Abstract
Male hypogonadism is not a risk associated with attention-deficit hyperactivity disorder (ADHD) stimulant medications, but recent studies have explored this connection. Though the pathophysiologic connection remains unclear, we predicted that long-term use of ADHD stimulant medications could increase the risk of hypogonadism in post-pubertal males. Utilizing TriNetX, LLC Research Network data from January 2000 through December 2019, men older than 18 with ADHD receiving long-term stimulant medication (>36 monthly prescriptions) were selected for the study population. Two control groups were constructed: individuals with ADHD but no stimulant medication use, and individuals without ADHD or stimulant medication use. A diagnosis of testicular hypofunction (ICD-10: E29.1) within five years of long-term ADHD stimulant medication use was the chosen primary outcome. After propensity score matching, 17,224 men were analyzed in each group. Of the men with long-term ADHD stimulant medication use, 1.20% were subsequently diagnosed with testicular hypofunction compared to 0.67% of individuals with ADHD without stimulant medication use (RR: 1.78, 95% CI: 1.42–2.23) and 0.68% in men without ADHD or stimulant medication use (RR: 1.75, 95% CI: 1.39–2.19). Therefore, chronic ADHD stimulant medication use was found to be significantly associated with a subsequent diagnosis of testicular hypofunction.
Similar content being viewed by others
Introduction
Attention-deficit hyperactivity disorder (ADHD) is one of the most diagnosed and treated psychiatric conditions in the United States. A 2019 Centers for Disease Control and Prevention estimation reported that over six million children, 9.4%, between the ages of two and seventeen in the United States had received a diagnosis of ADHD from a healthcare provider [1]. Of those children with a diagnosis, 62.0% were currently using medication. Though often considered a diagnosis in children, over 4% of adults are diagnosed with ADHD [2]. A mainstay of treatment for ADHD in both adults and children is stimulant medication such as methylphenidate (Ritalin) or amphetamine-dextroamphetamine (Adderall). These medications function by increasing the bioavailability of the catecholamines dopamine and norepinephrine within the brain [3].
Despite no reported risks of gonadal dysfunction associated with the medications, case studies and animal studies have recently suggested these medications could impact gonadal functioning, including fertility rates [4,5,6,7,8,9]. Dopamine has been shown to suppress the excitation of gonadotropin-releasing hormone (GnRH) neurons in both male and female mice [10]. GnRH acts upon the hypothalamus to release both luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which directly impact gonadal function and testosterone production [11]. Hypogonadism and, potentially, infertility can result when this signaling of the hypothalamic-pituitary-gonadal access is disrupted or suppressed.
To further assess this relationship, Wang et al. in 2019 utilized the National Health Insurance database in Taiwan to explore the relationship between the long-term use of methylphenidate and testicular dysfunction in boys [12]. This demonstrated an increased rate of testicular dysfunction within the population of males diagnosed with ADHD compared to their control group. ADHD medication use itself had no increased risk of testicular dysfunction [12].
We hypothesized that prolonged use of ADHD stimulant medications could negatively impact testosterone production in post-pubertal males. To assess this, we used electronic health records to build a retrospective cohort to evaluate testicular hypofunction rates among men with long-term use of ADHD medications compared to others.
Methods
Data source and study design
Data used in this study was collected and analyzed in January 2023 from the TriNetX, LLC Research Network (TriNetX LLC, Cambridge MA, USA), which provided access to electronic medical records (diagnoses, procedures, medications, and laboratory values), as well as insurance claims for approximately 108 million patients from 76 healthcare organizations. Information regarding demographics, diagnoses from International Classification of Disease (ICD) codes, procedures from Current Procedural Terminology (CPT) codes, and medications were all recorded and used for analysis. Medication data was obtained from prescriptions, orders, inpatient medication reconciliations, and charted medications and were identified in the database using the National Library of Medicine RxNorm classification system. Data from January 2000 through December 2019 were included.
The process by which the data was de-identified is attested to through a formal determination by a qualified expert as defined in Section §164.514(b)[1] of the Health Insurance Portability and Accountability Privacy Rule. Because this study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, this study was exempted from the Institutional Review Board.
Cohorts
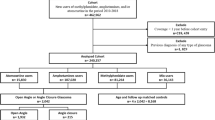
To evaluate the risk of long-term ADHD stimulant medication on developing a diagnosis of testosterone deficiency in post-pubertal males, we included adult men ages 20–40 years with a diagnosis of ADHD (ICD-10: F90) who did and did not have exposure to ADHD stimulant medications, including methylphenidate (RxNorm: 6901), dextroamphetamine (3288), lisdexamfetamine (700,810), amphetamine (725), and dexmethylphenidate (149,373). Men were defined as having chronic exposure if they had received at least 36 prescriptions of ADHD medications while control men with an ADHD diagnosis but had zero instances of these prescriptions including the above stimulants as well as non-stimulant ADHD medications including guanfacine (40,114) and atomoxetine (38,400). A second control group was additionally created including men with no diagnosis of ADHD and never having received any ADHD medications to serve as a population control. The primary outcome was a diagnosis of testicular hypofunction (ICD-10: E29.1) within five years of having been prescribed at least 36 prescriptions of ADHD medications (Fig. 1). We excluded men who did not meet these definitions, as well as those with identified genetic conditions such as Klinefelter syndrome.
Statistical analysis
Data was reported as mean and standard deviation or total counts. Baseline characteristics prior to propensity score matching were compared using T-test and Chi-squared. We then utilized propensity score matching – a statistical technique that utilizes logistic regression to build cohorts of equal size based on covariates of interest. We used 1:1 greedy nearest-neighbor propensity score matching to control for confounding variables through the TriNetX platform. In this analysis, we controlled for: age at Index, current age, race/ethnicity, hyperlipidemia (ICD-10: E78), overweight or obesity (E66), obstructive sleep apnea (G47.3), diabetes mellitus (E08-13), conduct disorder (F91), pervasive developmental disorders (F84), tic disorders (F95), and intellectual disabilities (F70-79). Statistical analysis was performed using Python and R software built into the TriNetX platform. We determined that the two groups had minimal differences after balancing, as the standardized differences between propensity scores were less than 0.1.
Results
Before matching, we identified 19,498 men with a diagnosis of ADHD and had received at least 36 prescriptions of ADHD stimulant medications and 147,441 men with a diagnosis of ADHD but never received any ADHD medications. After matching, a total of 17,224 men in each group were included in the analysis with an initial age of ADHD diagnosis occurring at 19.1 (10.8–27.4) years for those taking medications and 19.2 (11–27.4) years for those without medication use. The current age of those included was 28.9 (22.7-35.1) in the study group and 29 (22.8-35.1) in this control group (Table 1).
After propensity score matching, 1.20% of men who received at least 36 prescriptions of ADHD medications were found to have a subsequent diagnosis of testosterone hypofunction within five years compared to 0.67% in men with ADHD but had never received ADHD medications (risk ratio (RR) 1.78, 95% Confidence Interval (CI) 1.42–2.23).
Next, we compared these men with chronic ADHD medication use to the population without ADHD and who had never received any ADHD medication. Our study identified 2,416,153 men matching this criterion. After matching a total of 17,217 patients were included in each group. The age of ADHD diagnosis occurred at 19.1 (10.8–27.4) years and a current age of 28.9 (22.7–35.1) in the study group compared to an age at index of 19.2 (11−27.4) with a current age of 29 ± (22.9–35.1) for the control group (Table 2). Analysis revealed that 1.20% of men who received at least 36 prescriptions of ADHD medications had a subsequent diagnosis of testosterone hypofunction compared to 0.69% of men without a history of ADHD or ADHD medication use (RR: 1.75, 95% CI 1.39–2.19).
Discussion
This large-scale retrospective claims database study explored the impact stimulant medication use for ADHD has on future testicular hypofunction risk. The key finding is an increased relative risk for a subsequent diagnosis of hypogonadism among adult male patients who have received long-term pharmaceutical treatment for ADHD with dextroamphetamine, lisdexamfetamine, amphetamine, or dexmethylphenidate. This increased risk was found to be significant when compared to both individuals without ADHD and those with ADHD not using stimulant medications.
Our findings have major similarities and a key difference from the previous large Taiwanese cohort study, which found an association between an ADHD diagnosis and testicular dysfunction [12]. Both studies utilized large cohorts to compare rates of testosterone hypofunction in individuals with ADHD retrospectively. While our study did not evaluate the risk of testosterone hypofunction for individuals with ADHD regardless of medication use, we found a significant risk increase among individuals with long-term stimulant use. This conflicts with findings from Wang et al., who found no associated increase in the risk of testicular dysfunction with methylphenidate use among individuals with ADHD but did find a significant increase in testicular dysfunction risk with an ADHD diagnosis. Notably, our study evaluated multiple stimulant ADHD medications in addition to methylphenidate. Wang et al. focused on the effects of hypogonadism on development and puberty, a much younger study population with nearly a 10-year lower mean age [12].
Yet, our findings support conclusions made by the case study from Abdalla et al. that identified a case of reversible pituitary failure leading to hypogonadism believed to be caused by amphetamine-dextroamphetamine [4]. Another case report by Ramasamy et al. from 2014 reported on a case of testicular failure and delayed puberty in a 20-year-old male with a 17-year history of methylphenidate use despite cessation of drug use years prior. This patient was treated with supplemental testosterone and human chorionic gonadotropin [5].
One longitudinal study explored this between 2005 and 2011 and found a significantly decreased growth rate among adolescents with a 3-year history of stimulant medication use [13]. This treatment length matches the criteria used in our study, but the patient population of focus was on adolescents rather than adults, like the research by Wang et al. [12]. With hypothalamic influence over puberty and development involving the release of GnRH and subsequently, LH and FSH [14], a shared physiological pathway may be involved. Methylphenidate has also been found to decrease testosterone levels in male rhesus monkeys, and that dose-dependent decrease in testicular size further supports this idea [8]. The same axis is involved in folliculogenesis in females and was found to be impacted in rats with methylphenidate exposure [9]. Wang et al. postulated higher dosing relative to mass compared to typical pharmaceutical dosing in many of these animal studies as a reason they did not translate to the results of their research [12]. Our study did not have access to medication dosage, so we cannot comment on the potential impact on gonadal functioning.
To our knowledge, this study is the first to identify a significant relative risk of hypogonadism and testicular hypofunction with long-term ADHD medication use. With the number of patients found in TriNet, the power of this analysis is a particular strength, with most current literature supporting our hypothesis consisting of case reports [4,5,6]. The study size also allowed for propensity matching to minimize treatment selection bias in this population. Limitations of this study design include the lack of information available on the cause of hypogonadism or hormone levels. Also, an inherent bias exists because medication status was not randomized among individuals in the study, and factors such as symptom severity could impact who received pharmaceutical treatment. Despite these drawbacks, the study’s results are significant in helping determine all risks associated with the drug that should be considered by both the patient and healthcare provider when prescribing stimulant medication for ADHD.
Conclusions
Long-term ADHD stimulant medication use in men was found to be associated with a significant increase in relative risk for a subsequent testicular hypofunction diagnosis. This difference was found when compared to both those with ADHD not using pharmaceutical therapy and those without ADHD. These results indicate that impaired gonadal function is a potential side effect of stimulant medications. Future studies should explore the exact physiologic pathways responsible for this effect in men and women and the impact of the medications on GnRH, FSH, LH, testosterone levels, and fertility. This will enhance understanding of the adverse effects of stimulant use in treating ADHD.
Data availability
All data generated or analyzed during this study is included in this published article.
References
Bitsko RH, Claussen AH, Lichstein J, Black LI, Jones SE, Danielson ML, et al. Mental Health Surveillance Among Children–United States, 2013-9. MMWR Suppl. 2022;71:1–42.
Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–23.
Caye A, Swanson JM, Coghill D, Rohde LA. Treatment strategies for ADHD: an evidence-based guide to select optimal treatment. Mol Psychiatry. 2019;24:390–408.
Abdalla TE, Kotsonis D, Best J, Ramasamy R, Wood E. Stimulant-induced pituitary failure and reversible Azoospermia. Cureus. 2021;13:e14269.
Ramasamy R, Dadhich P, Dhingra A, Lipshultz L. Case Report: Testicular failure possibly associated with chronic use of methylphenidate. F1000Res. 2014;3:207.
Akaltun İ. Report of a 14-year-old boy whose testosterone level decreased after starting on Methylphenidate. J Child Adolesc Psychopharmacol. 2016;26:181.
Danborg PB, Simonsen AL, Gøtzsche PC. Impaired reproduction after exposure to ADHD drugs: Systematic review of animal studies. Int J Risk Saf Med. 2017;29:107–24.
Mattison DR, Plant TM, Lin HM, Chen HC, Chen JJ, Twaddle NC, et al. Pubertal delay in male nonhuman primates (Macaca mulatta) treated with methylphenidate. Proc Natl Acad Sci USA. 2011;108:16301–6.
Chatterjee-Chakrabarty S, Miller BT, Collins TJ, Nagamani M. Adverse effects of methylphenidate on the reproductive axis of adolescent female rats. Fertil Steril. 2005;84(Suppl 2):1131–8.
Liu X, Herbison AE. Dopamine regulation of gonadotropin-releasing hormone neuron excitability in male and female mice. Endocrinology. 2013;154(1):340–50.
Babu SR, Sadhnani MD, Swarna M, Padmavathi P, Reddy PP. Evaluation of FSH, LH and testosterone levels in different subgroups of infertile males. Indian J Clin Biochem. 2004;19:45–9.
Wang LJ, Lee SY, Chou WJ, Lee MJ, Tsai CS, Lee TL, et al. Testicular function after long-term methylphenidate treatment in boys with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2019;29:433–8.
Poulton AS, Melzer E, Tait PR, Garnett SP, Cowell CT, Baur LA, et al. Growth and pubertal development of adolescent boys on stimulant medication for attention deficit hyperactivity disorder. Med J Aust. 2013;198:29–32.
Spaziani M, Tarantino C, Tahani N, Gianfrilli D, Sbardella E, Lenzi A, et al. Hypothalamo-Pituitary axis and puberty. Mol Cell Endocrinol. 2021;520:111094.
Funding
No financial assistance was received or utilized for this study.
Author information
Authors and Affiliations
Contributions
TK and CD created the idea and design for the study. TK collected and analyzed the study’s data. GOW, KB, CD, and TK were involved in the drafting and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was exempt from the Institutional Review Board because only de-identified patient records were utilized. Individually identifiable data was never collected, used, or transmitted.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ostdiek-Wille, G.P., Bavitz, K.C., Kohn, T.P. et al. Attention-deficit hyperactivity disorder medication use is associated with testosterone hypofunction–results from a national claims database analysis. Int J Impot Res 36, 403–407 (2024). https://doi.org/10.1038/s41443-023-00805-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-023-00805-2
This article is cited by
-
Commentary on “Attention-deficit hyperactivity disorder medication use is associated with testosterone hypofunction”
International Journal of Impotence Research (2024)