the LDMC-K reagent. It also highlights how the conformational flexibility of the reagent coupled with protein-induced rigidity could enable the site selection in protein bioconjugation.
Late-stage modification and dual-probe installation
At this point, we decided to validate the potential of re-engineered linchpin (FX) for the installation of probes. In this perspective, we treated BLGA with the LDMC-K reagent (9d, Fig. 6a). After bioconjugation rendered cyclic protein (10d, >99% conv.), we subjected it to one-pot C-S/C-C bond dissociation rendering FX (17). Finally, we distributed the precursor to three reaction vials and subjected them to the parallel installation of probes through oxime formation. Here, the treatment of hydroxylamine derivative (21–23) of the probes delivered installation of 19F NMR tag (24, >99% conv. over two steps), biotin-based affinity probe (25, 86% overall conv.), and fluorophore (26, 91% overall conv.). The remarkable overall efficiency of the protocol is noteworthy. The independent sequencing of the products by MS, proteolytic digestion, peptide mapping (labeled KKY, residues 100–102; m/z 941.9, m/z 927.9, and m/z 875.3 [M + H]+), and MS-MS confirms the site-of-conjugation (K101, Supplementary Figs. 71–73). Next, we selected single-site engineered BLGA bioconjugates (24–26) for dual-probe installation. In a user-friendly chemoselective protocol, the CPM-maleimide derivative rendered dual-probe conjugates (28–30, 71–78%, Supplementary Figs. 75–77) and established its utility for applications in this perspective.
Challenges of serum albumins
At this stage, we decided to display the potential of LDMC-K with serum albumins. These proteins present additional complexity levels due to the display of a large number of solvent-accessible lysine residues and heterogeneous post-translational modifications (Supplementary Figs. 78 and 81). Besides, the solvent accessibility of Cys is much lower in comparison to BLGA. Interestingly, LDMC-K reagent 9d resulted in single-site modification of bovine serum albumin (BSA 8j, Supplementary Fig. 79). The proteolytic degradation, peptide mapping (labeled KVPQVSTPTLVEVSR, residues 413–427, m/z 645.69 [M + 3H]3+), and MS-MS confirmed the site-of-modification as K413 (Supplementary Fig. 79). Further, the single-site labeling of BSA at K431 with LDMC-K reagent 9g confirmed the modularity of the method (labeled SLGKVGTR, residues 428–435, m/z 570.82 [M + 2H]2+, Fig. S80). The level of precision becomes even more noteworthy in the backdrop of fifty-nine lysine residues in BSA, where more than twenty are in the proximity of cysteine. With these results in hand, we examined the potential of LDMC-K with human serum albumin (HSA, 8k). It contains fifty-seven lysine residues, with more than half of them in the proximity of cysteine. Gratifyingly, the LDMC-K reagent 9d delivered single-site modification of HSA (Supplementary Fig. 82). Further, sequencing confirmed the labeled peptide (LKCASLQK, residues 198–205, m/z 621.83 [M + 2H]2+) and the site-of-modification (K199, Supplementary Fig. 82). The observation of labeled LKCASLQK (residues 198–205) with LDMC-K reagents 9e (m/z 414.89 [M + 3H]3+) and 9f (m/z 621.83 [M + 2H]2+) and subsequent MS-MS re-validated the results (Supplementary Figs. 83–84). The esterase-like activity of HSA remains unperturbed after bioconjugation (Supplementary Fig. 109).
Protein-selectivity empowered by residue-pair selectivity coupled with a purification protocol
We selected a mixture of proteins to establish whether the method can sustain the biomolecular crowding and deliver simultaneous regulation of protein selectivity along with chemoselectivity and site-selectivity (Fig. 7a). At first, a structurally and functionally diverse mixture of myoglobin, lysozyme C, cytochrome C, α-lactalbumin, RNase A, ubiquitin, insulin, and HSA was treated with LDMC-K reagent (9d). The MS data confirmed the exclusive modification of HSA (8k, Supplementary Fig. 85). Next, we coupled the method with a purification protocol. In the process, we used acyl hydrazide functionalized beads to capture and enrich the HSA bioconjugate. After recovering the unreacted proteins, the immobilized HSA bioconjugate on-resin was released through transoximization using hydroxylamine derivative of coumarin (33). While the SDS-PAGE (see Supplementary Fig. 86) validated the purification protocol, the peptide mapping, and MS-MS (see Supplementary Fig. 86) of the isolated HSA bioconjugate confirmed the conjugation site (K199). It is noteworthy that the chemoselectivity and site-selectivity remained unaffected by the crowding of other proteins.
Single-site, single-protein labeling of human serum albumin in a mixture of proteins (MoP), and b cell lysate. Hydrazone formation captures and enriches the bioconjugate by ordered on-resin immobilization allowing recovery of unlabeled proteins. Next, the transoximization and centrifugal spin concentration render analytically pure single-site tagged protein bioconjugate.
Modification of a protein in cell lysate
Later, we challenged the performance of the LDMC-K method in a cell lysate derived from E. coli BL21 cells (Fig. 7b). The cell lysate was spiked with HSA (8k) and treated with LDMC-K reagent 9d. We were delighted to note protein-specific modification of HSA. We utilized the purification protocol involving hydrazone-oxime chemistry to enrich the HSA bioconjugate from the cell lysate and installed a coumarin tag in the process. After confirming the purity by SDS-PAGE (Supplementary Fig. 87), the proteolytic digestion, peptide mapping, and MS-MS confirmed the conservation of chemoselectivity and site-selectivity (K199, Supplementary Fig. 87) in combination with protein selectivity. It further revalidates that LDMC-K can negate the biomolecular crowding effect making it a suitable method for diverse applications.
Antibody-drug conjugates
The emergence of platforms for precision engineering of proteins has promised to meet protein-based therapeutics’ requirements. One of the biggest beneficiaries in this perspective has been the ADCs. These constructs provide a state-of-the-art platform for directed cancer chemotherapeutics. However, many efforts failed at the pre-clinical stages due to the lack of methods to access homogeneous ADCs. In this regard, the LDM platform offers promise. The ADC involves a monoclonal antibody conjugated to a drug molecule. While the antibody delivers the conjugate to tumor cells with overexpressed antigens, the drug is responsible for the low-dose cellular killing. The homogeneity of conjugation sites could ensure better control over the ADC attributes. Hence, we applied the LDMC-K method to synthesize antibody conjugates (AFC and ADC, Fig. 8a). At the onset, we treated the mAb with TCEP to reduce the disulfide and introduce free cysteine as per the established protocols40. Subsequent treatment with the LDMC-K reagent 9d led to the formation of cyclic adduct 36 that upon subsequent C-S/C-C dissociation yields the aldehyde-antibody conjugate (AAC, 37). Further, we sequenced the bioconjugate to establish the conjugation sites (K183, light chain, and K341, heavy chain, Supplementary Fig. 89) and aldehyde-antibody ratio (AAR 1.0, Supplementary Fig. 88). Next, the AAC (37) was treated with the hydroxylamine derivatives of coumarin (23) and emtansine (35a) to render AFC (38) and ADC (39), respectively. The SDS-PAGE revalidates the conjugation with both heavy and light chains (Supplementary Fig. 90). Next, the antiproliferative assay was performed to determine the efficacy of the ADC in the HER-2 overexpressing cell line SKBR-3. The results showed significant inhibition of cell growth at 0.25 nM (38%) and 0.5 nM doses (78%, Fig. 8b). Under the same conditions, Kadcyla (40) delivered 7 and 16% inhibition, respectively. Importantly, the LDMC-K ADC (0.5 nM) did not affect the viability of the HER-2 negative MDA-MB-231 cell line, while emtansine (DM1, 35) decreased its growth by 22% (Fig. 8c). Overall, the results establish the high efficacy and specificity of LDMC-K-ADC towards antiproliferation of HER-2 positive breast cancer cells.
a LDMC-K reagent 9d renders homogeneous trastuzumab conjugates with specific modifications of K183 and K341. Subsequently, AFC (38) and ADC (39) were prepared by late-stage installation of hydroxylamine derivative of fluorophore (coumarin, 23) and drug (emtansine, DM1, 35a). b Inhibition of cell proliferation by LDMC-K-ADC (39) as compared to trastuzumab (34), DM1 (35), and Kadcyla (40) in SKBR-3 (HER-2 positive) cancer cell line. The percentage inhibition was calculated using untreated cells as control. c Inhibition of cell proliferation by DM1 (35), ADC (39), and Kadcyla (40) at 0.5 nM concentration in HER-2 positive SKBR-3 as compared to HER-2 negative MDA-MB-231 cells. Data are presented as mean values (±SD), n = 3 biologically independent experiments.
Discussion
The precision engineering of proteins creates a hinge to connect chemistry, biology, and medicine. Hence, it is not surprising that it has drawn attention from diverse segments of science, specifically the rapidly growing biologics for precision therapeutics. However, the inherent challenges kept the principles for regulating precision elusive. As a result, the technological demand for specifically engineered proteins remained unmet, thrusting the use of heterogeneous bioconjugates. The efforts of our group and a few others helped gain insight into principles for rendering chemoselectivity and site-selectivity. However, simultaneous regulation of multiple selectivity attributes beyond protein-defined reactivity order remains challenging.
This manuscript presents an LDM that establishes the principles for creating a cysteine-based linchpin and directs single-site modification of a proximal lysine residue (LDMC-K). The method renders simultaneous regulation of reactivity, chemoselectivity, site-selectivity, modularity, and protein selectivity within the spectrum of optimized reaction parameters. The selection of low-frequency cysteine was critical to directly modifying a single lysine of a single protein within a complex mixture of proteins. However, finding an appropriate handle to serve multiple attributes around C-S bond formation and chemically orthogonal bond dissociation under mild physiological conditions was non-trivial. Hence, the major credit for the success of LDMC-K goes to the unique FC or nitroolefin chemistry that enabled the cysteine-linchpin by rendering an array of synchronized bond formation and dissociation processes. Besides, its successful transformation to aldehyde under mild conditions enables the integration of orthogonal late-stage transformations. Importantly, it drives a highly efficient purification protocol to deliver ordered protein immobilization and analytically pure single-site probe-tagged bioconjugates. Notably, the seamless operation extends to a complex biomolecular mixture to deliver protein selectivity coupled with single-site precision. Another critical attribute of the LDMC-K technology emerges from the adaptable rigidity of spacer that facilitates the placement of lysine selective group and irreversible bioconjugation. Notably, the overall protocol is user-friendly for non-experts. The platform proved highly efficient for synthesizing homogeneous dual-probe protein conjugates, antibody-fluorophore conjugate (AFC), and ADC. The LDMC-K-ADC exhibited excellent antiproliferative activity toward HER-2-positive breast cancer cells without negatively impacting the HER-2-negative cells. Further, the LDM technology offers principles that promise to bridge the gap between precision engineering of isolated proteins versus when they reside in a complex ecosystem.
Methods
General procedure of peptide labeling
The peptide stock solution was freshly prepared using acetonitrile with two drops of HPLC-grade methanol to enhance the solubility.
Michael addition to the peptide
Peptide containing free cysteine 12a (68 µg, 100 nmol) in acetonitrile (20 µl) from a freshly prepared stock solution was taken into a 1.5 ml HPLC vial containing phosphate buffer (140 µl, 50 mM, pH 7.0). To this solution, reagent 1 l (28 µg, 50 nmol) in acetonitrile (40 µl) was added and incubated at 25 °C. The overall concentration of the reaction was 500 µM. The progress of the Michael addition was monitored by LC-ESI-MS for up to 1 h.
Acylation with the peptide
Peptide containing protected cysteine and free lysine 12c (80 µg, 100 nmol) in acetonitrile (20 µl) from a freshly prepared stock solution was taken into a 1.5 ml HPLC vial containing phosphate buffer (140 µl, 50 mM, pH 7.0). To this solution, reagent 9d (40 µg, 100 nmol) in acetonitrile (40 µl) was added and incubated at 25 °C. The overall concentration of the reaction was 500 µM. The progress of the reaction was monitored by LC-ESI-MS for up to 1 h.
Peptide macrocyclization
Peptide containing free cysteine and lysine 12b (75 µg, 100 nmol) in acetonitrile (20 µl) from a freshly prepared stock solution was taken into a 1.5 ml HPLC vial containing phosphate buffer (140 µl, 50 mM, pH 7.0). To this solution, reagent 9d (40 µg, 100 nmol) in acetonitrile (40 µl) was added and incubated at 25 °C. The overall concentration of the reaction was 500 µM. The progress of the Michael addition followed by macrocyclization was monitored by LC-ESI-MS up to 1 h. This protocol was also used for peptides 12e–12g for examining the competition from Tyr, Ser, and His. The peptide 12h requires a few changes in the method (ESI) due to improved solubility in water.
Site-selective peptide modification
Peptide containing one free cysteine and two lysine residues 12d (116 µg, 100 nmol) in acetonitrile (20 µl) from a freshly prepared stock solution was taken into a 1.5 ml HPLC vial containing phosphate buffer (140 µl, 50 mM, pH 7.0). To this solution, reagent 9d (40 µg, 100 nmol) in ACN (40 µl) was added and incubated at 25 °C. The overall concentration of the reaction was 500 µM. The progress of the reaction was monitored by LC-ESI-MS up to 1 h. The site-of-modification was confirmed by MS-MS.
General procedure for site-selective modification of native proteins
Protein 8a (2 nmol) in phosphate buffer (70–80 µl, 50 mM, pH 7.0) was taken in a 1.5 ml microcentrifuge tube. To this solution, LDMC-K reagent (9d–9h, 2.4–20 nmol, each separately) in acetonitrile (20–30 µl) from a freshly prepared stock solution was added and incubated at 25 °C. The overall concentration of protein and LDMC-K reagent was 20 µM and 24–200 µM, respectively. After 1–24 h, the reaction mixture was diluted with water (500 µl), followed by centrifugation for 1 min at 9600×g to precipitate the unreacted LDMC-K reagent. The unreacted reagent and salts were removed using a centrifugal spin concentrator (0.5 mL, 10 kDa MWCO). The protein mixture was further washed with Grade I water (2 × 0.4 ml). The sample was analyzed by LC-ESI-MS or MALDI-ToF-MS. The sample was then buffer exchanged using NaHCO3 (100 µl, 0.1 M, pH 8.5) and incubated for 48 h. It resulted in the C-S bond dissociation, and a free aldehyde handle on the protein (17) was generated via the retro-Henry reaction. The sample was then buffer exchanged using phosphate buffer (100 µl, 50 mM, pH 7.0). To this solution, hydroxylamine hydrochloride 18 or O-benzylhydroxylamine hydrochloride 19 (2 µmol) in water (10 µl) from a freshly prepared stock solution was added for late-stage modification (oxime formation) and incubated for 3 h. The excess hydroxylamine and salts were removed by a centrifugal spin concentrator (0.5 ml, 10 kDa MWCO) and the sample was collected in an aqueous medium. The modification of protein was analyzed by LC-ESI-MS. The aqueous sample was concentrated by lyophilization before subjecting it to digestion, peptide mapping, and sequencing by MS-MS. [Note: The same protocol was followed for the single-site modification of BSA (8j) and HSA (8k)].
General procedure for molecular dynamics simulations
The atomistic classical molecular dynamics (MD) simulation studies were performed to understand LDM chemistry. The structures were solvated in TIP3P water box, extending 12 Å from the solute in all three directions using the LEAP module in AMBER20. Appropriate numbers of Na+ and Cl− counterions were added to neutralize the charges and to maintain 150 mM physiological salt concentration. AMBER ff14 force field was used to describe the interaction involving protein and Joung and Cheatham parameters for ions. The resulting solvated structures were subjected to minimization. Then MD was performed under constant pressure-constant temperature conditions (NPT) for 500 ps using a harmonic restraint on the solute with a force constant of 10 kcal/mol/Å2. This was followed by a production run of 1000 ns. Three independent trajectories were generated for the production runs. The LDMC-K reagent and the protein were simulated separately in water using similar protocols as reference systems.
The MD simulations were carried out using the PMEMD module of AMBER20 package with imposed 3D periodic boundary conditions. TIP3P water model was used to solvate the systems. A time step of 2 fs was used to integrate the equation of motion. The temperatures were maintained for the simulations using Langevin dynamics, while pressure was kept constant at 1 atm using Berendsen weak coupling method with anisotropic pressure scaling. The particle mesh Ewald method was used to calculate long-range electrostatic calculations with a real space cut-off distance of 10 Å where the vdW and direct electrostatic interactions were truncated. All bond lengths involving hydrogen atoms were held fixed using the SHAKE algorithm. All analyses were done using CPPTRAJ module of AMBER20 tools. Snapshot generations were done using (Visual Molecular Dynamics).
General procedure for single-site installation of tags
After the installation of free aldehyde handle on the protein (17) via retro-Henry process, various derivatives of hydroxylamine (21–23, 20–60 nmol) in H2O:ACN (1:1) from a freshly prepared stock solution were added for the late-stage modification (oxime formation) and incubated for 12–24 h. The excess hydroxylamine and salts were removed by a centrifugal spin concentrator (0.5 ml, 10 kDa MWCO), and the sample was collected in an aqueous medium. The modification of protein was analyzed by LC-ESI-MS. The aqueous sample was concentrated by lyophilization before subjecting it to digestion, peptide mapping, and sequencing by MS-MS.
General procedure for dual-probe installation
Protein 8a (2 nmol) in phosphate buffer (80 µl, 50 mM, pH 7.0) was taken in a 1.5 ml microcentrifuge tube. To this solution, LDMC-K reagent (9d, 20 nmol) in acetonitrile (20 µl) from a freshly prepared stock solution was added and incubated at 25 °C. The overall concentration of protein and LDMC-K reagent was 20 µM and 200 µM, respectively. After 6 h, the reaction mixture was diluted with water (500 µl), followed by centrifugation for 1 min at 9600×g to precipitate the unreacted LDMC-K reagent. The unreacted reagent and salts were removed using a centrifugal spin concentrator (0.5 mL, 10 kDa MWCO). The protein mixture was further washed with Grade I water (2 × 0.4 ml). The sample was analyzed by LC-ESI-MS. The sample was then buffer exchanged using NaHCO3 (100 µl, 0.1 M, pH 8.5) and incubated for 48 h. The C-S bond dissociation and retro-Henry reaction render the free aldehyde handle on the protein (17). The sample was then buffer exchanged using phosphate buffer (100 µl, 50 mM, pH 7.0). To this solution, various derivatives of hydroxylamine (21–23, 20–60 nmol) in ACN (10 µl) from the freshly prepared stock solution were added. The incubation for 12–24 h rendered the late-stage modification through oxime formation. Later to this solution, the CPM dye (27, 20 nmol) in acetonitrile (20 µl) from freshly prepared stock solution was added and incubated for 1 h. The excess hydroxylamine derivative, CPM dye, and salts were removed by a centrifugal spin concentrator (0.5 ml, 10 kDa MWCO), and the sample was collected in an aqueous medium. The modification of protein was analyzed by LC-ESI-MS.
Protein-selectivity coupled chemoselective and site-selective protein modification, enrichment, and late-stage tagging in a representative mixture of eight proteins
Ubiquitin (17 µg, 2 nmol), cytochrome C (24 µg, 2 nmol), RNase A (27 µg, 2 nmol), insulin (23 µg, 4 nmol), α-lactalbumin (28 µg, 2 nmol), lysozyme C (28 µg, 2 nmol), myoglobin (34 µg, 2 nmol), and HSA (25 µg, 0.4 nmol) in phosphate buffer (80 µl, 50 mM, pH 7.0) were mixed in a 1.5 ml microcentrifuge tube. Here, the HSA concentration was kept five times lower than the other proteins to present a substantial challenge to the method. To this solution, LDMC-K reagent 9d (1.6 µg, 4 nmol) in ACN (20 µl) from a freshly prepared stock solution was added and incubated at 25 °C. After 3 h, the reaction mixture was diluted with grade I water (500 µl). The unreacted LDMC-K reagent 9d and salts were removed by centrifugal spin concentrator (0.5 ml, 3 kDa MWCO) and the protein mixture was collected in the NaHCO3 buffer (100 µl, 0.1 M, pH 8.5). Next, the incubation for 48 h generates a free aldehyde handle on protein (32) via the retro-Henry reaction. Subsequently, the reaction mixture was diluted with phosphate buffer (500 µl, 50 mM, pH 7.0). The buffer exchange was performed by centrifugal spin concentrator (0.5 ml, 3 kDa MWCO). Subsequently, this reaction mixture was utilized for the enrichment of HSA.
In a 5 ml fritted polypropylene chromatography column with end tip closures, hydrazide beads (400 µl, hydrazide resin loading: 16 µmol/ml) were taken. The beads were washed with phosphate buffer (0.1 M, pH 7.0, 5 × 1 ml) and re-suspended (phosphate buffer, 100 µl, 50 mM, pH 7.0). The reaction mixture containing modified HSA 32 (40 nmol) in phosphate buffer (500 µl, 50 mM, pH 7.0) was added to the beads. Next, the end-to-end rotation (29 × g, rotary mixer) was performed at 25 °C for 24 h. The supernatant was collected, and the beads were washed with KCl (0.5 M, 6 × 1 ml) and phosphate buffer (0.1 M, pH 7.0, 4 × 1 ml) to remove the unreacted and adsorbed proteins from the resin. The beads were further washed with Grade I water (6 × 1 ml) and re-suspended (phosphate buffer, 450 µl, 50 mM, pH 6.5). To release the labeled protein from its immobilized derivative, the O-hydroxylamine derivative of coumarin 23 (20 µM) in ACN:H2O (50 µl) was added. The subsequent end-to-end rotation at 25 °C for 24 h led to transoximization. The supernatant was collected while the salts and 23 were removed using the centrifugal spin concentrator (10 kDa MWCO). The purity of the labeled protein 33 was confirmed by in-gel fluorescence (please see the source data file), peptide mapping, and MS-MS. The protocol results in coumarin tagged labeled protein 33 with excellent purity.
Single-site, single-protein labeling in cell lysate and its enrichment
E. coli BL21 cell lysate (2 µg/1 µl) in phosphate buffer (75 µl, 50 mM, pH 7.0) spiked with HSA 8k (25 µg, 0.4 nmol, in phosphate buffer, 5 µl, 50 mM, pH 7.0) were taken in a 1.5 ml microcentrifuge tube. To this solution, the LDMC-K reagent 9d (1.6 µg, 4 nmol) in ACN (20 µl) from a freshly prepared stock solution was added and incubated at 25 °C. After 3 h, the reaction mixture was diluted with Grade I water (500 µl). The unreacted LDMC-K reagent 9d and salts were removed by centrifugal spin concentrator (0.5 ml, 3 kDa MWCO). The reaction mixture was collected in NaHCO3 buffer (100 µl, 0.1 M, pH 8.5). The subsequent incubation for 48 h renders a free aldehyde handle on protein (32) via retro-Henry reaction. Next, the reaction mixture was diluted with phosphate buffer (500 µl, 50 mM, pH 7.0). The buffer exchange was performed by centrifugal spin concentrator (0.5 ml, 3 kDa MWCO). This reaction mixture was utilized for the enrichment of modified HSA in the subsequent step.
In a 5 ml fritted polypropylene chromatography column with end tip closures, hydrazide beads (400 µl, hydrazide resin loading: 16 µmol/ml) were taken. The beads were washed with phosphate buffer (0.1 M, pH 7.0, 5 × 1 ml) and re-suspended (phosphate buffer, 100 µl, 50 mM, pH 7.0). The cell lysate containing labeled and unlabeled HSA (combined batch: 2.6 mg, 40 nmol) in phosphate buffer (500 µl, 50 mM, pH 7.0) were added to the beads, followed by end-to-end rotation (29 × g, rotary mixer) at 25 °C for 24 h. The supernatant was collected, and the beads were washed with KCl (0.5 M, 6 × 1 ml) and phosphate buffer (0.1 M, pH 7.0, 4 × 1 ml) to remove the unreacted and adsorbed proteins from resin. The beads were further washed with Grade I water (6 × 1 ml) and re-suspended (phosphate buffer, 450 µl, 50 mM, pH 6.5). To release the labeled protein from its immobilized derivative, the O-hydroxylamine derivative of coumarin 23 (20 µM) in ACN:H2O (50 µl) was added. Next, the transoximization is facilitated by end-to-end rotation at 25 °C for 24 h. The supernatant was collected while the salts and 23 were removed using the centrifugal spin concentrator (10 kDa MWCO). The purity of the labeled protein 33 was confirmed by in-gel fluorescence (please see the source data file), peptide mapping, and MS-MS. The protocol results in coumarin tagged labeled protein 33 with excellent purity.
Site-selective modification of trastuzumab and synthesis of AFC
Trastuzumab 34 (300 µg, 2 nmol, Emcure, N7123B01) in phosphate buffer (80 µl, 50 mM, pH 7.0) was taken in a 1.5 ml microcentrifuge tube. TCEP (5 µg, 2 nmol) was added to this solution, and the reaction mixture was vortexed for 1 h. It was followed by the addition of reagent 9d (12 µg, 30 nmol) in ACN (20 µl) from a freshly prepared stock solution and vortexed for 8 h at 25 °C. The overall concentration of the trastuzumab 34 and LDMC-K reagent 9d was 20 µM and 300 µM, respectively. After 8 h, the reaction mixture was diluted with water (500 µl), followed by centrifugation for 1 min at 9600 × g to precipitate the unreacted LDMC-K reagent. The unreacted reagent and salts were removed using a centrifugal spin concentrator (0.5 mL, 10 kDa MWCO). The protein mixture was further washed with Grade I water (2 × 0.4 ml). The sample was analyzed by LC-ESI-MS. The sample was then buffer exchanged using NaHCO3 (100 µl, 0.1 M, pH 8.5) and incubated for 48 h. This step resulted in C-S bond dissociation to generate the free aldehyde handle on the protein (37) enabled by the retro-Henry reaction. This trastuzumab conjugate (37) can be stored after lyophilization for late-stage installation of desired probes through their hydroxylamine derivative. The labeled trastuzumab (37, 2 nmol) in phosphate buffer (80 µl, 50 mM, pH 7.0) was taken in a 1.5 ml microcentrifuge tube. To this solution, the hydroxylamine derivative of coumarin 23 (0.2 µmol) in Grade I water (10 µl) from a freshly prepared stock solution was added separately for the late-stage installation of probes. The reaction mixture was incubated for 3 h to yield AFC (38, please see the source data file). The excess hydroxylamine derivative and salts were removed by centrifugal spin concentrator (0.5 ml, 10 kDa MWCO).
Synthesis of ADC and antiproliferative assay
The hydroxylamine derivative of DM1 (35a, 2 µmol) in DMSO (20 µl) and 10% HCl (10 µl) from a freshly prepared stock solution was added to the antibody bioconjugate 37 in phosphate buffer (80 µl, 50 mM, pH 7.0). Subsequently, the reaction mixture was vortexed at 25 °C for 12 h to form the oxime derivative, i.e., ADC (39). The reaction mixture was frozen, lyophilized, followed by the addition of 100 µl of water. Under these conditions, the unreacted DM1 derivative 35a is insoluble, enabling its precipitation and removal. The centrifugal spin concentration (10 kDa MWCO) and volume reduction to 250 µl ensured the complete removal of 35a. The sample was lyophilized and stored for further studies.
SKBR-3 cells (104, source: NCCS Pune) were seeded in a 96-well plate (tissue culture grade, flat bottom) in a final volume of 100 μl of MacCoy’s 5 A culture medium. After seeding for 24 h, the cells were treated with various concentrations (0.25–0.5 nM) of ADC (39), Kadcyla (T-DM1, 40, Roche, N1037B18), DM1 (35), and trastuzumab (34) for the next 48 h and the total volume was kept 200 μl after addition of compounds. All the treatments were given in triplicate. The inhibition of cell proliferation was assessed using the MTT assay from Sigma Aldrich (Sigma Aldrich, Saint Louis, USA). Briefly, MTT reagent (100 μl, final concentration 0.5 mg/ml) was added after removing the medium, and the plates were incubated at 37 °C. After 1–1.5 h (depending upon the formation of crystals) of incubation, DMSO (100 μl) was added, and absorbance was taken on an ELISA plate reader (CYTATION 5, BioTeK) with a test wavelength of 570 nm and a reference wavelength of 630 nm. Relative growth inhibition rates for the untreated control were calculated and expressed as % inhibition of cell proliferation. In order to check the selectivity of ADC (39), we performed the MTT assay in HER-2 negative MDA-MB-231 cells (please see the source data file). MDA-MB-231 cells (source: ATCC, STR profiling authentication) were seeded in the DMEM medium, and the same protocol was followed as mentioned above.
Statistics and reproducibility
All the experiments were independently repeated at least three times. Data are presented as mean values ± SD (standard deviation) calculated using OriginPro 8.5 and GraphPad Prism 8.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the Article and its accompanying Supplementary Information file. The source data for Figs. 5c, 7a, b, 8b, c and Supplementary Figs. 65, 86a, 87b, 90, 98a, b, 98c, 108, 111, 112, is provided in the Source Data file. The raw data generated in this study have been deposited in the Figshare public repository https://doi.org/10.6084/m9.figshare.21219896. Source data are provided with this paper.
References
Reddi, R. N. et al. Tunable methacrylamides for covalent ligand directed release chemistry. J. Am. Chem. Soc. 143, 4979–4992 (2021).
Sutanto, F., Konstantinidou, M. & Dömling, A. Covalent inhibitors: a rational approach to drug discovery. RSC Med. Chem. 11, 876–884 (2020).
Gehringer, M. & Laufer, S. A. Emerging and re-emerging warheads for targeted covalent inhibitors: applications in medicinal chemistry and chemical biology. J. Med. Chem. 62, 5673–5724 (2019).
Novotny, C. J., Hamilton, G. L., McCormick, F. & Shokat, K. M. Farnesyltransferase-mediated delivery of a covalent inhibitor overcomes alternative prenylation to mislocalize K-Ras. ACS Chem. Biol. 12, 1956–1962 (2017).
Sun, X. et al. PROTACs: great opportunities for academia and industry. Sig. Transduct. Target Ther. 4, 64 (2019).
Paiva, S. L. & Crews, C. M. Targeted protein degradation: elements of PROTAC design. Curr. Opin. Chem. Biol. 50, 111–119 (2019).
Ahn, G. et al. LYTACs that engage the asialoglycoprotein receptor for targeted protein degradation. Nat. Chem. Biol. 17, 937–946 (2021).
Silva, M. J. S. A. et al. Efficient amino-sulfhydryl stapling on peptides and proteins using bifunctional NHS-activated acrylamides. Angew. Chem. Int. Ed. 60, 10850–10857 (2021).
Zhang, Y., Zhang, Q., Wong, C. T. T. & Li, X. Chemoselective peptide cyclization and bicyclization directly on unprotected peptides. J. Am. Chem. Soc. 141, 12274–12279 (2019).
Kumar, M., Reddy, N. C. & Rai, V. Chemical technologies for precise protein bioconjugation interfacing biology and medicine. Chem. Commun. 57, 7083–7095 (2021).
Drago, J. Z., Modi, S. & Chandarlapaty, S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 18, 327–344 (2021).
Forte, N., Chudasama, V. & Baker, J. R. Homogeneous antibody-drug conjugates via site-selective disulfide bridging. Drug Discov. Today Technol. 30, 11–20 (2018).
Cornish, V. W., Hahn, K. M. & Schultz, P. G. Site-specific protein modification using a ketone handle. J. Am. Chem. Soc. 118, 8150–8151 (1996).
Carrico, I. S., Carlson, B. L. & Bertozzi, C. R. Introducing genetically encoded aldehydes into proteins. Nat. Chem. Biol. 3, 312–322 (2007).
Raj, M., Wu, H., Blosser, S. L., Vittoria, M. A. & Arora, P. S. Aldehyde capture ligation for synthesis of native peptide bonds. J. Am. Chem. Soc. 137, 6932–6940 (2015).
Rosen, C. B. & Francis, M. B. Targeting the N terminus for site-selective protein modification. Nat. Chem. Biol. 13, 697–705 (2017).
Macdonald, J. I., Munch, H. K., Moore, T. & Francis, M. B. One-step site-specific modification of native proteins with 2-pyridinecarboxyaldehydes. Nat. Chem. Biol. 11, 326–331 (2015).
Chen, D., Disotuar, M. M., Xiong, X., Wang, Y. & Chou, D. H. C. Selective N-terminal functionalization of native peptides and proteins. Chem. Sci. 8, 2717–2722 (2017).
Schoffelen, S. et al. Metal-free and pH-controlled introduction of azides in proteins. Chem. Sci. 2, 701–705 (2011).
Singudas, R., Adusumalli, S. R., Joshi, P. N. & Rai, V. A phthalimidation protocol that follows protein defined parameters. Chem. Commun. 51, 473–476 (2015).
Jia, S., He, D. & Chang, C. J. Bioinspired thiophosphorodichloridate reagents for chemoselective histidine bioconjugation. J. Am. Chem. Soc. 141, 7294–7301 (2019).
Joshi, P. N. & Rai, V. Single-site labeling of histidine in proteins, on-demand reversibility, and traceless metal-free protein purification. Chem. Commun. 55, 1100–1103 (2019).
Chen, X., Muthoosamy, K., Pfisterer, A., Neumann, B. & Weil, T. Site-selective lysine modification of native proteins and peptides via kinetically controlled labeling. Bioconjug. Chem. 23, 500–508 (2012).
Chilamari, M., Kalra, N., Shukla, S. & Rai, V. Single-site labeling of lysine in proteins through a metal-free multicomponent approach. Chem. Commun. 54, 7302–7305 (2018).
Lin, S. et al. Redox-based reagents for chemoselective methionine bioconjugation. Science 355, 597–602 (2017).
Hoyt, E. A., Cal, P. M. S. D., Oliveira, B. L. & Bernardes, G. J. L. Contemporary approaches to site-selective protein modification. Nat. Rev. Chem. 3, 147–171 (2019).
Ohata, J. et al. An activity-based methionine bioconjugation approach to developing proximity-activated imaging reporters. ACS Cent. Sci. 6, 32–40 (2020).
Chen, G. et al. Reactivity of functional groups on the protein surface: development of epoxide probes for protein labeling. J. Am. Chem. Soc. 125, 8130–8133 (2003).
Tsukiji, S., Miyagawa, M., Takaoka, Y., Tamura, T. & Hamachi, I. Ligand-directed tosyl chemistry for protein labelling in vivo. Nat. Chem. Biol. 5, 341–343 (2009).
Faustino, H., Silva, M. J. S. A., Veiros, L. F., Bernardes, G. J. L. & Gois, P. M. P. Iminoboronates are efficient intermediates for selective, rapid and reversible N-terminal cysteine functionalisation. Chem. Sci. 7, 5052–5058 (2016).
Bandyopadhyay, A., Cambray, S. & Gao, J. Fast and selective labeling of N-terminal cysteines at neutral pH via thiazolidino boronate formation. Chem. Sci. 7, 4589–4593 (2016).
Ren, H. et al. A biocompatible condensation reaction for the labeling of terminal cysteine residues on proteins. Angew. Chem. Int. Ed. 48, 9658–9662 (2009).
Casi, G., Huguenin-Dezot, N., Zuberbühler, K., Scheuermann, J. & Neri, D. Site-specific traceless coupling of potent cytotoxic drugs to recombinant antibodies for pharmacodelivery. J. Am. Chem. Soc. 134, 5887–5892 (2012).
Purushottam, L. et al. Single-site glycine-specific labeling of proteins. Nat. Commun. 10, 2539 (2019).
Adusumalli, S. R. et al. Single-site labeling of native proteins enabled by a chemoselective and site-selective chemical technology. J. Am. Chem. Soc. 140, 15114–15123 (2018).
Adusumalli, S. R. et al. Chemoselective and site-selective lysine-directed lysine modification enables single-site labeling of native proteins. Angew. Chem. Int. Ed. 59, 10332–10336 (2020).
Rawale, D. G. et al. Linchpins empowers promiscuous electrophiles to enable site-selective modification of histidine and aspartic acid in proteins. Chem. Sci. 12, 6732–6736 (2021).
Kalia, D., Malekar, P. V. & Parthasarathy, M. Exocyclic olefinic maleimide: synthesis and application for stable and thiol-selective bioconjugation. Angew. Chem. Int. Ed. Engl. 55, 1432–1435 (2016).
Zhang, Y. et al. Thiol Specific and tracelessly removable bioconjugation via michael addition to 5-methylene pyrrolones. J. Am. Chem. Soc. 139, 6146–6151 (2017).
Nunes, J. P. M. et al. Functional native disulfide bridging enables delivery of a potent, stable and targeted antibody–drug conjugate (ADC). Chem. Commun. 51, 10624–10627 (2015).
Acknowledgements
This work was supported by SERB, DST, DBT, SERB-PACE (IPA/20221/000148), and IISER Bhopal. N.C.R., R.M., P.N.J., S.T.K., I.B., and J.K. are recipients of a research fellowship from CSIR, UGC, S.N. Bose Centre, and IISER Bhopal. S.S. is a recipient of the DBT-Wellcome fellowship (IA/I/16/2/502719). V.R. is a recipient of the Swarnajayanti Fellowship (DST/SJF/CSA-01/2018-19 and SB/SJF/2019-20/01). We thank Mr. Atanu Nandy and Prof. S. Mukherjee for their help with fluorescence spectroscopy.
Author information
Authors and Affiliations
Contributions
V.R. and N.C.R. conceived the research. N.C.R., R.M., and P.N.J. designed and performed the bioconjugation experiments. R.K.M., S.T.K., and J.K. designed and performed cell-based experiments. S.S. and N.K. designed and performed the antiproliferative assay for ADCs. S.C. and I.B. designed and performed the simulations. All the authors wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
V.R. is the founder of Plabeltech Private Limited. A patent application (US 11,149,058 B2; Applicant: IISER Bhopal and DBT; Inventors: Vishal Rai and Srinivasa Rao Adusumalli) has been granted on the LDM. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reddy, N.C., Molla, R., Joshi, P.N. et al. Traceless cysteine-linchpin enables precision engineering of lysine in native proteins. Nat Commun 13, 6038 (2022). https://doi.org/10.1038/s41467-022-33772-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33772-1
This article is cited by
-
Non-symmetric stapling of native peptides
Nature Reviews Chemistry (2024)
-
Bioinspired one-pot furan-thiol-amine multicomponent reaction for making heterocycles and its applications
Nature Communications (2023)
-
A simple method for developing lysine targeted covalent protein reagents
Nature Communications (2023)
-
Bioinspired 5-caffeoylquinic acid capped silver nanoparticles using Coffee arabica leaf extract for high-sensitive cysteine detection
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.