Abstract
Type 2 spinal muscular atrophy with lower extremity dominance (SMALED2) is caused by bicaudal D cargo adaptor 2 (BICD2) variants. However, the SMALED2 genotype and phenotype correlation have not been thoroughly characterized. We identified de novo heterozygous BICD2 missense variants in two fetuses with severe, prenatally diagnosed multiple arthrogryposis congenita. This report provides further insights into the genetics of this rare disease.
Similar content being viewed by others
Spinal muscular atrophy (SMA) with lower extremity dominance (SMALED), a rare SMA type, is characterized by musculature weakness and atrophy due to motor neuron dysfunction predominantly affecting the lower extremities and is caused by one of two autosomal dominantly inherited genes. SMALED2 (OMIM number 615290), a SMALED subtype, is caused by mutations in bicaudal D cargo adaptor 2 (BICD2) at 9q22.31. Forty-five disease-causing BICD2 variants have been reported in the PubMed database. SMALED2A (OMIM number 615290) is a classical form with childhood-to-adulthood onset of muscular weakness and atrophy, predominantly affecting the lower extremities. SMALED2B (OMIM number 618291) is a more severe form with prenatal onset, congenital myopathy, and arthrogryposis1. The correlations between the genotypes and phenotypes of SMALED2 remain unclear. We encountered two patients with BICD2 de novo variants associated with severe multiple arthrogryposis congenita. Here, we present genetic variant information and the phenotypic prenatal course of the disease.
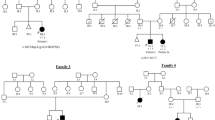
In Case 1, a 24-year-old nulliparous pregnant woman without significant medical or family history was referred to our hospital for a fetus with multiple fetal arthrogryposis at 17+6 weeks of gestation (Fig. 1a). Fetal ultrasonographic findings revealed flexed fetal wrist and elbow joints, adducted and flexed hip joints, extended knee and foot joints with talipes equinovarus, and no fetal movement (Fig. 1b and c). Despite thorough counseling, she chose to terminate the pregnancy at 19+6 weeks of gestation.
a Pedigree of Case 1. b, c Prenatal ultrasonographic images of Case 1 at 18+2 weeks of gestation. d Pedigree of Case 2. e, f Prenatal ultrasonographic images of Case 2 at 18+1 weeks of gestation. The arrow indicates the pterygium at the elbow joints. g Prenatal ultrasonographic images of Case 2 at 23+1 weeks of gestation. h Postnatal autopsy radiograph of Case 2. i, j Postnatal gross images of Case 2.
In Case 2, a 36-year-old nulliparous pregnant woman with no significant medical or family history was referred to our hospital for a fetus with multiple fetal arthrogryposis at 18+1 weeks of gestation (Fig. 1d). Fetal ultrasonographic findings revealed no fetal movement, fixed and extended knees and ankles, adducted and flexed hip joints, and fixed upper extremities with flexed wrists and elbow joints. Pterygia was identified in both elbow joints (Fig. 1e and f). Single-nucleotide polymorphism microarray analysis using an amniocentesis sample revealed no chromosomal abnormalities. Fetal hydrops, subcutaneous edema, pleural effusion, and ascites developed at 20+1 weeks of gestation. The status of fetal hydrops gradually worsened (Fig. 1g), and intrauterine fetal demise (IUFD) was confirmed at 28+1 weeks of gestation. Labor was induced at 29+5 weeks of gestation. Autopsy radiographs revealed no abnormal findings in skeletal structures, deformities, shortening of bones, or calcification of the joints (Fig. 1h). The gross findings included multiple pterygia on the neck, bilateral axilla, cubital, and hip joints; hyperextended knee joints; and arthrogryposis (Fig. 1i and j).
We performed trio whole-exome sequencing in both cases after approval from the Institutional Review Boards of Jikei University School of Medicine and the National Center for Child Health and Development (IRB numbers: 27-060 [7945] and 926, respectively). DNA was extracted from fetal umbilical cords and parental peripheral blood. Whole-exome libraries were prepared and sequenced using a previously described method2. We extracted variants with minor allele frequencies <0.01 in the Integrated Japanese Genome Variation Database (ToMMo [iJGVD] 3.5KJPN; https://jmorp.megabank.tohoku.ac.jp) and the Human Genetic Variation Database (HGVD; https://www.hgvd.genome.med.kyoto-u.ac.jp) and a CADD Phred score >20. We also selected candidate variants that had a read coverage threshold >8. We identified a novel heterozygous BICD2 missense mutant variant (NM_001003800.2:c.2200A>G, p.Lys734Glu) in the Case 1 fetus and a BICD2 mutant variant (NM_001003800.2:c.2081G>A, p.Arg694His) in the Case 2 fetus. Neither variant was detected in the respective parents. Neither variant has been reported in any control genome database, such as the International Genome Sample Resource (https://www.internationalgenome.org), HGVD, or ToMMo (iJGVD). In silico analyses predicted a deleterious effect on protein function for p.Lys734Glu (Case 1) (SIFT, 0.002; PolyPhen2 HDIV, 0.969; PolyPhen2 HVAR, 0.793; and CADD score, 29.5) and p.Arg694His (Case 2) (SIFT, 0; PolyPhen2 HDIV, 1; PolyPhen2 HVAR, 0.999; and CADD score, 34). According to the American College of Medical Genetics and Genomics and the Association of Molecular Pathology guidelines, both variants were classified as “likely pathogenic” (PS2, PM1, and PM2 for Case 1 and PS2, PM1, PM2, and PP3 for Case 2)3. Therefore, the fetuses were diagnosed with SMALED2B caused by de novo BICD2 missense mutations.
Figure 2 shows BICD2 single-gene variants and the clinical symptoms reported as SMALED2B4,5,6,7,8,9,10,11. Most of the variants (94%) were associated with arthrogryposis congenita with decreased or absent fetal movement. Fetal hydrops and multiple pterygia were observed in a few cases. Ten fetuses that survived until delivery developed permanent respiratory insufficiency, with the exception of one neonate who required respiratory support for only 3 weeks. Multiple arthrogryposis congenita and decreased fetal movement were observed in Cases 1 and 2. Talipes equinovarus was detected only in Case 1. Cases 1 and 2 both followed a disease course similar to those of patients with other SMALED2B variants. Talipes equinovarus and multiple pterygia may present minor phenotypic differences among several BICD2 variants.
A BICD2 single gene variants with clinical symptoms reported as SMALED2B, including the two present cases. The numbers in the solid boxes represent the exon number in the BICD2 gene. B Table comparing the clinical symptoms of BICD2 variants causing SMALED2B. TOP termination of pregnancy, GW gestational week, IUFD intrauterine fetal demise.
In ClinVar, an in-frame deletion variant at the same location as the variant in Case 1, NM_001003800.2 (BICD2):c.2200_2202del (p.Lys734del) (Variation ID: 982816), is registered as likely pathogenic and causes childhood-onset proximal spinal muscular atrophy with contractures. A previous study reported another disease-causing missense variant, c.2202G>T (p.Lys734Asn), at a location close to that of the variant in Case 1 (Fig. 2B)12, resulting in a prenatal onset phenotype with multiple congenital articular contractures with leg and thorax deformities and multiple muscular atrophy. However, this patient survived for >16 years, confirming a relatively milder form of SMALED2B caused by this variant compared with other variants. Since the pregnancy was terminated in Case 1, a thorough comparison of the clinical course of these three variants, Lys734Glu, Lys734Asn, and Lys734del, is difficult. The disease onset at the 17th week of gestation with severe multiple arthrogryposis in Case 1 might have been associated with a relatively more severe course among the three variants. The Lys734 codon is located in the coiled-coil 3 region, which interacts with RANBP2 and RAB6, indicating the important function of BICD2. Further clinical cases need to be studied to define the phenotype of variants at this amino acid.
A single previous study reported the variant c.2081G>A (p.Arg694His), the same variant detected in Case 2 (Fig. 2B); fetal hydrops with akinesia was detected at 19 weeks of gestation, and labor was induced owing to IUFD at 21 weeks of gestation. However, the clinical course was not detailed in the report13. This article details the clinical symptoms and outcomes of this variant, which follows a course similar to that reported previously. A total of four cases were previously reported with variants located at the same amino acid, p.Arg6944,10,13, as in Case 2. All patients with the p.Arg694Cys variant survived until live birth but developed respiratory insufficiency. However, fetuses with p.Arg694His, including the fetus in Case 2, developed fetal hydrops and did not survive until birth. The Arg694 codon is also located in the coiled-coil 3 region, which interacts with RANBP2 and RAB6, indicating the important function of BICD2.
In conclusion, we presented BICD2 missense mutation variants from two independent fetuses with severe multiple arthrogryposes. We highlight the importance of reporting new cases to provide further insights into the genetic basis of this rare disease.
HGV database
The relevant data from this Data Report are hosted at the Human Genome Variation Database at https://doi.org/10.6084/m9.figshare.hgv.3427, https://doi.org/10.6084/m9.figshare.hgv.3430, and https://doi.org/10.6084/m9.figshare.hgv.3246.
References
Koboldt, D. C., Waldrop, M. A., Wilson, R. K. & Flanigan, K. M. The genotypic and phenotypic spectrum of BICD2 variants in spinal muscular atrophy. Ann. Neurol. 87, 487–496 (2020).
Kobayashi, Y. et al. Novel missense COL2A1 variant in a fetus with achondrogenesis type II. Hum. Genome Var. 9, 40 (2022).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Storbeck, M. et al. Phenotypic extremes of BICD2-opathies: from lethal, congenital muscular atrophy with arthrogryposis to asymptomatic with subclinical features. Eur. J. Hum. Genet. 25, 1040–1048 (2017).
Chin, H. L. et al. An infant with congenital respiratory insufficiency and diaphragmatic paralysis: a novel BICD2 phenotype? Am. J. Med. Genet. A 188, 926–930 (2022).
Chandler, N., Brace, P., Roberts, R. & Mellis, R. Likely pathogenic variant in the BICD2 gene in fetus presenting with non-immune hydrops. Prenat. Diagn. 43, 727–729 (2023).
Ueda, Y., Suganuma, T., Narumi-Kishimoto, Y., Kaname, T. & Sato, T. A case of severe autosomal dominant spinal muscular atrophy with lower extremity predominance caused by a de novo BICD2 mutation. Brain Dev. 43, 135–139 (2021).
Koboldt, D. C. et al. In-frame de novo mutation in BICD2 in two patients with muscular atrophy and arthrogryposis. Cold Spring Harb. Mol. Case Stud. 4, a003160 (2018).
Marchionni, E. et al. Fetal early motor neuron disruption and prenatal molecular diagnosis in a severe BICD2-opathy. Am. J. Med. Genet. A 185, 1509–1514 (2021).
Ravenscroft, G. et al. Recurrent de novo BICD2 mutation associated with arthrogryposis multiplex congenita and bilateral perisylvian polymicrogyria. Neuromuscul. Disord. 26, 744–748 (2016).
Ahmed, A. A. et al. Arthrogryposis and pterygia as lethal end manifestations of genetically defined congenital myopathies. Am. J. Med. Genet. A 176, 359–367 (2018).
Rudnik-Schöneborn, S. et al. Autosomal dominant spinal muscular atrophy with lower extremity predominance: a recognizable phenotype of BICD2 mutations. Muscle Nerve 54, 496–500 (2016).
Al-Kouatly, H. B. et al. High diagnosis rate for nonimmune hydrops fetalis with prenatal clinical exome from the Hydrops-Yielding Diagnostic Results of Prenatal Sequencing (HYDROPS) Study. Genet. Med. 23, 1325–1333 (2021).
Acknowledgements
The authors are grateful to the patients and their family members for permission to use their clinical information in this article. This study was partly supported by a research grant from JSPS KAKENHI (grant numbers: JP 23K15822 and 23K07256).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Masuda, L., Hasegawa, A., Kamura, H. et al. Missense BICD2 variants in fetuses with congenital arthrogryposis and pterygia. Hum Genome Var 11, 32 (2024). https://doi.org/10.1038/s41439-024-00290-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41439-024-00290-z