Abstract
We previously showed that castration of rats reduced erectile function over time; when testosterone replacement therapy was started 4 weeks after castration, erectile function improved. In this study, we examined the mechanism of improvement in erectile function following testosterone replacement therapy in rats. Thirty 12-week-old rats were divided into castrated (Cast), castrated with subcutaneous administration of testosterone (Cast + T), and sham (Sham) groups. Erectile function and mRNA and protein expression were evaluated in the rats by using standard methods. To assess erectile function, we measured the intracavernosal pressure, mean arterial pressure, mRNA expression of endothelial growth factors, and protein expression of endothelial nitric oxide synthase (eNOS). The intracavernosal pressure/mean arterial pressure ratio was significantly lower in the Cast group, and testosterone administration significantly improved (P = 0.017). Compared to the Cast group, the Cast+T group exhibited significantly increased mRNA expressions of vascular endothelial growth factor A (VEGF-A), intercellular adhesion molecule 1 (ICAM-1), transforming growth factor-β (TGF-β), nerve growth factor (NGF), α-smooth muscle actin (α-SMA), caveolae associated protein 1 (Cavin-1), Cavin-2, Cavin-3, sirtuin 1 (Sirt-1), sphingosine-1-phosphate 1 (S1P1), S1P2, and S1P3 and eNOS protein expression. Testosterone replacement therapy improved erectile function in castrated rats by increasing growth factors and eNOS protein.
Similar content being viewed by others
Introduction
Testosterone plays an important role in sexual function, such as erection and ejaculation, and the development of libido. However, blood testosterone in men decreases with age; by the age of 80 years, the testosterone secretion rate is approximately half that of young men [1]. Decreased testosterone has been reported to induce apoptosis in smooth muscle cells [2] and reduce nitric oxide (NO) expression in the endothelium and neurons [3]. It also causes pathogenic changes, such as the promotion of fat cell accumulation [4, 5] and placement of elastic fibers and connective tissue in the corpus cavernosum structure, which contribute to erectile dysfunction (ED) [6]. These factors cause sexual function to decline as testosterone declines, leading to the development of ED [7].
A meta-analysis study demonstrated that testosterone replacement therapy improved sexual function in patients with decreased sexual function due to testosterone deficiency [8]. In animal experiments, erectile function was restored by testosterone replacement therapy in castrated rats [9, 10]. However, because testosterone replacement therapy is performed immediately after castration in most animal experiments, this therapy may not improve but rather maintain erectile function. In clinical practice, testosterone replacement therapy is administered to patients with testosterone deficiency and impaired erectile function. Therefore, in animal experiments, it is necessary to wait for a period after castration until erectile function declines before beginning testosterone replacement therapy to determine the effects of this therapy. We previously showed that castration of rats continued to reduce erectile function over time, with a significant decrease compared to in the control group at 4 weeks [11]. Additionally, when testosterone replacement therapy is started at 4 weeks after castration, erectile function improves with the improvement of the corpus cavernosum tissue structure over time, with significant effects observed at 8 weeks compared to in the castration group [12]. Studies are also needed to clarify the mechanism of improvement of erectile function following testosterone replacement therapy. We predicted that testosterone replacement therapy affects growth factors that control the corpus cavernosum structure. In this study, we examined the mechanism of improvement in erectile function by testosterone replacement therapy in rats.
Material and methods
Animals and treatment protocols
Thirty 12-week-old male Wistar/ST rats were purchased from Japan SLC, Inc. (Hamamatsu, Japan). All experimental protocols were approved by the ethics review board of Nagoya City University and conducted in accordance with our institutional standards for the care and use of animals (H25-P-09). The rats were kept in a temperature- and humidity-controlled room, with a 12-h/12-h light/dark cycle and free access to normal water.
Treatment protocols
Rats were assigned to the following groups: castrated (Cast, n = 10), castrated with subcutaneous administration of testosterone (Cast + T, n = 10), and sham (Sham, n = 10) (Fig. 1A). Rats in the Cast and Cast+T groups were castrated as reported previously [11, 12]. In the Sham group, rats underwent sham laparotomy, and the incision was sutured. In the Cast+T group, rats were administered monthly doses of testosterone undecanoate (25 mg/kg; Matrix Scientific, Elgin, SC, USA) subcutaneously starting 4 weeks after the operation for 8 weeks. The testosterone solution contained testosterone undecanoate dissolved in a vehicle composed of sesame oil (Nacalai Tesque, Kyoto, Japan) and 0.01% benzyl alcohol (Wako Pure Chemical Industries, Osaka, Japan). The Sham and Cast groups were injected with the vehicle on the same schedule. In this study, because of castration surgery, we could distinguish by the morphology of the rats, and we did not perform a blind test.
A Experimental design. Rats were assigned to the following groups: castrated (Cast), castrated with subcutaneous testosterone (Cast + T), and sham (Sham). Sham rats underwent sham laparotomies, and the incision was sutured. In the Cast+T group, rats were administered daily subcutaneous doses of testosterone undecanoate (25 mg/kg/month) starting 4 weeks after the operation for 8 weeks. After this period, the rats were subjected to erectile function testing in vivo and in vitro. B Total testosterone levels in serum. Data are presented as a box-and-whisker plot (n = 4). **P < 0.01 vs. each group using analysis of Tukey test.
Blood samples and measurement of biological parameters
Blood samples were obtained from all rats via the vena cava. After coagulation and centrifugal separation at 800 × g for 20 min at 4 °C, serum samples were stored at −80 °C until analysis. The serum testosterone level was measured using a Testosterone ELISA kit (ALPCO, Salem, NE, USA). The absorbance was measured using a Nivo 3 S (PerkinElmer, Waltham, MA, USA).
Examination of erectile function
Intracavernosal pressure (ICP) was measured by electrical stimulation as previously reported [11,12,13]. Rats from each group (n = 6) were anaesthetized using isoflurane with an inhalation anesthesia apparatus (Nakazawa Seisaku-sho, Funabashi, Japan). The mean arterial pressure (MAP) was measured from the carotid artery, and the ICP was measured from the left crus of the corpus cavernosum using a 23-G needle. A data acquisition board (PowerLab 2/26, ADInstruments Pty. Ltd., New South Wales, Australia) with an amplifier was used to monitor these pressures via a pressure transducer. The cavernous nerve was electrically stimulated using stainless steel bipolar wire electrodes (Unique Medical, Osaka, Japan) connected to a pulse generator (Nihon Kohden, Tokyo, Japan). The parameters were 1 min at 5 V, 1–16 Hz, and a square wave duration of 5 ms. The erectile function of rats was evaluated using the maximum ICP/MAP ratio.
Real-time quantitative polymerase chain reaction
Real-time quantitative polymerase chain reaction (qRT-PCR) analysis was performed as previously reported [13]. Total RNA was extracted from the corpus cavernosum samples using the TriPure Isolation Reagent (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions (n = 4). RNA concentration and quality were measured using spectrophotometric analysis at 260 and 280 nm. Total RNA (1 µg) was reverse transcribed to complementary DNA (cDNA) using a ReverTra Ace-α kit (Toyobo, Osaka, Japan). cDNA (1 µL) was used for reverse transcription-polymerase chain reaction (RT-PCR) using KAPA SYBR Fast qPCR Kit (Roche, Basel, Switzerland). The reaction cycle consisted of incubations for 50 °C for 2 min, 95 °C for 10 min, 40 cycles each of 95 °C for 15 s and 60 °C for 1 min, and 95 °C for 15 s using a CFX96 Real-time System (Bio-Rad, Hercules, CA, USA). The forward and reverse PCR primers are shown in Table 1. Target gene expression was quantified relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression by using the comparative cycle threshold (CT) method.
Western blot analysis
Western blot analysis was performed as previously reported [13]. Total protein was extracted from the corpus cavernosum using PRO-PREPTM (iNtRON Biotechnology, Gyeonggi-do, Korea) according to the manufacturer’s instructions (n = 4). The protein concentration was quantified using PierceTM BCA Protein Assay Reagent (Thermo Fisher Scientific, Waltham, MA, USA). Separated total protein (25 μg) was transferred onto polyvinylidene difluoride membranes (Merck, Kenilworth, NJ, USA). Mouse monoclonal anti-endothelial nitric oxide synthase (eNOS) (1:100; Abcam, Cambridge, UK), mouse monoclonal anti-β-actin (1:5,000; Sigma), and anti-mouse immunoglobulin G conjugated with horseradish peroxidase (1:20,000; GE Healthcare, Little Chalfont, UK) were used for detecting protein. The protein expression was evaluated with the bands using enhanced chemiluminescence (Amersham Biosciences, Amersham, UK) and ImageQuant LAS 4000 mini (GE Healthcare). Pictures were analyzed using the ImageJ 1.43 u software (NIH, Bethesda, MD, USA).
Statistical analyses
Data were expressed as the mean ± standard error of the mean. Statistical significance was determined by a one-way analysis of variance following Tukey comparison test using in EZR on R commander ver. 1.41 [14]. P values <0.05 were considered to indicate statistically significant results.
Results
Biological parameters
Figure 1 shows the serum total testosterone levels after testosterone administration. Cast rats showed significantly lower levels of serum total testosterone compared to their Sham counterparts (Sham, 2.52 ± 0.64 ng/mL; Cast, 0.10 ± 0.02 ng/mL, P = 0.0057). However, Cast+T and Sham rats exhibited similar levels of testosterone (Cast + T, 2.22 ± 0.24 ng/mL, P = 0.0012).
Erectile function
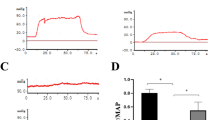
Figure 2A shows the representative tracings of the ICP and atrial pressure changes during electrical stimulation of the cavernous nerve. ICPs in the L-OHP group were lower than those in the Sham group. Figure 2B shows the ICP/MAP ratios for different stimulation frequencies. The Cast group (0.31 ± 0.05) exhibited significantly lower ratios than the Sham group (0.67 ± 0.08; P = 0.0069) at 16 Hz. The Cast+T group (0.70 ± 0.07) showed significantly higher ratios than the Cast group (P = 0.017).
A Representative tracings of intracavernous pressure (ICP) and arterial pressure changes during electrical stimulation (16 Hz) of the cavernous nerve in Sham, Cast and Cast+T rats. B Erectile function according to the ICP/mean arterial pressure (MAP) ratio. Data are presented as a box-and-whisker plot (n = 6). *P < 0.05, **P < 0.01 vs. each group using analysis of Tukey test.
mRNA expression analysis
Figure 3 shows the mRNA expression levels in the corpus cavernosum. The expression of vascular endothelial growth factor A (VEGF-A: P = 0.0082 and P = 0.0058), intercellular adhesion molecule 1 (ICAM-1: P = 0.0018 and P = 0.0014), transforming growth factor-β (TGF-β: P = 0.0049 and P = 0.0035), and nerve growth factor (NGF: P = 0.0133 and P = 0.0164) were higher in the Cast+T group than in the Sham and Cast groups. The expression of α-smooth muscle actin (α-SMA: P = 0.0095 and P = 0.0060), caveolae associated protein 1 (Cavin-1: P = 0.0098 and P = 0.0096), Cavin-2 (P = 0.0311 and P = 0.0301), and Cavin-3 (P = 0.0491 and P = 0.0436) were higher in the Cast+T group than in the Sham and Cast groups. Further, the expression of sirtuin 1 (Sirt-1: P = 0.0063 and P = 0.0070), sphingosine-1-phosphate 1 (S1P1: P = 0.0048 and P = 0.0063), S1P2 (P = 0.0068 and P = 0.0062), and S1P3 (P = 0.0072 and P = 0.0031) were higher in the Cast+T group than in the Sham and Cast groups.
eNOS protein analysis
Figure 4 shows the eNOS protein expression levels. The eNOS protein level in the Cast group was significantly lower than that in the Sham group (P = 0.0424). eNOS expression tended to be higher in the Cast+T group than in the Cast group (P = 0.0933).
Discussion
We investigated a mechanism for improving erectile function using testosterone replacement therapy in castrated rat models. Castrated rats were treated with testosterone undecanoate for 8 weeks. Based on blood testosterone measurement using enzyme-linked immunosorbent assay, the blood testosterone level was significantly reduced by castration. In addition, testosterone administration significantly increased the blood testosterone level compared to in the Cast group and was similar to that in the Sham group, indicating that the testosterone dose was appropriate for testosterone replacement therapy.
According to a previous study, testosterone administration improved the corpus cavernosum structure, with consequent improvements in the erectile function of castrated rats; [12] therefore, in this study, we investigated the mRNA expression of growth factors involved in cell proliferation. VEGF-A is an important growth factor that acts specifically on vascular endothelial cells during angiogenesis and promotes lumen formation and the migration of endothelial cells [15, 16]. Testosterone has been reported to stimulate the secretion of VEGF and potent angiogenesis and vasodilators in castrated animal glandular epithelial cells [17, 18].
We observed a significant increase in VEGF mRNA expression in the Cast+T group compared to in the Cast group. Thus, testosterone replacement therapy appears to increase VEGF and improve the corpus cavernosum structure. In addition, ICAM-1 has been reported to activate angiogenesis [16, 19]. ICAM-1 is an intercellular adhesion molecule in vascular endothelial cells that is induced and expressed in endothelial cells by VEGF [20]. Based on our results, ICAM-1 mRNA expression was significantly increased in the Cast+T group compared to in the Cast group. Thus, testosterone replacement therapy may improve the corpus cavernosum structure by increasing ICAM-1. Furthermore, the increased expression of factors constituting the corpus cavernosum, such as TGF-β and NGF, was observed following administration of testosterone.
With increases in the levels of these growth factors in the corpus cavernosum, increases in α-SMA, Cavin, Sirt-1, and S1P were also observed. In addition, eNOS protein was increased following testosterone administration. The caveolae has been reported to play an important role in signal transduction [21] and to mediate increases in endocytosis, which is associated with activation of NO production by eNOS [22]. Caveolae is a target for the S1P1 receptor and contributes to S1P-induced eNOS activation [23]. Studies using a knockout mouse model showed that Cavin-1 is required for formation of the caveolae structures [24], and Cavin-2 and Cavin-3 are also associated with caveolae [25, 26]. Loss of Cavin-3 reduces Cavin-1 in smooth muscle of blood vessels and bladder by approximately 40% and the amount of caveolae in smooth muscle by 40–45% [27]. According to our results, the mRNA expression levels of Cavin-1, Cavin-2, and Cavin-3 were significantly increased in the Cast+T group compared to in the Cast group. Therefore, testosterone replacement therapy may improve erectile function by increasing the expression of Cavin-1, Cavin-2, and Cavin-3, forming and regulating caveolae, and increasing eNOS.
In addition, testosterone replacement therapy increased Sirt-1 mRNA expression. Sirtuins have been reported to be involved in NO production via eNOS [28, 29], and the increase in Sirt-1 may have increased eNOS. S1P is involved in controlling NO production [23, 30,31,32]. S1P is produced in endothelial cells and is involved in numerous processes such as cell proliferation, apoptosis suppression, and neurotransmitter release [30]. It has been reported that S1P acts on the S1P receptor present in vascular endothelial cells to activate eNOS and induce NO production [23, 31, 32]. Additionally, VEGF specifically induces the expression of S1P1 receptor, enhances the intracellular signal transduction response to S1P, enhances S1P-mediated vascular relaxation, and significantly stimulates angiogenesis synergistically with S1P [33, 34]. In this study, the mRNA expression levels of S1P1, S1P2, and S1P3 were significantly increased in the Cast+T group compared to in the Cast group. Based on these results, testosterone replacement therapy appears to activate eNOS by increasing the expression of growth factors and inducing NO production to improve erectile function.
Our results are limited to the mRNA level, and further studies are needed to directly measure NO production at the protein level. In addition, it is unclear whether the blood concentration in rats represents that in humans. This study investigated the effects of testosterone on castrated rats; however, in humans, testosterone is rarely administrated to patients who have undergone castration surgery. Since the results of this experiment show the effect of testosterone administration on a rapid decrease in testosterone in the castrated rats, it is necessary to examine with another testosterone deficiency animal model. However, testosterone replacement therapy may be effective for treating ED (Fig. 5). In addition, ED is rarely treated with testosterone alone in older men, with phosphodiesterase-5 (PDE-5) inhibitors often used in combination. It has also been reported that testosterone changes the expression of PDE-5 [35], and it will be useful to investigate the effects of the combined use of testosterone and PDE-5 inhibitors in the future.
Conclusion
Testosterone replacement therapy improved the erectile function of castrated rats by increasing growth factors and eNOS protein.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Amplified nocturnal luteinizing hormone (LH) secretory burst frequency with selective attenuation of pulsatile (but not basal) testosterone secretion in healthy aged men: possible Leydig cell desensitization to endogenous LH signaling–a clinical research center study. J Clin Endocrinol Metab. 1995;80:3025–31.
Bandyk MG, Sawczuk IS, Olsson CA, Katz AE, Buttyan R. Characterization of the products of a gene expressed during androgen-programmed cell death and their potential use as a marker of urogenital injury. J Urol. 1990;143:407–13.
Kavoussi PK, Smith RP, Oliver JL, Costabile RA, Steers WD, Brown-Steinke K, et al. S-nitrosylation of endothelial nitric oxide synthase impacts erectile function. Int J Impot Res. 2019;31:31–8.
Traish AM, Toselli P, Jeong SJ, Kim NN. Adipocyte accumulation in penile corpus cavernosum of the orchiectomized rabbit: a potential mechanism for veno-occlusive dysfunction in androgen deficiency. J Androl. 2005;26:242–8.
Kovanecz I, Ferrini MG, Vernet D, Nolazco G, Rajfer J, Gonzalez-Cadavid NF. Pioglitazone prevents corporal veno-occlusive dysfunction in a rat model of type 2 diabetes mellitus. BJU Int. 2006;98:116–24.
Shen ZJ, Zhou XL, Lu YL, Chen ZD. Effect of androgen deprivation on penile ultrastructure. Asian J Androl. 2003;5:33–6.
Petrone L, Mannucci E, Corona G, Bartolini M, Forti G, Giommi R, et al. Structured interview on erectile dysfunction (SIEDY): a new, multidimensional instrument for quantification of pathogenetic issues on erectile dysfunction. Int J Impot Res. 2003;15:210–20.
Corona G, Torres LO, Maggi M. Testosterone therapy: what we have learned From trials. J Sex Med. 2020;17:447–60.
Hwang I, Lee HS, Yu HS, Kim ME, Lee JS, Park K. Testosterone modulates endothelial progenitor cells in rat corpus cavernosum. BJU Int. 2016;117:976–81.
Li R, Meng X, Zhang Y, Wang T, Yang J, Niu Y, et al. Testosterone improves erectile function through inhibition of reactive oxygen species generation in castrated rats. PeerJ 2016;4:e2000.
Kataoka T, Hotta Y, Maeda Y, Kimura K. Testosterone deficiency causes endothelial dysfunction via elevation of asymmetric dimethylarginine and oxidative stress in castrated rats. J Sex Med. 2017;14:1540–8.
Kataoka T, Hotta Y, Yamamoto Y, Fukamoto A, Takeuchi M, Maeda Y, et al. Effect of late androgen replacement therapy on erectile function Through structural changes in castrated rats. Sex Med. 2021;9:100348.
Kataoka T, Mori T, Suzuki J, Kawaki Y, Kito Y, Hotta Y, et al. Oxaliplatin, an anticancer agent, causes erectile dysfunction in rats due to endothelial dysfunction. J Sex Med. 2021;18:1337–45.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature 2000;407:242–8.
Gho YS, Kleinman HK, Sosne G. Angiogenic activity of human soluble intercellular adhesion molecule-1. Cancer Res. 1999;59:5128–32.
Joseph IB, Nelson JB, Denmeade SR, Isaacs JT. Androgens regulate vascular endothelial growth factor content in normal and malignant prostatic tissue. Clin Cancer Res. 1997;3:2507–11.
Häggström S, Lissbrant IF, Bergh A, Damber JE. Testosterone induces vascular endothelial growth factor synthesis in the ventral prostate in castrated rats. J Urol. 1999;161:1620–5.
Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature 1995;376:517–9.
Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–20.
Shvets E, Ludwig A, Nichols BJ. News from the caves: update on the structure and function of caveolae. Curr Opin Cell Biol. 2014;29:99–106.
Chen Z, Bakhshi FR, Shajahan AN, Sharma T, Mao M, Trane A, et al. Nitric oxide-dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Mol Biol Cell. 2012;23:1388–98.
Igarashi J, Michel T. Agonist-modulated targeting of the EDG-1 receptor to plasmalemmal caveolae. eNOS activation by sphingosine 1-phosphate and the role of caveolin-1 in sphingolipid signal transduction. J Biol Chem. 2000;275:32363–70.
Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, et al. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 2008;8:310–7.
Hansen CG, Bright NA, Howard G, Nichols BJ. SDPR induces membrane curvature and functions in the formation of caveolae. Nat Cell Biol. 2009;11:807–14.
McMahon KA, Zajicek H, Li WP, Peyton MJ, Minna JD, Hernandez VJ, et al. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J. 2009;28:1001–15.
Zhu B, Swärd K, Ekman M, Uvelius B, Rippe C. Cavin-3 (PRKCDBP) deficiency reduces the density of caveolae in smooth muscle. Cell Tissue Res. 2017;368:591–602.
Suades R, Cosentino F. Sirtuin 1/soluble guanylyl cyclase: a nitric oxide-independent pathway to rescue ageing-induced vascular dysfunction. Cardiovasc Res. 2019;115:485–7.
Kilic U, Gok O, Elibol-Can B, Uysal O, Bacaksiz A. Efficacy of statins on sirtuin 1 and endothelial nitric oxide synthase expression: the role of sirtuin 1 gene variants in human coronary atherosclerosis. Clin Exp Pharm Physiol. 2015;42:321–30.
Kimura T, Sato K, Kuwabara A, Tomura H, Ishiwara M, Kobayashi I, et al. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J Biol Chem. 2001;276:31780–5.
Nofer JR, van der Giet M, Tölle M, Wolinska I, von Wnuck Lipinski K, Baba HA, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–81.
Theilmeier G, Schmidt C, Herrmann J, Keul P, Schäfers M, Herrgott I, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation 2006;114:1403–9.
Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999;99:301–12.
Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci USA. 2003;100:10664–9.
Zhang XH, Morelli A, Luconi M, Vignozzi L, Filippi S, Marini M, et al. Testosterone regulates PDE5 expression and in vivo responsiveness to tadalafil in rat corpus cavernosum. Eur Urol. 2005;47:409–16.
Acknowledgements
We acknowledge the assistance of the Research Equipment Sharing Center at the Nagoya City University.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research (KAKENHI, 20K09563) from the Japan Society for the Promotion of Science (JSPS) and the 24th General Assembly of the Japanese Association of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Conceptualization, TK and KK; Data curation, TK, IH, TM, YH, and YM; Formal analysis, TK and IH.; Funding acquisition, TK, YH, and KK; Investigation, TK, IH, and TM; Methodology, TK and YH; Project administration, TK; Supervision, YH, AS, YM, YFH, and KK; Writing-original draft, TK and KK; Writing-review & editing TK and KK.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kataoka, T., Ito, H., Mori, T. et al. Testosterone improved erectile function by upregulating transcriptional expression of growth factors in late androgen replacement therapy model rats. Int J Impot Res (2022). https://doi.org/10.1038/s41443-022-00627-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41443-022-00627-8