Abstract
Our aim was to investigate the effects of an intervention that comprised a brief counseling session on lifestyle habits together with the use of the EVIDENT (Lifestyles and arterial aging) application on the modification of markers that indicated different hemodynamic functions, such as arterial stiffness, blood pressure or wave reflections. The EVIDENT II study (Lifestyles and arterial aging) is a randomized clinical trial with two parallel groups. Each participant had an initial visit and two follow-up visits at 3 and 12 months where the peripheral augmentation index-PAIx75 was measured by tonometry, while an oscillometric method was used for the cardio-ankle vascular index-CAVI and brachial-ankle pulse wave velocity-baPWV. Both groups (intervention-IG and control-CG) were briefly counseled on diet and exercise. The IG also received instructions on the use of the EVIDENT II application during the 3 months. The sample (N = 597; 52 ± 11 years; 64% women) was randomized into IG = 291 and CG = 306. Comparing groups, there was a decrease in the PAIx75 favorable to the IG at 3 months (−4.9%, 95% CI: −7.7 to −2.1) and 12 months (−3.9%, 95% CI: −6.8 to −1.0). There was also a nonsignificant reduction of the CAVI at 3 months in the IG. A group effect was observed in the measurement of the PAIx75 over the 12-month follow-up (p = 0.003). This interaction was only observed in women (p = 0.017). In conclusion, the use of the EVIDENT II application achieved a modification of the PAIx75, a marker of wave reflection and vascular tone, in women through lifestyle improvements (physical activity and adherence to the Mediterranean diet). No significant results were found regarding blood pressure or arterial stiffness markers.
Similar content being viewed by others
Introduction
Mobile health or mHealth is a fundamental communication link between health professionals and patients. mHealth includes the use of mobile phones, patient monitoring devices and other wireless devices, and it has been used in the prevention, diagnosis and treatment of chronic diseases, such as diabetes mellitus 2 [1, 2] or arterial hypertension [3], as well as in the promotion of healthy lifestyles [4].
One of the most recent reviews on this topic shows that interventions with mobile phones improved physical activity and adiposity markers (evidence level I-A), with greater effectiveness achieved by combining these interventions with individualized advice [4]. Similarly, mobile-based interventions increased the consumption of fruit and vegetables by between 2 and 4 servings per day, without otherwise affecting the total caloric and sugary drink intake [5, 6]. The effects on physical activity may be substantial, both in terms of increasing weekly physical activity (1.5 to 153 min/week) [4] and the daily number of steps (800 to 1104 steps/day) [7].
Lifestyles have various effects on health and are most noticeable in the prevention of cardiovascular diseases, cardiovascular mortality, hypertension or vascular aging [8,9,10,11]. However, to date, very few studies have highlighted the effects of the improvement of lifestyles through mHealth in terms of cardiovascular events and mortality, probably because although these devices have widespread use [12], they have not been used long enough for testable results to be obtained. Nevertheless, studies have yielded positive results of mHealth interventions on relevant clinical variables (blood pressure, weight, chronic diseases or diabetic control), which are all important given their role in morbidity and mortality, especially cardiovascular. Comparing the use of mHealth versus standard care, the McLean review [13] found an average reduction of 3.7 mmHg for systolic blood pressure and 2.4 mmHg for diastolic blood pressure. Along the same lines, Pal’s review [14] showed a 0.5% reduction in Hb1Ac among patients with Diabetes Mellitus 2, similar to that found by Hamine et al. [15] in a more recent review. With regard to weight loss, a review by Liu F et al. [16]. established that interventions involving mobile phones achieved an average reduction of 1.4 kg compared to a control group.
The role played by arterial stiffness in the development and prediction of serious cardiovascular events is widely covered in the literature [17]. However, although these measures of arterial stiffness can be considered intermediate markers in the development of atherosclerosis [18, 19], the effect of the use of mHealth on these stiffness parameters has not previously been reported. In this regard, the results of the present study may open up a new field of research. Another aspect to be addressed is the different responses in the upper and lower arterial segments of acute aerobic exercise that are not analyzed in this work but have been shown in the work of Mutter et al. [20].
The objective of this study is to investigate the effect of an intervention consisting of the usual practice in primary care consultations (brief counseling on healthy lifestyles) together with the use of the EVIDENT (Lifestyles and arterial aging) application for 3 months on the modification of markers that indicate different hemodynamic functions, such as the arterial stiffness, blood pressure, or wave reflections, in a general population sample randomly selected in Spanish health centers.
Methods
Design and scope
The EVIDENT II study was a randomized clinical trial with two parallel groups. The follow-up period was 12 months. The measurements were obtained at baseline and in months 3 and 12 after randomization. The intervention period lasted 3 months after randomization.
The trial was performed in a primary care setting. Participants were recruited in six Spanish cities (Salamanca, Valladolid, Barcelona, Bilbao, Talavera de la Reina and Zaragoza). The study was completed in September 2016.
Participants
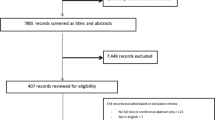
All participants in the EVIDENT I study (1553 individuals) were eligible for the study. After confirming the inclusion/exclusion criteria (refer to subsequent description), 720 individuals were excluded for different reasons, including 250 individuals who were over 70 years old, 85 individuals who did not meet the inclusion criteria, 325 individuals who declined to participate, and 60 individuals who were excluded for other reasons. Finally, 833 individuals were included (415 individuals were included in the intervention group and 418 individuals were included in the control group). During the follow-up, 63 subjects in the Intervention group-IG and 55 subjects in the control group-CG dropped out of the study, with no group differences in terms of age and sex. The analyses of this study focus on the 597 individuals for whom arterial stiffness measurements were obtained. The average age of this subsample is 3 years older than the general sample; however, it does not differ in any other variable. The requirements for study participation included an age under 70 years and no history of cardiovascular events. Individuals with musculoskeletal disease that limits walking, severe respiratory, renal or hepatic disease, a diagnosis of oncological processes in the last 5 years, and pregnant women were excluded from the study.
Ethics approval and consent to participate
The study protocol was approved by the Clinical Research Ethics Committee of the Health Care Area of Salamanca on June 21, 2013 and was published in 2014 [21]. All participants were duly informed and provided signed informed consent.
Sample size
With 597 participants (291 participants in the intervention group and 306 participants in the control group), the strength of the hypothesis test is >90% for detecting a statistically significant difference in the mean peripheral augmentation index-PAIx75 found at 3 months between the intervention group (86%) and the control group (91%), given an alpha risk of 0.05 in the bilateral contrast.
Randomization
The participants were randomized to one of the two clinical trial arms: intervention group (IG) and control group (CG). A standardized program (Consellería de Sanidade, X.d.G., Epidat 4.0, Santiago de Compostela, Spain) [22] was used to generate the randomization sequence, which was hidden until the test arms were assigned.
Intervention
The two groups (IG and CG) were counseled on healthy lifestyles at the end of the baseline visit and prior to randomization. The counseling session, which can be considered standard in primary care consultations, lasted approximately 30 min and included recommendations for increasing habitual physical activity and adherence to the Mediterranean diet.
The IG group participants had a further counseling session of ~30 min with research staff. During this visit, a smartphone with the EVIDENT application was provided, and each participant was instructed in its use. These instructions included explanations of the specific sections of the application: the daily record of food with the selection of food and quantities, the daily record of physical activity (type and quantity) and the number of steps, visualization and comparison of all data input compared to reference values for both nutrient intake (carbohydrates 50–60%, proteins 10–15%, fats 30–35%, fiber > 22 g/day) and number of daily steps (>10,000 steps/day). The application presents daily recommendations to help achieve individualized goals for the improvement of lifestyles based on the information provided by each participant. At the end of the exposure time to the intervention (3 months), the participants in the intervention group returned the smartphone, and an analysis of adherence to the application was performed, with categorization as low adherence (used <1 month), medium adherence (used between 1 and 2 months) and high adherence (used more than 2 months).
Outcome measures
The main outcomes were changes in the PAIx75, cardio-ankle vascular index-CAVI and brachial-ankle pulse wave velocity-baPWV figures in the intervention group with respect to the control group. The other outcomes measured included the blood pressure (central and peripheral), pulse pressure (central and peripheral), pressure amplification, and heart rate.
Measurements of wave reflections
Peripheral augmentation index measurements were obtained with Pulse Wave Application Software (B-pro (A-Pulse)-Health STATS International). The device has been validated in Asian and Caucasian populations [23, 24] and captures the radial pulse by tonometry, estimating the central arterial pressure by means of an equation. The peripheral augmentation index can be used as an indicator of arterial aging. It is a measurement obtained directly from the peripheral pulse wave; it is defined as the ratio between the late systolic pressure and early systolic pressure [25] and is calculated with the following formula: (Second peak systolic blood pressure [SBP2] − diastolic blood pressure [DBP])/(first peak SBP − DBP) × 100 (%). Due to the potential influence of the heart rate at the point of measurement, the peripheral augmentation index is standardized to a heart rate of 75 bpm (PAIx75) following the equation [26] PAIx75 = ((Heart rate − 75) × 0.39) + PAIx. The validity and reliability of the measurement of the central arterial pressure and augmentation index by means of this device were previously reported on 104 subjects at the beginning of the study [23]. To ensure that there was minimal difference between the measures of the study and the measurements obtained during validation, the same person performed all measurements. In addition, this analysis was performed under the same standardized measurement conditions that were subsequently used.
Measurements of arterial stiffness
The CAVI and the baPWV were obtained using the Vasera device VS-1500® (Fukuda Denshi). This device measures the CAVI, which is an index that reflects the stiffness of the arteries from the heart to the ankles. The CAVI estimates arterial elasticity through an oscillometric method and is used as a measure of arterial stiffness independent of blood pressure [27]. The CAVI index is calculated according to the stiffness parameter β, expressed by the following equation: 2ρ × 1/(Ps − Pd) × ln (Ps/Pd) × PWV2, where ρ represents the blood density and Ps and Pd are the SBP and DBP in mmHg. The baPWV was calculated with the following equation = (0.5934 × height (cm) + 14.4724)/tba (tba is the time interval between the arm and ankle waves). These measurements were obtained in a supine position after at least 10 min of rest in a room with a stable temperature and after being instructed not to smoke or perform physical activity in the hour prior to the test.
Measurements of blood pressure
Clinical blood pressure (systolic and diastolic) was measured using an OMRON model M10-IT sphygmomanometer (Omron Health Care, Kyoto, Japan) in accordance with the recommendations of the European Society of Hypertension [28]. The mean of the second and third measurements using the dominant arm was used for the analyses. Pressure amplification was defined as the difference between the peripheral and central blood pressure values.
Additional details regarding the process of measuring other variables were described in the study protocol [21].
Blinding strategy
Due to the nature of the intervention, the participants cannot be blinded. However, strategies were used to achieve the highest possible level of blindness. The researcher who visited the intervention group to explain the application was different from the nurse responsible for performing the assessments and providing the standardized counseling to both groups. In addition, the researcher in charge of performing the statistical analysis did not know the group assignments. To prevent contamination between groups, no additional advice or reinforcement was provided during the 3-month assessment visit. In addition, the application will not be available for download on the web until the end of the study so the control group will not be able to make use of it in any way.
Statistical analysis
Intention-to-treat analysis was used for the main analyses of the results. In the descriptive study of the baseline data, the results were expressed as the mean ± standard deviation. Student’s t was used to compare the means between two independent groups. To analyze the changes within the same group at 3 and 12 months postrandomization in the main and secondary outcomes, Student’s t for paired data was used; to analyze the effect of the intervention and compare the changes observed between the IG and the CG, an ANCOVA model was used. Furthermore, the interaction between the variables assessed and the group (IG or CG) was analyzed to assess the group effect via an analysis of variance of repeated measures. The results were adjusted for the baseline value of each variable and the presence of medications, such as statins and angiotensin-converting enzyme-ACE inhibitors, at the baseline. For the hypothesis test, a statistical significance level of 0.05 was established. The data were analyzed using SPSS version 23.0 (IBM Corp, Armonk, NY, USA).
Results
Sample description (baseline data)
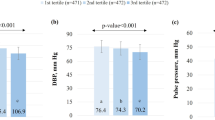
The sample consisted of 597 individuals (291 IG and 306 CG) (Table 1). There were no differences in the sex distribution (381 women (64%) and 216 men (36%)). The mean age was 52 ± 11 years (p > 0.05 in both). There were no group differences (IG and CG) in the baseline measurements in terms of blood pressure (122.8/75.5 mmHg), heart rate (69.3 bpm), PAIx75 (89.6%), CAVI (7.61), baPWV (12.55 m/s), or any other variable analyzed (central arterial pressure, central pulse pressure and pressure amplification). Among the 291 participants in the intervention group, 72 participants (24.7%) used the application less than one month, 45 participants (15.5%) used it between one and two months and 174 participants (59.8%) used it more than two months.
Intragroup changes at 3 and 12 months postintervention
The PAIx75 decreased in the IG at 3 months (−2.8%, −5.1 to −0.5), and these values were maintained at 12 months (−2.5%, −4.8 to −0.2) (Table 2). There were no significant changes in the values of the CAVI and baPWV, although there was a decrease at 3 months, which was not maintained at 12 months. In the CG, there was a nonsignificant increase in the values for the PAIx75 and baPWV at 3 and 12 months. The CAVI values increased along the same lines, reaching significance at 12 months (0.25; 0.11 to 0.39). The central and peripheral blood pressure decreased in both groups at 3 and 12 months. The IG was not subject to changes in the percentage of individuals on antihypertensive treatment, while this percentage increased in the control group from the baseline visit (21.2%) to 3 months (23.4%, p < 0.05) and 12 months (25.5%, p < 0.05).
Intergroup changes (IG − CG) in terms of wave reflections and arterial stiffness (Table 3).
A postintervention decrease in the PAIx75 was found in the intervention group at 3 months (−4.9%; −7.7 to −2.1) and 12 months (−3.9%; −6.8 to −1.0). A similarly favorable, albeit nonsignificant, effect was observed in the IG with a decrease in the CAVI values at 3 (−0.05; −0.21 to 0.11) and 12 (−0.13; −0.32 to 0.06) months. No changes were observed in the other variables analyzed (peripheral and central blood pressure, pulse pressure, pressure amplification and heart rate). The results by sex are presented in Table 4. There was no significant difference in the consumption of statins and ACE inhibitors between the study groups in any of the evaluation visits as shown in Table 5.
Assessment of group effects
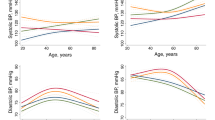
A group effect was observed in the PAIx75 measurements throughout the 12-month follow-up (p = 0.003) (Figs. 1–3). When analyzed by sex, this interaction was only observed among women (p = 0.017), whereas it disappeared among men (p = 0.226). None of the other variables analyzed showed significant group interaction effects in the measurements: CAVI (p = 0.464), baPWV (p = 0.421), central systolic blood pressure (p = 0.445), central pulse pressure (p = 0.245) or pressure amplification (p = 0.200).
Discussion
An intervention to improve lifestyles that consists of a brief healthy lifestyle counseling session, which is considered standard practice in primary care nursing consultations, plus the use of information technologies (the EVIDENT II application) has achieved a decrease in the PAIx75 (a marker of vascular tone) compared to counseling alone in a sample of the general population attending Spanish health centers. Analysis by sex shows that the reduction is more pronounced in women. In the other variables analyzed (CAVI and baPWV), all related to arterial stiffness, a tendency towards a reduction was observed at 3 months of follow-up; however, this tendency disappeared after 12 months postintervention.
The potential link between lifestyles and PAIx75 values was widely reported in the first phase of the EVIDENT study [29]. An inverse association between daily physical activity (amount and intensity) and PAIx75 was observed [30]. Sedentary behavior was also independently associated with this marker [31]. Among dietary habits, an inverse association with the glycemic index of the usual diet stood out [32]. However, these findings all originated from an observational study where adequate causal relationships could not be established. The use of the EVIDENT II application achieved a reduction of the PAIx75 at 3 months in the intervention group through the increase of physical activity and improvements in eating habits. At 3 months, the IG yielded a significant increase in the self-reported time of moderate/vigorous physical activity with an average increase of 29 min/week (p = 0.02), while this increase was not reported by the control group (counseling only) (mean 17 min/week (p = 0.38)). Adherence to the Mediterranean diet increased in both groups approximately half a point [33]. However, the PAIx75 was not further reduced at 12 months compared to 3 months in the IG. This finding may be explained because the physical activity analyzed by the 7-day PAR did not show changes at 12 months in any of the groups (p value for tendency = 0.25) [34]. Moreover, an increase in the Mediterranean diet adherence was observed in both groups at 12 months with no differences between them (p value for tendency = 0.46). High values of PAIx are a marker of increased systemic vascular resistance and indicate an increase in the systolic load and myocardial hypertrophy. The evidence for a relationship between the PAIx and cardiovascular morbidity and mortality is less than the central augmentation index-CAIx; however, several studies have shown that the PAIx was independently predictive of adverse cardiac events [35, 36]. One of the main determinants of the augmentation index is the blood pressure at the time of measurement [37]. However, these values do not vary between groups in any of the evaluation visits. The improvement in the PAIx75 values is only achieved in women, which reinforces the idea that the development of smartphone applications should take into account the specific target users according to their age or sociodemographic characteristics, although this may affect a proliferation of specific applications [38].
The CAVI and baPWV directly measure arterial elasticity, and their use is widespread in Asian countries as a predictive tool for arterial stiffness [39]. These measures have more recently been validated and introduced into Caucasian populations, and their prognostic value regarding cardiovascular disease is high and equivalent to other measures more commonly used, such as the cfPWV [40]. The effect of modifiable lifestyles on CAVI values is not sufficiently described, although there are studies that relate it to smoking (lower CAVI values when the habit is completely or partially stopped) [41] and the performance of aerobic physical activity (the more physical activity, the lower the CAVI values) [42]. Similarly, the SAPALDIA 3 study [43], which employed an observational design, has shown an association between the CAVI index and the performance of vigorous physical activity in a cohort of Swiss elderly individuals. Increased arterial stiffness (CAVI ≥ 9, upper tertile) was inversely and significantly associated with vigorous PA (odds ratio (OR): 0.65, 95% confidence interval (CI): 0.48–0.88). In this regard, the results of the EVA study may be interesting [44]. This study aims to analyze the factors that influence the early stages of vascular aging and analyze the CAVI index and lifestyles in 500 participants of 5 age groups. The results of the EVIDENT study show a slight decrease in the CAVI values in the intervention group compared to an increase in the control group at the 3-month assessment visit, a result of the use of the EVIDENT II application during this period. However, this trend is not maintained in the long term (12 months), and this finding only occurs in women. One potential explanation of the latter can be found in the recently published EVIDENT II results [33]. This study yields a beneficial effect among women in the intervention group with an increase in self-reported physical activity of 130.3 METs min/week compared to women in the control group. In contrast, men in the intervention group decreased physical activity by 80.0 METs min/week compared to those in the control group. None of these differences reached statistical significance; however, they suggest a beneficial effect of the use of the application in women that could result in more marked effects on arterial stiffness.
Among the main limitations of this work, the following issues should be noted: The study design prevents the blinding of the participants. Self-reported questionnaires were used for lifestyle assessment, although all assessments were validated in the reference population. Despite participants being instructed not to use any other application that could interfere with the objectives of this study for its duration, there are no guarantees to ensure this occurred. Finally, the exposure time to the intervention (3 months) might not be sufficient to identify positive results in changing lifestyles and the related blood structural properties.
In conclusion, the use of the EVIDENT II application achieved a modification of the PAIx75, a marker of wave reflection and vascular tone, in women through lifestyle improvements (physical activity and adherence to the Mediterranean diet). No significant results were found in blood pressure or arterial stiffness markers. Thus, the results of this study suggest that information technologies can have a positive impact on the modification of clinical variables related to cardiovascular disease.
References
Garabedian LF, Ross-Degnan D, Wharam JF. Mobile phone and smartphone technologies for diabetes care and self-management. Curr Diab Rep. 2015;15:109.
Valentiner LS, Ried-Larsen M, Karstoft K, Brinklov CF, Brons C, Nielsen RO, et al. Long-term effect of smartphone-delivered Interval Walking Training on physical activity in patients with type 2 diabetes: protocol for a parallel group single-blinded randomised controlled trial. BMJ Open. 2017;7:e014036.
Parati G, Torlasco C, Omboni S, Pellegrini D. Smartphone applications for hypertension management: a potential game-changer that needs more control. Curr Hypertens Rep. 2017;19:48.
Afshin A, Babalola D, McLean M, Yu Z, Ma W, Chen CY, et al. Information technology and lifestyle: a systematic evaluation of internet and mobile interventions for improving diet, physical activity, obesity, tobacco, and alcohol use. J Am Heart Assoc. 2016;5:e003058.
Norman GJ, Kolodziejczyk JK, Adams MA, Patrick K, Marshall SJ. Fruit and vegetable intake and eating behaviors mediate the effect of a randomized text-message based weight loss program. Prev Med. 2013;56:3–7.
Spring B, Schneider K, McFadden HG, Vaughn J, Kozak AT, Smith M, et al. Multiple behavior changes in diet and activity: a randomized controlled trial using mobile technology. Arch Intern Med. 2012;172:789–96.
Jee H. Review of researches on smartphone applications for physical activity promotion in healthy adults. J Exerc Rehabil. 2017;13:3–11.
Veronese N, Li Y, Manson JE, Willett WC, Fontana L, Hu FB. Combined associations of body weight and lifestyle factors with all cause and cause specific mortality in men and women: prospective cohort study. BMJ. 2016;355:i5855.
Berstad P, Botteri E, Larsen IK, Loberg M, Kalager M, Holme O, et al. Lifestyle changes at middle age and mortality: a population-based prospective cohort study. J Epidemiol Community Health. 2017;71:59–66.
Bai G, Zhang J, Zhao C, Wang Y, Qi Y, Zhang B. Adherence to a healthy lifestyle and a DASH-style diet and risk of hypertension in Chinese individuals. Hypertens Res. 2017;40:196–202.
Nowak KL, Rossman MJ, Chonchol M, Seals DR. Strategies for achieving healthy vascular aging. Hypertension. 2018;71:389–402.
Statista. Number of smartphone users worldwide from 2014 to 2019. https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/ (2016). Accessed 05 Oct 2017.
McLean G, Band R, Saunderson K, Hanlon P, Murray E, Little P, et al. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens. 2016;34:600–12.
Pal K, Eastwood SV, Michie S, Farmer A, Barnard ML, Peacock R, et al. Computer-based interventions to improve self-management in adults with type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2014;37:1759–66.
Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17:e52.
Liu F, Kong X, Cao J, Chen S, Li C, Huang J, et al. Mobile phone intervention and weight loss among overweight and obese adults: a meta-analysis of randomized controlled trials. Am J Epidemiol. 2015;181:337–48.
Liao J, Farmer J. Arterial stiffness as a risk factor for coronary artery disease. Curr Atheroscler Rep. 2014;16:387.
Rosenbaum D, Giral P, Chapman J, Rached FH, Kahn JF, Bruckert E, et al. Radial augmentation index is a surrogate marker of atherosclerotic burden in a primary prevention cohort. Atherosclerosis. 2013;231:436–41.
Saiki A, Sato Y, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, et al. The role of a novel arterial stiffness parameter, cardio-ankle vascular index (CAVI), as a surrogate marker for cardiovascular diseases. J Atheroscler Thromb. 2016;23:155–68.
Mutter AF, Cooke AB, Saleh O, Gomez YH, Daskalopoulou SS. A systematic review on the effect of acute aerobic exercise on arterial stiffness reveals a differential response in the upper and lower arterial segments. Hypertens Res. 2017;40:146–72.
Recio-Rodriguez JI, Martin-Cantera C, Gonzalez-Viejo N, Gomez-Arranz A, Arietaleanizbeascoa MS, Schmolling-Guinovart Y, et al. Effectiveness of a smartphone application for improving healthy lifestyles, a randomized clinical trial (EVIDENT II): study protocol. BMC Public Health. 2014;14:254.
Consellería de Sanidade XdG, España; Organización Panamericana de la salud (OPS-OMS); Universidad CES, Colombia. Epidat: programa para análisis epidemiológico de datos. Versión 4.2 Julio; 2016.
Garcia-Ortiz L, Recio-Rodriguez JI, Canales-Reina JJ, Cabrejas-Sanchez A, Gomez-Arranz A, Magdalena-Belio JF, et al. Comparison of two measuring instruments, B-pro and SphygmoCor system as reference, to evaluate central systolic blood pressure and radial augmentation index. Hypertens Res. 2012;35:617–23.
Williams B, Lacy PS, Yan P, Hwee CN, Liang C, Ting CM. Development and validation of a novel method to derive central aortic systolic pressure from the radial pressure waveform using an n-point moving average method. J Am Coll Cardiol. 2011;57:951–61.
Munir S, Guilcher A, Kamalesh T, Clapp B, Redwood S, Marber M, et al. Peripheral augmentation index defines the relationship between central and peripheral pulse pressure. Hypertension. 2008;51:112–8.
Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(Pt 1):263–70.
Shirai K, Hiruta N, Song M, Kurosu T, Suzuki J, Tomaru T, et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb. 2011;18:924–38.
O’Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23:697–701.
Garcia-Ortiz L, Recio-Rodriguez JI, Martin-Cantera C, Cabrejas-Sanchez A, Gomez-Arranz A, Gonzalez-Viejo N, et al. Physical exercise, fitness and dietary pattern and their relationship with circadian blood pressure pattern, augmentation index and endothelial dysfunction biological markers: EVIDENT study protocol. BMC Public Health. 2010;10:233.
Garcia-Ortiz L, Recio-Rodriguez JI, Schmidt-Trucksass A, Puigdomenech-Puig E, Martinez-Vizcaino V, Fernandez-Alonso C, et al. Relationship between objectively measured physical activity and cardiovascular aging in the general population—the EVIDENT trial. Atherosclerosis. 2014;233:434–40.
Recio-Rodriguez JI, Gomez-Marcos MA, Patino-Alonso MC, Romaguera-Bosch M, Grandes G, Menendez-Suarez M, et al. Association of television viewing time with central hemodynamic parameters and the radial augmentation index in adults. Am J Hypertens. 2013;26:488–94.
Recio-Rodriguez JI, Gomez-Marcos MA, Patino-Alonso MC, Rodrigo-De Pablo E, Cabrejas-Sanchez A, Arietaleanizbeaskoa MS, et al. Glycemic index, glycemic load, and pulse wave reflection in adults. Nutr Metab Cardiovasc Dis. 2015;25:68–74.
Recio-Rodriguez JI, Agudo-Conde C, Martin-Cantera C, Gonzalez-Viejo MN, Fernandez-Alonso MD, Arietaleanizbeaskoa MS, et al. Short-term effectiveness of a mobile phone app for increasing physical activity and adherence to the mediterranean diet in primary care: a randomized controlled trial (EVIDENT II Study). J Med Internet Res. 2016;18:e331.
Garcia-Ortiz L, Recio-Rodriguez JI, Agudo-Conde C, Patino-Alonso MC, Maderuelo-Fernandez JA, Repiso Gento I, et al. Long-term effectiveness of a smartphone app for improving healthy lifestyles in general population in primary care: randomized controlled trial (Evident II Study). JMIR Mhealth Uhealth. 2018;6:e107.
Izzo JL Jr., Brachial vs. central systolic pressure and pulse wave transmission indicators: a critical analysis. Am J Hypertens. 2014;27:1433–42.
Fischer-Rasokat U, Brenck F, Zeiher AM, Spyridopoulos I. Radial augmentation index unmasks premature coronary artery disease in younger males. Blood Press Monit. 2009;14:59–67.
Nurnberger J, Dammer S, Opazo Saez A, Philipp T, Schafers RF. Diastolic blood pressure is an important determinant of augmentation index and pulse wave velocity in young, healthy males. J Hum Hypertens. 2003;17:153–8.
Tonkin E, Brimblecombe J, Wycherley TP. Characteristics of smartphone applications for nutrition improvement in community settings: a scoping review. Adv Nutr. 2017;8:308–22.
Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, et al. Cardio-ankle vascular index is a new noninvasive parameter of arterial stiffness. Circ J. 2007;71:1710–4.
Gomez-Sanchez L, Garcia-Ortiz L, Patino-Alonso MC, Recio-Rodriguez JI, Frontera G, Ramos R, et al. The association between the cardio-ankle vascular index and other parameters of vascular structure and function in caucasian adults: MARK tudy. J Atheroscler Thromb. 2015;22:901–11.
Hata K, Nakagawa T, Mizuno M, Yanagi N, Kitamura H, Hayashi T, et al. Relationship between smoking and a new index of arterial stiffness, the cardio-ankle vascular index, in male workers: a cross-sectional study. Tob Induc Dis. 2012;10:11.
Lindholm H, Punakallio A, Lusa S, Sainio M, Ponocny E, Winker R. Association of cardio-ankle vascular index with physical fitness and cognitive symptoms in aging Finnish firefighters. Int Arch Occup Environ Health. 2012;85:397–403.
Endes S, Schaffner E, Caviezel S, Dratva J, Autenrieth CS, Wanner M, et al. Physical activity is associated with lower arterial stiffness in older adults: results of the SAPALDIA 3 cohort study. Eur J Epidemiol. 2016;31:275–85.
Gomez-Marcos MA, Martinez-Salgado C, Gonzalez-Sarmiento R, Hernandez-Rivas JM, Sanchez-Fernandez PL, Recio-Rodriguez JI, et al. Association between different risk factors and vascular accelerated ageing (EVA study): study protocol for a cross-sectional, descriptive observational study. BMJ Open. 2016;6:e011031.
Members EVIDENT Investigators Group
Coordinating center: La Alamedilla Health Center (Health Service of Castilla y León): Luis Garcia-Ortiz, Jose I Recio-Rodriguez, Manuel A. Gómez-Marcos, Emiliano Rodríguez-Sánchez, Jose A Maderuelo-Fernández, Jose A Iglesias-Valiente, Maria C Patino-Alonso, Diana Pérez-Arechaederra, Sara Mora-Simón, Cristina Agudo-Conde, Maria C Castaño-Sánchez, Carmela Rodríguez-Martín, Benigna Sánchez-Salgado, Angela de Cabo-Laso, Rosario Alonso-Domínguez, and Natalia Sánchez-Aguadero.
Collaborating centers: Passeig de Sant Joan Health Center (Catalan Health Service): Carlos Martín-Cantera, Jose Canales-Reina, Epifania Rodrigo de Pablo, Maria L Lasaosa-Medina, Maria J Calvo-Aponte, A Rodríguez-Franco, Carmen Martin-Borras, Anna Puig-Ribera, Ruben Colominas-Garrido, and Elisa Puigdomenech Puig.
Ca N’Oriac Health Center (Catalan Health Service): Monserrat Romaguera-Bosch.
Sant Roc Health Center (Catalan Health Service): Sandra Maneus.
Río Tajo Health Center (Health Service of Castilla-La Mancha): Yolanda Schmolling-Guinovart, Beatriz Rodríguez-Martín, Alicia Fernández del Rio, José A Fernández-Díaz, José B Calderón-Ubeda, José L Menéndez-Obregón, Antonio Segura-Fragoso, Carmen Zabala-Baños, Vicente Martínez-Vizcaíno, and María Martínez-Andrés.
Casa de Barco Health Center (Health Service of Castilla y León): Maria C Fernández-Alonso, Amparo Gómez-Arranz, Aventina de la Cal de la Fuente, Marta Menéndez-Suarez, and Irene Repiso-Gento.
San Pablo Health Center (Health Service of Castilla y León): Maria I Arranz-Hernando, Maria I Pérez-Concejo, Maria A Alonso-Manjarres, Maria E Villarroya, Maria J Arribas de Rodrigo, Margarita Pérez de Lis, Maria D de Arriba-Gómez, A Arqueaga-Luengo and Maria M López-Arroyo.
Torre Ramona Health Center (Health Service of Aragón): Natividad González-Viejo, Jose F Magdalena-Belio, Luis Otegui-Ilarduya, Francisco J Rubio-Galán, Amor Melguizo-Bejar, Ines Sauras-Yera, Maria J Gil-Train, Marta Iribarne-Ferrer, Olga Magdalena-González, and Miguel A Lafuente-Ripolles.
Primary Care Research Unit of Bizkaia (Basque Health Service-Osakidetza): Gonzalo Grandes, Álvaro Sanchez, Verónica Arce, Maria S Arietaleanizbeaskoa, Nere Mendizabal, and Eguskiñe Iturregui-San Nicolas.
CGB Computer Company, Salamanca, Spain (contribution to technical development of the application EVIDENT II).
Funding
This study was funded by the Spanish Ministry of Science and Innovation (MICINN) and Carlos III Health Institute/European Regional Development Fund (ERDF) (FIS: PI13/00618, PI13/01526, PI13/00058, PI13/01635, PI13/02528, PI12/01474; RETICS: RD12/0005, RD16/0007), Regional Health Management of Castilla and León (GRS 1191/B/15, GRS 909/B/14, GRS 770/B/13) and the Infosalud Foundation. None of the funders were involved in the design, implementation, analysis or interpretation of the data.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of “EVIDENT Investigators group” are above References.
Rights and permissions
About this article
Cite this article
Recio-Rodríguez, J.I., Rodriguez-Sanchez, E., Martin-Cantera, C. et al. Combined use of a healthy lifestyle smartphone application and usual primary care counseling to improve arterial stiffness, blood pressure and wave reflections: a Randomized Controlled Trial (EVIDENT II Study). Hypertens Res 42, 852–862 (2019). https://doi.org/10.1038/s41440-018-0182-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0182-6
Keywords
This article is cited by
-
Mobile health strategies for blood pressure self-management in urban populations with digital barriers: systematic review and meta-analyses
npj Digital Medicine (2021)
-
Perspectives of patients and physicians regarding hypertensive management from an online survey for excellence: a subanalysis of the PARADOX study by physician categories
Hypertension Research (2020)
-
Association between ideal cardiovascular health score trajectories and arterial stiffness: the Kailuan Study
Hypertension Research (2020)
-
Physician and patient perspectives on hypertension management and factors associated with lifestyle modifications in Japan: results from an online survey
Hypertension Research (2020)