Abstract
Vein ligation for veno-occlusive erectile dysfunction is being abandoned due to the recurrence rate. Among the reasons for failure is inability to ligate the deep system of veins; the internal pudendal vein. The vein exits the pelvis in the gluteal region, from the lesser sciatic foramen to the greater sciatic foramen, coursing over the ischial spine and sacro-spinous ligament, under the gluteus maximus. This work aims to verify feasibility of the first surgical procedure to ligate the internal pudendal vein through the gluteal approach. This cadaveric study involved five formalin-fixed cadavers. A surface anatomical landmark was designed to identify the ischial spine, at the intersection of two lines: a vertical line from posterior superior iliac spine to ischial tuberosity, and a horizontal line extending from sacro-coccygeal joint, laterally. An incision is cut encompassing the target point. Subcutaneous fat is dissected down to the gluteus maximus, which is split along the direction of its fibers. The vein can be found crossing over the ischial spine. “Shaeer’s Vein Ligation – I” appears to be surgically feasible. A protocol for a surgical study is registered at clinicaltrials.gov, and is open for participation.

Similar content being viewed by others
Introduction
Erectile dysfunction (ED) is a global health problem. Prevalence of ED is estimated to be 13.1–71.2% of males, variable with age and comorbidities [1]. Etiology is often multifactorial. Potential underlying etiologies can be psychogenic or organic. With a perspective on penile hemodynamics which drive the process of erection, organic etiologies often translate into either arteriogenic ED (arterial stenosis) or veno-occlusive dysfunction (VOD). VOD is when there is failure of penile corporal rigidity despite adequate arterial influx, with a high venous efflux. This is demonstrated in penile duplex studies by consistently normal peak systolic velocities and high end-diastolic velocities (EDV > 5 cm/sec) [2].
In case of failure of medical therapy for ED, the main surgical option is penile prosthesis implantation (PPI), with patient satisfaction rates exceeding 85% [3]. Yet, PPI can be costly, and prone to complications (though rare), such as prosthetic infection and mechanical failure [4, 5]. Vein ligation surgery had been described as early as 1897 [6] and has since been modified and re-examined. This mainly refers to deep dorsal vein ligation (DVL) [7] or embolization [8]. Currently, this surgery is rarely performed considering the failure rates and recurrence rates [9]. The low or short-lived efficacy of DVL is multifactorial, including the pathogenesis behind it being endothelial and smooth muscle damage rather than excessive venous outflow [10], which is the consensus at the time of performing the present work.
Regardless the pathogenesis, rapid efflux of blood through certain veins occurs, resulting in loss of rigidity. If effective occlusion of those veins could be achieved, this would possibly enhance rigidity. Hence the use of vacuum suction with basal compression in a subset of patients. Otherwise, the only option for many such cases refractory to treatment comes down to PPI. One of the proposed causes for the low efficacy and short-lived sustainability of vein ligation is missing some venous outlets at the time of surgery [11, 12], such as the deep system of veins. The penis is drained by 3 systems of veins, the superficial, intermediate, and deep. The deep system is formed of the crural and cavernosal veins. Both drain mainly into the internal pudendal vein (IPV) [13], though may communicate with the periprostatic (retropubic) plexus [11]. IPV has so far been surgically inaccessible.
The IPV courses through Alcock’s canal, along with the internal pudendal artery and the pudendal nerve (pudendal neurovascular bundle). They emerge from the pelvis posteriorly, through the lesser sciatic foramen which is bounded superiorly by the ischial spine (IS) and sacrospinous ligament (SSL). The neurovascular bundle crosses over the IS and SSL, covered by the gluteus maximus muscle (GM). The bundle then dips back into the pelvis through the greater sciatic foramen.
This work is a cadaveric study describing Shaeer’s Vein Ligation-I (SVL-I), the first surgical technique for ligation of IPV as it courses superficial to the IS, deep to the GM, through a gluteal incision. To our knowledge, this is the first report of such an approach.
Subjects and methods
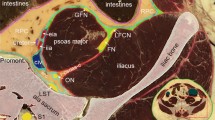
Approval from the ethical committee was obtained before commencing the study (approval number MD-282-2023). The study was conducted at the Department of Anatomy and Embryology, Kasr Al Aini Faculty of Medicine, Cairo University, Egypt. SVL-I was performed unilaterally in five formalin-fixed cadavers, with the gluteal region intact. Surface anatomical landmarks were used to identify the IS, at the intersection between two lines: a vertical line bridging the posterior superior iliac spine to the ischial tuberosity, and a horizontal line extending from the sacro-coccygeal joint, laterally (Fig. 1). This point is the surface anatomy of the IS. The IPV courses on or just medial to the IS, deep to the GM.
a: The posterior superior iliac spine. b: Sacro-coccygeal joint. c: Surface anatomy landmark for the ischial spine. d: Ischial tuberosity. e: Internal pudendal vein. f: Gluteus maximus. The ischial spine at the intersection between two lines:—Line (a–d): extending from the posterior superior iliac spine (a) to the ischial tuberosity (d).—Line b: extending laterally from the sacro-coccygeal joint (b).—The internal pudendal vein is on or immediately medial to the ischial spine (c).
A vertical incision encompassing the target point for the IS was cut, lateral to the anal cleft, and extended laterally in a hockey-stick fashion as needed. Subcutaneous fat was dissected down to the GM, which was split along the direction of its fibers. The IPV was identified deep to GM, coursing between the pudendal nerve medially and the pudendal artery laterally. IPV was ligated. The split fibers of GM were approximated. Subcutaneous fat and skin were sutured closed. Video of the surgical technique is available at the Video Journal of Sexual Medicine [14].
Results
In five cadavers, IPV was successfully identified and ligated at the target point. Figure 1 shows the anatomical landmarks which lead to the IS and subsequently the IPV. Those anatomical landmarks were verified to point to the IPV in all cases.
Figure 2 shows the skin incision in the gluteal region. Extension of the incision in a hockey-stick fashion was required in all cases (Fig. 2.1). The mean length of the incision was 9.3 ± 0.6 cm (range: 9–10 cm) (9–10 cm) (Fig. 2.2), varying with the bulk of tissue down to the IPV.
Figure 3 shows the dissection of the subcutaneous fat.
Figure 4 shows the splitting of the GM fibers along their direction (coursing downwards and laterally) (a), and the inferior gluteal neurovascular bundle within the muscle (b).
In all cases, IPV width was 0.5 cm, IPV length was 2.7 ± 1 cm (1.8–4 cm), and the distance from IPV to sciatic nerve was 5.1 ± 1 cm (4–6 cm). IPV was retrieved at a depth of 5.2 ± 1.6 cm (3–8 cm) from the skin surface Figs. 5–7. The procedure in the first cadaver took approximately 40 min time. This came down to 20–30 min in the subsequent four cadavers. Although the formalin-fixed cadavers were firm, there was no noticeable difficulty in reaching the IPV. This should be even easier with frozen-thawed cadavers (which were not available), and most importantly with live surgery.
7.1: Ligated and cut internal pudendal vein (arrows). 7.2: The distance between internal pudendal vein (a) and lateral wall of sacrum (b). 7.3: The distance between sciatic nerve (a) and internal pudendal vein (b). 7.4: The depth from skin surface to reach the internal pudendal vein. 7.5: The internal pudendal vein diameter in cut-section.
Discussion
Venous surgery for ED
In the 1980’s and 90’s, DVL and its variants were extensively tried. This included classic DVL [7], DVL and spongiolysis [15], with further modifications down the road such as excision of the deep dorsal vein [16], venous stripping surgery and refined venous stripping [17], embolization through the DDV [18] and crural ligation [19]. Leak-afflicted systems can be identified by cavernosography or computed tomography cavernosography for patient selection and surgical planning [20]. Deep system leak could be indentified in 94.2% of cases suffering VOD, using computed tomography cavernosography [21]. However, the root of the deep system of veins (IPV) remained surgically inaccessible.
Shaeer’s Vein ligation—I (SVL-I)
“SVL-I” is to our knowledge the first technique for ligation of the IPV. The surgical technique has been established in this cadaveric study. SVL-I can be performed unilaterally or bilaterally, with or without retrograde embolization, and with or without dorsal vein occlusion. This should be dictated by cavernosography. Efficacy and sustainability are yet to be tested. A theoretical risk for SVL-I may be priapism. Patients selected for SVL-I should be those refractory to medical treatment for ED, and are destined for a penile implant. If refractory priapism actually occurs, the patient will have PPI performed promptly.
Surgical tips
Avoiding false identification of the IPV
In the first case, we initially miss-identified a longitudinal facia-covered structure within the deeper bulk of the gluteus maximus as being the IPV. We decided to explore further down to confirm our finding. We eventually retrieved the IPV at a deeper level, surrounded by fat and directly related to bone. We verified the correct identification of the IPV by identifying the adjacent artery and two nerves (internal pudendal artery, pudendal nerve, and nerve to obturator internus), by identifying the glistening white striated fibers of the SSL below IPV, and by dissecting along the same plane to the sciatic nerve laterally, and to the sacral bone medially. We believe that the initially miss-identified structure could have been ramifications of the inferior gluteal neurovascular bundle within the muscle tissue; pure muscle tissue with no fat around. Accordingly, identification of IPV should only be confirmed deep to the GM muscle fibers, within fat, not within muscle, and with the sacral bone medial Fig. 6. The fore mentioned exploration was not required in subsequent cadavers, and will not be required in live surgery. This exploration in the first cadaver is the reason why it took 40 min, compared to an average of 25 min for the subsequent cases.
Expected depth for dissection down to IPV
The depth at which the IPV was encountered ranged from 3 to 8 cm, variable with the bulk of gluteal fat and muscle. In patients with obesity, a surgical headlight should be helpful, particularly if extent of the skin incision is to be confined to the shortest possible length.
Avoiding injury of adjacent structures
The inferior gluteal nerve (IGN)
The IGN is a primarily motor nerve. It typically courses underneath the piriformis muscle before emerging at its caudal border medial to the sciatic nerve and dividing into several branches, that enter the inferior third of the GM on its deep surface [22]. The IGN can be found at the junction of a line connecting the most prominent lateral border of the greater trochanter horizontally, with a perpendicular vertical line from the ischial tuberosity [22]. Median distance from IGN to ischial spine was 28.5 mm (range, 6-53 mm) [23].
Cutaneous branches of the IGN may occur in the lower outer quadrant of the GM and travel in an inferosuperior or inferolateral direction. These branches had a mean distance of 12.5 cm from the midline [24].
Injury to the nerve is mostly iatrogenic and leads to an altered gait pattern known as gluteus maximus ‘lurch.’ Injury is more likely when a muscle-splitting incision is across the GM rather than along the muscle fibers [25]. A triangular-shaped anatomic area that contains the IGN was described, formed by connecting the following points: posterior inferior iliac spine, ischial tuberosity and the greater trochanter. This triangle can further be divided into lower triangle and the upper triangle. The latter is the “danger zone” that contains the IGN and its branches [26]. Yet, procedures involving severing the GM are commonly performed, an example being the posterior approach for hip arthroplasty [27]. Injuries without nerve transection can be managed conservatively [28].
The main trunk of IGN enters the gluteus maximus 5–6 cm medial to the tip of the greater trochanter well away from our field. It courses down medial and adjacent to the sciatic nerve which we measured to be 5.1 ± 1 cm lateral to the IPV. The cutaneous branches emerge in the lateral gluteal region, again away from our field. SVL-I is performed on the medial perimeter of the danger zone [26] rather than within. Therefore -in our opinion- injury of the main trunk of IGN far-fetched.
In order to avoid injury of smaller branches of the IGN, we recommend that GM splitting is maintained medially, dissection and retraction of the muscle should be with blunt instruments, and along the direction of its fibers rather than across them. We also recommend that once fat appears beneath the muscle, finger dissection/sweeping should be employed to split the muscle further, thus avoiding injury of underlying vessels and of the IGN by the surgical instruments.
When suturing the gluteus maximus closed, we recommend sparing the deeper layer of the muscle where the nerve courses, and confining suturing to the intermediate and superficial layers of muscle bulk. Pre-marking the anatomical landmark of the IGN [22] and the “danger zone” [26] on the gluteal skin can be employed if needed.
Sciatic nerve
The sciatic nerve is 5.1 ± 1 cm lateral to our anatomical target point and the IPV. Therefore there should be no risk of injury to the sciatic nerve, Sciatic nerve cannot be mistaken for any other structure, considering its large caliber, one of the largest nerves in the lower limb. IPV emerges from the pelvis and dips back in, for a short length. This is in contrast to the sciatic nerve which runs freely down the lower limb.
Internal pudendal artery, pudendal nerve, and nerve to obturator internus
Identification, dissection and separation of the IPV from the adjacent artery and nerves was relatively easy and did not require optical magnification, considering its caliber (0.5 cm). We found IPV to be the most lateral of the internal pudendal neurovascular bundle structures, though there may be anatomical variations. We expect identifications of the IPV to be even easier in live surgery compared to formalin-fixed cadavers. However, if needed, identification in live surgery can be further facilitated by induction of artificial erection, thereby augmenting arterial pulsations and vein engorgement. Working with cavernosography under C-arm is another possible addition.
Concealment of the incision
We performed a hockey-stick incision in this cadaveric study. This was required considering the tissue stiffness encountered with formalin-fixed cadavers. Average length was 9.3 ± 0.6 cm. In live surgery, we recommend starting with a shorter vertical incision, and only extend length and adopt the hockey-stick form if needed, particularly with obese patients.
The more medial the incision is, the more concealed it will be, within the anal cleft. In one case, the incision was more medial than planned. The sacral bone was therefore encountered once dissection was beyond the gluteus maximus. This was simply resolved by sliding laterally, and the IPV was identified with ease. So it appears that a more medial incision is feasible, serving both concealment and protection against injury to the branches of the IGN.
Finally, despite the debate over pathogenesis of VOD and the controversial prior results for venous surgery, vein occlusion has some logic behind it, if only its results could be improved. In the current era, with a plethora of emerging novel solutions for ED (such as regenerative therapies), there is always a need for innovation. High ligation of the IPV in the gluteal region (SVL–I) appears to be surgically feasible. It is yet to be seen whether SVL-I is efficacious and if the effect is sustained, in clinical trials. A multi-center clinical study has been registered at clinicaltrials.gov, and is open for participation from interested centers [29].
Data availability
Data are available within the published article.
References
Kessler A, Sollie S, Challacombe B, Briggs K, Van Hemelrijck M. The global prevalence of erectile dysfunction: a review. BJU Int. 2019;124:587–99.
Jung DC, Park SY, Lee JY. Penile Doppler ultrasonography revisited. Ultrasonography. 2018;37:16–24.
Barton GJ, Carlos EC, Lentz AC. Sexual quality of life and satisfaction with penile prostheses. Sex Med Rev. 2019;7:178–88.
Kohn TP, Rajanahally S, Hellstrom WJG, Hsieh TC, Raheem OA. Global trends in prevalence, treatments, and costs of penile prosthesis for erectile dysfunction in men. Eur Urol Focus. 2022;8:803–13.
Byrne ER, Ungerer GN, Ziegelmann MJ, Kohler TS. Complications and troubleshooting in primary penile prosthetic surgery-a review. Int J Impot Res. 2023;35:679–85.
Tracy EE. Ligation of the Dorsal Vein of the Penis for Impotency. South Med Rec. 1897;27:616–8.
Wespes E, Schulman CC. Venous leakage: surgical treatment of a curable cause of impotence. J Urol. 1985;133:796–8.
Maiza D, Courtheoux P, Henriet JP, Maiza C, Vaislic C. [Preliminary results 6 months after embolization of the deep dorsal vein of the penis in erectile insufficiencies of venous origin]. J Mal Vasc. 1984;9:327.
Cakan M, Yalçinkaya F, Demirel F, Ozgünay T, Altuğ U. Is dorsale penile vein ligation (dpvl) still a treatment option in veno-occlusive dysfunction? Int Urol Nephrol. 2004;36:381–7.
Montorsi F, Adaikan G, Becher E, Giuliano F, Khoury S, Lue TF, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med. 2010;7:3572–88.
Lewis RW. After a Decade of Venous Surgery - Is there a Place for it in 1996? In: Porst H (ed). Penile Disorders, vol. 1. Springer: Germany, 1996, pp 230-1.
Lunglmayr G, Nachtigall M, Gindl K. Long-term results of deep dorsal penile vein transsection in venous impotence. Eur Urol. 1988;15:209–12.
Ellsworth PI. Penis Anatomy. https://emedicine.medscape.com/article/1949325-overview?form=fpf#a2, 2017. Accessed Nov. 11th 2023.
Shaeer O, El-Shaarawy E, Emam H, Elsisi I, Sokar E. Shaeer’s Vein Ligation – I. VJSM. https://www.vjsm.info/videos/all/pudendal-vein-for-management-of-veno-occlusive-erectile-dysfunction-the-cadaveric-study, 2024.
Gilbert P, Sparwasser C, Beckert R, Treiber U, Pust R. Venous surgery in erectile dysfunction. The role of dorsal-penile-vein ligation and spongiosolysis for impotence. Urol Int. 1992;49:40–7.
Vale JA, Feneley MR, Lees WR, Kirby RS. Venous leak surgery: long-term follow-up of patients undergoing excision and ligation of the deep dorsal vein of the penis. Br J Urol. 1995;76:192–5.
Hsu GL, Chen HS, Hsieh CH, Lee WY, Chen KL, Chang CH. Clinical experience of a refined penile venous stripping surgery procedure for patients with erectile dysfunction: is it a viable option? J Androl. 2010;31:271–80.
Allaire E, Sussman H, Zugail AS, Hauet P, Floresco J, Virag R. Erectile dysfunction resistant to medical treatment caused by cavernovenous leakage: an innovative surgical approach combining pre-operative work up, embolisation, and open surgery. Eur J Vasc Endovasc Surg. 2021;61:510–7.
Flores S, Tal R, O’Brien K, Mulhall JP. Outcomes of crural ligation surgery for isolated crural venous leak. J Sex Med. 2011;8:3495–9.
Hoppe H, Diehm N. Venogenic erectile dysfunction: diagnosis on computed tomography cavernosography and endovascular treatment using an anterograde access via deep dorsal penile vein. CVIR Endovasc. 2022;5:10.
Ye T, Li J, Li L, Yang L. Computed tomography cavernosography combined with volume rendering to observe venous leakage in young patients with erectile dysfunction. Br J Radio. 2018;91:20180118.
Skalak AF, McGee MF, Wu G, Bogie K. Relationship of inferior gluteal nerves and vessels: target for application of stimulation devices for the prevention of pressure ulcers in spinal cord injury. Surg Radio Anat. 2008;30:41–5.
Florian-Rodriguez ME, Hare A, Chin K, Phelan JN, Ripperda CM, Corton MM. Inferior gluteal and other nerves associated with sacrospinous ligament: a cadaver study. Am J Obstet Gynecol. 2016;215:646.e641–646.e646.
Iwanaga J, Simonds E, Vetter M, Patel M, Oskouian RJ, Tubbs RS. The inferior gluteal nerve often has a cutaneous branch: a discovery with application to hip surgery and targeting gluteal pain syndromes. Clin Anat. 2018;31:937–41.
Hasija R, Kelly JJ, Shah NV, Newman JM, Chan JJ, Robinson J, et al. Nerve injuries associated with total hip arthroplasty. J Clin Orthop Trauma. 2018;9:81–6.
Apaydin N, Bozkurt M, Loukas M, Tubbs RS, Esmer AF. The course of the inferior gluteal nerve and surgical landmarks for its localization during posterior approaches to hip. Surg Radio Anat. 2009;31:415–8.
Quitmann H. Supercapsular percutaneously assisted (SuperPath) approach in total hip arthroplasty : Surgical technique and preliminary results. Oper Orthop Traumatol. 2019;31:536–46.
Grant GA, Goodkin R, Kliot M. Evaluation and surgical management of peripheral nerve problems. Neurosurgery. 1999;44:825–39.
Shaeer O. Shaeer’s Vein Ligation-I: internal pudendal vein sub-gluteal ligation for veno occlusive erectile dysfunction. https://clinicaltrials.gov/study/NCT06121687?term=shaeer%27s%20vein&rank=2, 2023. Accessed Nov. 12th 2023.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Osama Shaeer: Conceptualization, Methodology, Investigation, Writing – Original Draft, Writing – Review & Editing, Supervision. Ehab A. A. El-Shaarawy: Methodology, Investigation, Writing – Original Draft, Writing – Review & Editing, Supervision, Resources. Hossam Yahia Sayed Emam: Methodology, Investigation, Resources. Islam Elsisi: Investigation. Eslam Sokar: Investigation, Methodology. Kamal Shaeer: Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaeer, O., El-Shaarawy, E.A.A., Emam, H.Y. et al. Sub-gluteal ligation of the internal pudendal vein for management of veno-occlusive erectile dysfunction (Shaeer’s Vein Ligation–I): the cadaveric study. Int J Impot Res (2024). https://doi.org/10.1038/s41443-024-00846-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41443-024-00846-1