Abstract
Scrotal hematoma is a challenging complication of penile prosthesis surgery. We characterize the risk of hematoma formation with implementation of standardized techniques to mitigate hematomas and assess for any associated factors in a large multi-institutional penile implant cohort. This was a retrospective review from February 2018 to December 2020 of all patients who underwent inflatable penile prosthesis implantation at 2 high volume implant centers. Cases were defined as “complex” if they involved revision, salvage with removal/replacement, or were performed with concurrent penile, scrotal or intra-abdominal surgeries. The incidence of scrotal hematoma among primary and complex IPP recipients was measured and modifiable and innate risk factors associated with hematoma formation within the two cohorts were tracked. Of 246 men who underwent penile prosthesis surgery, 194 (78.9%) patients underwent primary implantation and 52 (21.1%) were complex. Although hematoma formers in the complex group had comparable drain outputs to primary patients on postoperative day 0 (66.8cc ± 32.5 vs 48.4 ± 27.7, p = 0.470) and postoperative day 1 (40.3cc ± 20.8vs 21.8 ± 11.3 p = 0.125), hematomas in the complex group had a higher propensity for OR evacuation (p = 0.03). Difference in duration of temporary device inflation between 2 (64, 26%) and 4 weeks (182, 74%) did not contribute to hematoma formation (p = 0.562). The incidence of postoperative hematoma formation in complex cases was 9.6% (5/52) and 3.6% in primary cases (7/194) (HR = 2.61, p = 0.072). Complex IPP surgery performed for revision or with ancillary procedures are more likely to result in clinically significant hematomas that require surgical management, suggesting a need for heightened caution in managing these individuals.
Similar content being viewed by others
Introduction
Penile prosthesis implantation is a highly beneficial surgical treatment option for medical refractory erectile dysfunction [1, 2]. Contemporary optimization of perioperative patient management has drastically improved device survival and minimized patient morbidity [3, 4]. Serious adverse events can occur during or following inflatable penile prosthesis (IPP) surgery [5, 6]. Scrotal hematoma formation after IPP surgery is a serious adverse event that remains relatively underexplored in the literature [7, 8].
Hematoma formation is an unfavorable complication with a wide variation in incidence, ranging from 0.2% to 22.2% in primary implant recipients [9,10,11,12,13]. Prevention of postoperative IPP bleeding complications correlates with shorter recovery time and decreased risk of device infection [7, 8]. Numerous strategies have been developed to help mitigate postoperative IPP scrotal bleeding, including running corporotomy closures, use of clotting agents at corporotomies, penoscrotal pressure dressing (e.g. “Mummy wrap”), complete or partial device inflation to minimize bleeding from the corporotomy, and/or closed suction device placement [8, 14]. Inconsistent adoption and reporting of these strategies limits evaluation of their efficacy. Hematomas in patients undergoing revision/replacement implantation or those having concomitant procedures have yet to be clearly defined.
We report hematoma rates in a large series of penile implant recipients that underwent standardized perioperative management from two experienced penile implant surgeons. We report associated risk factors for development of postoperative hematoma and hypothesize hematoma rates in “complex” implant recipients to be higher than virginal IPP patients. Further we delineate whether timing of device deflation in the postoperative recovery period influences hematoma formation.
Methods
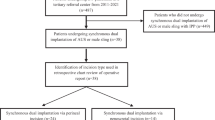
This is a multi-institutional, IRB approved retrospective study of hematoma formation in all three-piece IPP insertion cases performed between February 2018 to December 2020 by two penile implant surgeons. Primary cases were classified as IPP-naive patients, while complex cases were defined as single-component revision cases, entire device removal/replacement cases, or IPPs performed with concurrent procedures as listed in Table 1. The rationale for this classification system was based on surgeon knowledge and experience pertaining to surgical history altering normal tissue planes and concomitant surgery deviating from standard operation, with both introducing an opportunity for additional scrotal dissection and manipulation. All patients underwent placement of a three-piece IPP made by either Coloplast (Minneapolis, MN, USA) or Boston Scientific (Marlborough, MA, USA) with all penoscrotal surgical approaches included in the cohort analysis. Malleable penile implants were excluded.
Peri-operative factors, including timing of anticoagulation/antiplatelet therapy discontinuation, were based on American Urological Association (AUA) guideline recommendations as well as surgeon preference [15]. The suspension timeframe ranged from 3 to 7 days prior to surgery depending on the specific agent taken by the patient and the degree of surgical manipulation anticipated on a case-by-case basis.
Surgical technique
Intra-operative surgical techniques were standardized between institutions, including antimicrobial irrigation for hydrophilic-coated devices. All patients received a modified no-touch penoscrotal technique with small (<2 cm) corporotomies [16]. No other intraoperative technical complications (e.g corporal crossover, corporal perforation or urethral injury) occurred during the study period. All recipients received a 10 French Jackson Pratt (JP) scrotal closed suction drain (CSD) postoperatively, exiting superior and lateral to scrotum the groin through a stab incision. Patients underwent placement of a postoperative compression dressing applied to the penile shaft and scrotum according to the Mummy Wrap technique previously published by Henry [14].
Postoperative management
The standard protocol for immediate postoperative management was adjusted during the study period to adhere to institutional policy and CDC recommendations pertaining to SARS-CoV-2 coronavirus (COVID-19) [17]. Prior to COVID-19, the protocol for IPP recipients included a standardized 23-hour overnight hospitalization for observation with the CSD removed on postoperative day (POD) 1 prior to discharge based on volume of output (specifically with volumes <100cc/23 h). Following the onset of COVID-19, the protocol shifted to same-day discharge. As a result, patients were either instructed to a self-discontinuation of CSD at home on POD1 or scheduled for an office nurse visit for CSD removal between POD1–3. Most patients were left partially inflated (roughly 50–70% based on patient tolerance) during the first 4 weeks of recovery followed by device teaching and usage. A small subset of motivated patients underwent an expedited recovery pathway with device manipulation and teaching at 2 weeks post-operatively.
The decision to reinitiate anticoagulant/antiplatelet therapy was based on degree of intraoperative dissection and hemostasis at case conclusion but was not sooner than 72 h postoperatively. Extensive counseling was performed throughout the perioperative period regarding activity restrictions and modifications as well as the importance of complying with recommended compressive undergarments during the first few weeks of recovery.
Outcomes assessment
The main outcome measure of the study was development of postoperative hematoma. This was diagnosed by clinical examination, with or without confirmatory radiographic imaging. Other data points included time-frame of presentation from operative date, status of anticoagulation (AC) therapy and management modality. Conservative management of scrotal hematomas was attempted in all patients. Patients underwent surgical management if the performing surgeon felt evacuation would mitigate risk of device compromise or due to patient intolerance. Secondary measures collected included CSD outputs on POD0 and POD1, Visual Analog Score (VAS) pain scores and total morphine equivalents (TMEs) at interval periods during their hospitalization in Post-Anesthesia Care Unit (PACU) and on POD0 and POD1. Further, demographic, intraoperative, and postoperative data was compared between the cohort of men who developed hematomas and those who did not, along with comparative analyses between virginal and complex IPP cases.
Statistical analyses were performed with STATA Statistical Package Version 17.0 (StataCorp, College Station, TX: StataCorp LLC). Correlation between categorical variables was made using the Chi-square test. T tests were used when comparing means for categorical variables with only two groups. Differences between the hematoma and non-hematoma cohorts were evaluated using the Mann–Whitney U test (continuous variables) and Fisher’s exact test (categorical variables). A P value of < 0.05 was considered statistically significant.
Results
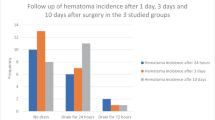
A total of 246 patients met inclusion criteria during the study period and were included in the final analysis. 194 (78.9%) qualified as primary recipients while 52 (21.1%) were complex. Our cohort was predominately middle-aged (50–79) and obese (BMI > 30). By protocol, 179 (72.8%) CSDs were removed on POD1 and 64 (26.0%) removed between POD1-POD3 with 3 (1.2%) of patients lacking sufficient documentation to determine CSD removal. The average CSD output from 0–12 h was 49.7cc (±47.7) and 21.7cc (±25.1) from 12 to 24 h. Postoperative device deactivation occurred with 64 (26.0%) patients at 2 weeks and 182 (74.0%) at 4 weeks. Of the cohort, 12 patients developed postoperative scrotal hematoma, presenting at a median of 12.5 days postoperatively (POD1–32 days). A majority were diagnosed based on clinical examination alone (n = 8, 66.7%). Table 2 summarizes the study population patient characteristics and Table 3 highlights comorbidities by group. No specific comorbidity was associated with overall risk of hematoma formation.
Patients who ultimately developed scrotal hematomas—in both primary and complex cases— experienced increased pain on the postoperative night immediately following surgery compared to non-hematoma formers with a VAS score of 5.3 ± 2.4 vs 3.2 ± 1.1 (p = 0.012). In assessing drain output as a predictive factor for subsequent hematoma formation, CSD output in the acute postoperative period of POD0–1 was comparable amongst the two groups with 66.8cc ± 32.5 vs 48.4cc ± 27.7 on POD0 (p = 0.470) and 40.3cc ± 21.8 vs 20.7cc ± 11.3 on POD1 (p = 0.125). All hematomas captured during the study period occurred prior to COVID-19 and no further analysis was therefore performed assessing the impact of protocol changes—hospitalization vs same-day surgery—had on the incidence of this postoperative complication.
The incidence of postoperative hematoma formation in complex cases (5/52, 9.6%) was more than double that of primary cases (7/194, 3.6%) but did not reach significance (HR = 2.61, p = 0.072). Of primary cases with scrotal hematomas, four (80%) were anticoagulated and had resumed their medication at least several days prior to presentation while 2 (40%) complex cases with scrotal hematoma were anticoagulated at time of presentation. Complex IPP hematomas had a higher propensity for OR evacuation with all 5 (100%) patients requiring operative intervention compared to 2 (28.6%) primary patients (p = 0.028). In the complex patients who underwent operative intervention, all were taken to the OR within 24 h after diagnosis of scrotal hematoma due to patient discomfort. Of the complex, anticoagulated IPP patients, both were late presentation cases (>20 days postoperatively) and had been cleared to resume their oral AC within 7 days of hematoma formation. There were no documented device infection, failure or revision/explant documented in the 12 hematoma cases by end of study period. Difference in duration of temporary device inflation between 2 and 4 weeks did not contribute to hematoma formation (p = 0.562). However, complex IPP patients who developed a scrotal hematoma in a delayed fashion were more likely to have had device deactivation at 4 weeks and require OR decompression (p = 0.026).
Discussion
This study investigated the impact of preoperative, intraoperative and postoperative factors on hematoma formation following IPP implantation. We found that the risk of developing a clinically significant scrotal hematoma requiring operative evacuation was statistically higher in complex cases (revisions, removal/replacement or concomitant surgeries) while primary cases were successfully managed expectantly. All complex IPP patients who required operative evacuation were taken to surgery within 24 h of diagnosis and successfully maintained all device components without secondary device failure or infection [12,13,14,15,16,17,18]. No intraoperative factors were linked to hematoma formation in either cohort. Furthermore, primary IPP recipients were more likely to be on anticoagulants/antiplatelet therapy at the time of presentation with a clinically significant scrotal hematoma than were complex counterparts.
Despite advancements in prosthetic devices, surgical techniques and postoperative management strategies over the past decade, hematoma formation remains a major postoperative complication [3, 19,20,21,22]. Our series demonstrates an overall rate of 5% hematoma formation, and we further identify complex IPP cases as a risk factor for clinically significant scrotal hematoma formation with an incidence of 10% among complex IPP cases [23]. Studies assessing hematoma formation following augmentation mammoplasty or revision arthroplasty may provide important insight into these findings. In breast surgery, hematoma after breast enhancement is attributed to a chronic inflammatory reaction to the implant with bleeding in response to the rupture of vulnerable vessels in the tissue capsule [24]. Similarly, revision arthroplasty has reported that mechanical friction between the textured surface of the implant and the high vascular capsule may result in an intracapsular hematoma formation [25, 26]. Analogously, a majority of complex IPP cases included removal of a previously placed implant along with its pseudo-capsule. Due to the capsular formation, complex procedures inherently require additional scrotal manipulation with an increased surface area involved, introducing the opportunity for increased bleeding. The lower threshold to intervene operatively on complex hematomas highlighted in our study may be due in part to high-volume prosthetic surgeons understanding of these principles and recognizing the benefit of mitigating the risk prolonged conservative recovery may have on subsequent delayed infection and need for further salvage procedures.
Other surgical specialties and subspecialties, including neurosurgery, orthopedics, and urologic oncology, have examined means to reduce postoperative bleeding complications associated with a variety of procedures. The use of hemostatic agents in conjunction with standard hemostatic strategies has demonstrated promising results in this area [27,28,29]. In prosthetic surgery specifically, the use of oxidized regenerated cellulose pledgets at corporotomy sites for primary IPPs has demonstrated successful reduction in postoperative scrotal bleeding without risk of device infection or explanation [30, 31]. This series highlights contrasting management strategies utilized for hematoma formation following complex and primary cases. An initial strategy consisting of conservative management proved successful in the primary IPP setting. Importantly, expectant management did not lead to device failure or infection necessitating secondary procedure for revision or removal/replacement during the study period.
Implanters ought to have a heightened awareness when confronting a “complex” penile implant recipient for several reasons. First, our work demonstrates these patients to face a substantially increased risk of hematoma formation than virginal counterparts. Second, most hematoma patients in the complex penile implant cohort ultimately developed a clinically actionable hematoma within 72 h of implantation, despite experiencing acceptable postoperative drain outputs within the first 24 h. In this hematoma cohort, all ultimately proceeded with definitive surgical decompression within 24 h of diagnosis based on patient symptoms and surgeon concern that delayed intervention would place the implant at untoward risk. Due to the surgical take-back risk and patient morbidity, complex IPP recipients may best be served by referral to specialized centers with experienced surgeons [4, 21, 32].
Standard approaches to minimize hematomas postoperatively after IPP include watertight corporotomy closure, meticulous hemostasis, use of a Mummy wrap, and partial device inflation [3, 9, 20, 33]. Other factors remain controversial. Some have argued that early removal of CSD (less than 72 h), reduced degree of device inflation, and early deactivation increase risk of bleeding complications by introducing reduced tamponade and increased scrotal manipulation [10, 34]. We did not identify any intraoperative factor as an independent risk factors for hematoma formation in either primary or complex hematoma group40. Nevertheless, these results provide compelling evidence that early and swift action with surgical intervention is advisable in management of hematoma formation in complex IPP recipients.
Scrotal hematoma formation following penile implant surgery has often been attributed to premature anticoagulation resumption, noncompliance with postoperative activity limitations, and scrotal support instructions. We found the risk of bleeding associated with blood thinner status appeared higher in primary cases. Current recommendations regarding anticoagulation/antiplatelet therapy in prosthetic surgery are based on evidence derived from other surgical specialties. The AUA has published guidelines pertaining to shock wave lithotripsy, ureteroscopy, percutaneous nephrolithotomy as well as higher risk procedures including radical prostatectomy and partial nephrectomy [15]. Suspension plans are based on the risk for hemorrhagic complications secondary to the procedure (low or high) paired with the patient’s cardiovascular risk category. In general, it is recommended that in elective, higher-risk procedures, medications like warfarin or clopidogrel be stopped at least 5 days preoperatively while novel ACs like rivaroxaban apixaban and dabigatran be discontinued 2–5 days prior. Anticoagulation/antiplatelet guidelines for prosthetic surgery do not exist beyond stating therapies can be resumed based on clinician gestalt [15]. Furthermore, the AUA guidelines on the surgical management of ED do not provide clarity in labeling implant surgery as either a low or high-risk procedure for hemorrhagic complications [15]. This study suggests judicious consideration of prolonged anticoagulation suspension may be warranted to reduce hematoma formation in the primary setting.
Our study is not without limitations. As a retrospective analysis, the data may have captured the clinically significant scrotal hematomas without identifying the true incidence of hematoma within the cohort. Second, the time-frame of our study was from February 2018 to December of 2020, which includes the COVID-19 pandemic lockdown period. As such, operative volume was significantly lower and standardized protocol shifted to adhere to clinical care changes. Furthermore, the lockdown’s impact on postoperative activity levels during the recovery phase is unknown.
Conclusions
We found that complex IPP surgeries have a high rate of both clinically significant postoperative scrotal hematomas and operative re-intervention. These results provide implanters with valuable data to improve patient counseling and medical optimization prior to penile implant surgery.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Brantley Scott F, Bradley WE, Timm GW. Management of erectile impotence use of implantable inflatable prosthesis. Urology. 1973;2:80–2. https://doi.org/10.1016/0090-4295(73)90224-0.
Levine LA, Becher E, Bella A, Brant W, Kohler T, Martinez-Salamanca JI, et al. Penile prosthesis surgery: current recommendations from the international consultation on sexual medicine. J Sex Med. 2016;13:489–518. https://doi.org/10.1016/j.jsxm.2016.01.017.
Chung E, Bettocchi C, Egydio P, Love C, Osmonov D, Park S, et al. The International Penile Prosthesis Implant Consensus Forum: clinical recommendations and surgical principles on the inflatable 3-piece penile prosthesis implant. Nat Rev Urol. 2022;19:534–46. https://doi.org/10.1038/s41585-022-00607-z.
Ohl DA, Brock G, Ralph D, Bogache W, Jones L, Munarriz R, et al. Prospective evaluation of patient satisfaction, and surgeon and patient trainer assessment of the coloplast titan one touch release three-piece inflatable penile prosthesis. J Sex Med. 2012;9:2467–74. https://doi.org/10.1111/j.1743-6109.2012.02819.x.
Palma-Zamora I, Sood A, Dabaja AA. 30-day adverse event rates following penile prosthesis surgery: an American College of Surgeons National Surgical Quality Improvement Program based evaluation. Transl Androl Urol. 2017;6 Suppl 5:S767–73. https://doi.org/10.21037/tau.2017.04.25.
Vakalopoulos I, Kampantais S, Gkagkalidis K, Ioannidis S, Dimitriadis G, Patsialas C, et al. Complications of inflatable penile prostheses implantation classified according to the modified clavien system. Adv Androl. 2014;2014:1–5. https://doi.org/10.1155/2014/127693.
Wilson S, Cleves M, Delk J. Hematoma formation following penile prothesis implantation: to drain or not to drain. J Urol. 1996;55:634A.
Osmonov D, Ragheb A, Otero J, Bettocchi C, Van Renterghem K, Jünemann K. et al. To drain or not to drain an inflatable penile prosthesis implantation? A multi-institutional experience tracking scrotal hematoma and infection occurrence. Eur Urol. 2021;79 Suppl 1:S687–8. https://doi.org/10.1016/s0302-2838(21)00880-0.
Wilson SK, Delk JR, Salem EA, Cleves MA. Long-term survival of inflatable penile prostheses: single surgical group experience with 2,384 first-time implants spanning two decades. J Sex Med. 2007;4:1074–9. https://doi.org/10.1111/j.1743-6109.2007.00540.x.
Mirheydar H, Zhou T, Chang DC, Hsieh TC. Reoperation rates for penile prosthetic surgery. J Sex Med. 2016;13:129–33. https://doi.org/10.1016/j.jsxm.2015.11.013.
Diego P, Mariangela P, Carlotta P. Penile prosthesis surgery in Italy: personal experiences, complications and considerations after 552 cases. Int Arch Urol Complicat. 2018;4:1–9. https://doi.org/10.23937/2469-5742/1510050.
Minervini A, Ralph DJ, Pryor JP. Outcome of penile prosthesis implantation for treating erectile dysfunction: experience with 504 procedures. BJU Int. 2006;97:129–33. https://doi.org/10.1111/j.1464-410X.2005.05907.x.
Natali A, Olianas R, Fisch M. Penile implantation in Europe: Successes and complications with 253 implants in Italy and Germany. J Sex Med. 2008;5:1503–12. https://doi.org/10.1111/j.1743-6109.2008.00819.x.
Henry GD. The Henry Mummy WrapTM and the Henry Finger SweepTM surgical techniques. J Sex Med. 2009;6:619–22. https://doi.org/10.1111/j.1743-6109.2008.01200.x.
Culkin DJ, Exaire EJ, Green D, Soloway MS, Gross AJ, Desai MR, et al. Anticoagulation and antiplatelet therapy in urological practice: ICUD/AUA review paper. J Urol. 2014;192:1026–34. https://doi.org/10.1016/j.juro.2014.04.103.
Eid JF. No-touch technique. J Sex Med. 2011;8:5–8. https://doi.org/10.1111/j.1743-6109.2010.02137.x.
COVID-19: Guidance for triage of non-emergent surgical procedures. American College of Surgeons. 2020. https://www.facs.org/for-medical-professionals/covid-19/clinical-guidance/triage/. Accessed 13 Oct 2022.
Garber BB, Bickell M. Delayed postoperative hematoma formation after inflatable penile prosthesis implantation. J Sex Med. 2015;12:265–9. https://doi.org/10.1111/jsm.12728.
Henry GD, Karpman E, Brant W, Christine B, Kansas BT, Khera M, et al. The who, how and what of real-world penile implantation in 2015: the PROPPER registry baseline data. J Urol. 2016;195:427–33. https://doi.org/10.1016/j.juro.2015.07.109.
Garber BB. Inflatable penile prosthesis: site-specific malfunction analysis. Int J Impot Res. 2003;15:22–5. https://doi.org/10.1038/sj.ijir.3900942.
Onyeji IC, Sui W, Pagano MJ, Weinberg AC, James MB, Theofanides MC, et al. Impact of surgeon case volume on reoperation rates after inflatable penile prosthesis surgery. J Urol. 2017;197:223–9. https://doi.org/10.1016/j.juro.2016.08.083.
O’Rourke TK, Erbella A, Zhang Y, Wosnitzer MS. Prevention, identification, and management of post-operative penile implant complications of infection, hematoma, and device malfunction. Transl Androl Urol. 2017;6:S832–48. https://doi.org/10.21037/tau.2017.06.07.
Henry GD, Donatucci CF, Conners W, Greenfield JM, Carson CC, Wilson SK, et al. An outcomes analysis of over 200 revision surgeries for penile prosthesis implantation: a multicenter study. J Sex Med. 2012;9:309–15. https://doi.org/10.1111/j.1743-6109.2011.02524.x.
Kaoutzanis C, Winocour J, Gupta V, Kumar NG, Sarosiek K, Wormer B, et al. Incidence and risk factors for major hematomas in aesthetic surgery: analysis of 129,007 patients. Aesthet Surg J. 2017;37:1175–85. https://doi.org/10.1093/asj/sjx062.
Matsuda S, Kaku N, Tabata T, Tsumura H. Progressive osteolysis with hematoma following revision total hip arthroplasty using hydroxyapatite mesh: a case report. J Orthop Case Rep. 2018;8:25–8. https://doi.org/10.13107/jocr.2250-0685.1142.
Galat DD, McGovern SC, Hanssen AD, Larson DR, Harrington JR, Clarke HD. Early return to surgery for evacuation of a postoperative hematoma after primary total knee arthroplasty. J Bone Joint Surg Am. 2008;90:2331–6. https://doi.org/10.2106/JBJS.G.01370.
Krzastek SC, Smith R. An update on the best approaches to prevent complications in penile prosthesis recipients. Ther Adv Urol. 2019;11:1–9. https://doi.org/10.1177/1756287218818076.
Wang JQ, Chen LY, Jiang BJ, Zhao YM. Oxidized regenerated cellulose can reduce hidden blood loss after total hip arthroplasty: a retrospective study. J Invest Surg. 2019;32:716–22. https://doi.org/10.1080/08941939.2018.1458166.
Sundaram CP, Keenan AC. Evolution of hemostatic agents in surgical practice. Indian J Urol. 2010;26:374–8. https://doi.org/10.4103/0970-1591.70574.
Wolfe AR, Davenport MT, Rozanski AT, Shakir NA, Ward EE, West ML, et al. An update on oxidized regenerated cellulose (fibrillarTM) in reducing postoperative corporal bleeding following inflatable penile prosthesis surgery. Transl Androl Urol. 2020;9:43–9. https://doi.org/10.21037/tau.2019.08.05.
Cohen SD, Francois Eid J. Hemostatic matrix during corporotomy closure. J Sex Med. 2014;11:869–72. https://doi.org/10.1111/jsm.12510.
Henry GD, Kansal NS, Callaway M, Grigsby T, Henderson J, Noble J, et al. Centers of excellence concept and penile prostheses: an outcome analysis. J Urol. 2009;181:1264–8. https://doi.org/10.1016/j.juro.2008.10.157.
Montague DK. Penile prosthesis corporotomy closure: a new technique. J Urol. 1993;150:924–5. https://doi.org/10.1016/S0022-5347(17)35650-1.
Apoj M, Rodriguez D, Barbosa P, Pan S, Rajender A, Biebal M, et al. Closed suction drain outputs at 12 and 24 h after primary three-piece inflatable penile prosthesis surgery. Int J Impot Res. 2020;32:117–21. https://doi.org/10.1038/s41443-019-0130-2.
Author information
Authors and Affiliations
Contributions
AEB designed the work that led to submission, contributed to data extraction and drafted the manuscript. AS and RP contributed to data extraction and provided feedback on the report; DS helped write the manuscript; JS and MSG conceived the work that led to the submission, revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
Consultants for Coloplast (JS and MG) and Boston Scientific (JS).
Ethical approval
Approval by Einstein Medical Center’s Institutional Review Board (IRB) was granted prior to initiation of retrospective data collection.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Braun, A.E., Swerdloff, D., Sudhakar, A. et al. Defining the incidence and management of postoperative scrotal hematoma after primary and complex three-piece inflatable penile prosthesis surgery. Int J Impot Res (2023). https://doi.org/10.1038/s41443-023-00697-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41443-023-00697-2
This article is cited by
-
Perioperative outcomes of penile prosthesis implantation in Germany: results from the GRAND study
International Journal of Impotence Research (2023)
-
Commentary: Value of prolonged scrotal drainage after penile prosthesis implantation: a multicentre prospective nonrandomized pilot study
International Journal of Impotence Research (2023)