Abstract
Cystoid macular oedema (CMO), which is defined as a macular thickening and cystic changes due to accumulation of fluid, could be asymptomatic and only diagnosed using paraclinical techniques. Fluorescein angiography (FA) and optical coherence tomography (OCT) are useful in detecting CMO in clinical practice. Non-leaking CMO, also known as angiographically silent CMO, is referred to as cases of CMO without leakage in fluorescein angiography. This type of CMO has been reported in some retinal dystrophies, in cases of maculopathy as a side effect of certain drugs, and also in some systemic disorders. The exact mechanism and treatment options for this type of CMO are still not clear. This literature review aims to discuss different causes of non-leaking CMO, proposed mechanisms, and management options. Three sections including drugs, retinal dystrophies, and systemic disorders are discussed in this review.
摘要
黄斑囊样水肿 (CMO) 定义为液体蓄积造成的黄斑区增厚和囊样改变, 可能无症状, 并且只能由临床辅助检查所诊断。荧光血管造影 (FA) 和光学相干断层扫描 (OCT) 是临床上检测CMO的有效手段。无渗漏的CMO, 在造影中以“安静的CMO”著称, 特指那些在荧光素血管造影中没有荧光渗漏的CMO。在某些视网膜营养不良、药物副作用及全身性疾病中可见这种类型的CMO。该类型CMO的发病机制和治疗方法仍不明确。本综述旨在讨论造成无渗漏CMO的不同病因、潜在机制和管理方法。本综述讨论了药物、视网膜营养不良及系统性疾病三个部分。
Similar content being viewed by others
Introduction
Cystoid macular oedema (CMO) is defined as increased macular thickness and cystic changes at the macula due to the accumulation of fluid. CMO could be asymptomatic and only diagnosed using paraclinical techniques [1]. Fluorescein angiography can demonstrate leakage of fluorescein from the vessels in a petaloid pattern, leakage from macular capillaries, and pooling in the cystoid cavities [2]. Optical coherence tomography (OCT) shows elevated macular thickness and hyporeflective spaces in retinal layers [1]. The most common causes of CMO include retinal vascular disorders, uveitis, neovascular age-related macular degeneration, cataract surgery, retinal dystrophies, and certain drugs [1]. CMO may happen either due to fluid accumulation in extracellular spaces, which is known as vasogenic oedema, or fluid might accumulate inside the cells because of cytotoxic oedema and cell swelling [3]. In a healthy retina, fluids are cleared from the outer and inner retinal layers by the retinal pigment epithelium (RPE) and glial cells, respectively. Extracellular oedema occurs due to the passage of fluid from vessels into the tissue because of blood-retina barrier breakdown in the setting of ischemia, inflammation, or vascular disorders [3]. In intracellular oedema, the cystic spaces are formed by swollen Müller cells. Swelling of glial cells can occur because of increased intracellular osmotic pressure due to different mechanisms [3]. CMO without leakage in FA, also known as angiographically silent CMO, occurs in cases without significant capillary leakage but the exact mechanism is still unclear. This type of CMO has been reported in some cases of retinal dystrophies, in cases of maculopathy as a side effect of certain drugs, and also in some systemic disorders. In dystrophies such as choroideremia, Goldmann-Favre syndrome, Enhanced S cone syndrome, clumped pigmentary retinal degeneration, juvenile x-linked retinoschisis, and Usher syndrome genes involved in the maintenance of retinal architecture are mutated and this can lead to non-leaking CMO [4].
Macular schisis could be considered synonymous with non-leaking CMO. Macular schisis first starts as cystic spaces in the retina and can eventually lead to macular detachment due to migration of the fluid under the neurosensory retina [5]. Morning glory syndrome, optic nerve head pits and colobomas are described as causes of macular schisis. Myopic foveoschisis and splitting of macular inner layers can occur in high myopic patients [6]. Macular schisis also can occur in patients without any known etiological factors [5]. Causes of non-leaking CMO should be kept in mind in differential diagnosis of cases with unexplained macular schisis.
Many aspects of non-leaking CMO are not yet clear. Therefore, a comprehensive search of the PubMed and google scholar databases was performed to identify different reported cases on this subject. This literature review aims to discuss different causes of non-leaking CMO, proposed mechanisms and management approaches. Three sections including drugs, retinal dystrophies, and systemic disorders are discussed in this review.
Causes of fluorescein angiography silent CMO
A. Drugs
Niacin
Niacin is a drug used for treating hyperlipidaemia [7]. The mechanism of macular oedema caused by niacin could be a breakdown of the blood–ocular barrier and subsequent extracellular fluid accumulation or swelling in Muller cells and intracellular fluid accumulation through a toxic effect [7]. Dajani and Lauer reported bilateral cystoid spaces in outer plexiform and inner nuclear layers and increased central retinal thickness detected by optical coherence tomography (OCT) in a patient who had been taking niacin 3 grams daily for several months. Fluorescein angiography(FA) did not show any leakage and 4 weeks after cessation of niacin, visual acuity normalized and OCT abnormalities disappeared [7]. The severity and incidence of macular oedema with this agent are dose-dependent [7] and usually occur in doses of more than 3 grams [8]. However, Domanico and colleagues reported bilateral cystoid spaces and retinal thickening without angiographic leakage in a patient who was taking 18 milligrams of niacin per day for 4 weeks which resolved after discontinuation of the drug [8]. In another study cystoid spaces in ganglion cell layer, outer and inner nuclear layers in OCT after taking 2 g of niacin daily for 5 months were described which reversed 1 month after drug discontinuation. In this study fundus autofluorescence (FAF) and FA were normal [9]. Cai et al reported a case of niacin maculopathy with ellipsoid zone disruption in addition to cystoid spaces in inner and outer nuclear layers. Ellipsoid zone disruptions persisted after drug discontinuation and resolution of macular oedema [10]. Niacin-induced macular oedema disappears with discontinuation of the agent and in one study macular oedema and visual symptoms improved after a decrease in dosage without a need for drug cessation [11].
Taxanes
Taxane agents including paclitaxel, nanoparticle albumin-bound (nab) paclitaxel and docetaxel stabilize microtubules and are used as chemotherapy drugs [12]. These drugs can induce angiographically silent macular oedema which usually resolves after cessation of the responsible drug [12,13,14]. In a study on 1533 patients who were receiving paclitaxel, 0.3% of them developed CMO [15]. The exact mechanism of CMO is not clear and many mechanisms have been proposed. Since the oedema is mostly located in the outer layers of the retina, RPE dysfunction due to disturbances in the microtubule structures of RPE cells could be a possible cause [16]. Also, Shih and Lee describe a case of paclitaxel-induced CMO with decreased Arden ratio in electrooculogram (EOG) which shows impaired RPE function [17]. Nomi et al. reported two cases of paclitaxel maculopathy with late petaloid hyperfluorescence in ICGA and suggested accumulation of intracellular fluid in the retina and minimal dysfunction of the blood-retina barrier as a possible mechanism [18]. ICGA in another case of paclitaxel maculopathy showed focal hypoperfusion of choroid along with choriocapillaris dropout in EDI-OCT and choriocapillaris impairment was proposed as a possible mechanism [17]. In that case, pentoxifylline which can increase choroidal blood flow was started [17]. However, in another case report of CMO due to paclitaxel in ICGA there was no sign of choroidal hyperpermeability and the authors concluded that choroidal vessels are not involved in the pathology [19]. Nakao et al suggested Müller cells toxicity as a possible mechanism of CMO due to paclitaxel because of observed delayed and reduced amplitude of b wave in ERG [20] but in another study the b wave of ERG was normal in paclitaxel-induced macular oedema [17]. In a study by Perez and colleagues fovea avascular zone was intact in OCTA suggesting a lack of vascular compromise [21]. In their study continuous inner and outer plexiform layers were consistent with CMO due to taxanes compared with other causes of CMO [21].
Kaya and colleagues reported that mean central macular thickness is significantly thicker in patients receiving taxane compared to the control group even without the presence of CMO [22]. Increased macular thickness especially in parafoveal and perifoveal areas, with relative sparing of the fovea, was reported in patients who were being treated with taxanes and did not have significant macular oedema [23].
Although reported symptoms and imaging findings often disappear after discontinuation of the drug, bilateral sub-macular hyperreflectivity of outer retinal layers without improvement after discontinuation of docetaxel has been reported [24]. In another case report, the authors described hyperpigmentation of RPE after resolution of nab-paclitaxel-induced CMO which was probably due to irreversible damage to RPE function after 4 years of using this drug [25].
The exact risk factors of developing CMO by using taxanes are not clear. Nghiem-Buffet et al described a case of cystoid macular oedema and foveal ellipsoid zone interruption due to docetaxel which was possibly potentiated by concomitant tamoxifen use [26] and in another study docetaxel-induced maculopathy was hypothesized to be potentiated by concurrent usage of hydroxychloroquine [27]. Bilateral docetaxel-induced CMO has also been reported in a patient with retinitis pigmentosa with a good response to drug discontinuation and nepafenac eye drop [28].
In addition to discontinuation of the responsible drug, other treatments have been reported in the literature. One of the options is a topical carbonic anhydrase inhibitor. In the case of nab-Paclitaxel-induced CMO a monocular trial of dorzolamide, three times daily was initiated in one eye in addition to drug cessation. After 2 weeks there was a marked decrease in CMO in the treated eye compared with the other eye [29]. In a case of paclitaxel-induced CMO with hypertrophic retinal pigment epithelial changes which was refractive to drug cessation topical dorzolamide 2% three times a day was started and after 4 weeks the vision improved and CMO resolved [30]. In another case of CMO secondary to paclitaxel without response to drug cessation, dorzolamide 1% eye drop 3 times a day led to resolution of CMO and since some cystoid spaces persisted at the parafovea for a year, dorzolamide was restarted to regress these spaces [31].In a case of docetaxel-induced CMO without response to drug discontinuation, 4 months of treatment with oral acetazolamide 250 MG TID led to CMO resolution [32]. In a case of CMO secondary to paclitaxel use, after improvement of macular oedema, paclitaxel was resumed and a maintenance dose of methazolamide was administered [33]. Similarly, in another case, paclitaxel was continued in combination with acetazolamide (250 mg twice a day) [34]. Another case of CMO secondary to paclitaxel was managed with acetazolamide 125 mg TDS and ketorolac 0.5% eye drops qid [35]. Another treatment option is intravitreal bevacizumab. In a case of bilateral CMO following nab-paclitaxel treatment, monthly intravitreal bevacizumab injections 2 times in the right eye and 3 times in the left eye along with the continuation of nab-paclitaxel led to stabilization of VA but CMO persisted on OCT [36]. In a case of bilateral nab-Paclitaxel-induced CMO after a complete cycle of chemotherapy, the authors randomized one eye to intravitreal bevacizumab injection every month and the other eye to three times daily dorzolamide 2% eye drops. The improvement in the eyes was approximately equal however there were some limitations to this interpretation including different VA and CMT in the eyes and the possible effect of IVB injection on the other eye [37]. However, in another case, the patient developed bilateral CMO due to nab-paclitaxel despite receiving intravenous bevacizumab and the CMO resolved with drug cessation [38]. Matsuoka et al reported a case of CMO due to nab-paclitaxel which was treated with subtenon injections of triamcinolone acetonide and they observed a mild decrease of CRT in the treated eye only after the first subtenon injection. The patient recovered after discontinuation of the drug which was 3 months after the second subtenon injection [39]. Similarly, in another case, intravitreal dexamethasone implants decreased CRT in the eyes but CMO persisted and 2 months later discontinuation of nab-paclitaxel led to the resolution of CMO [40]. Topical steroids and NSAIDs along with discontinuation of nab-paclitaxel [41], bromfenac eye drop, and cessation of paclitaxel [31] and nepafenac 3 mg/mL eye drop daily together with paclitaxel cessation [42] caused resolution of CMO in reported cases.
Pentosan polysulfate sodium
Pentosan polysulfate sodium (PPS) is an analog of biologic glycosaminoglycans which is used for the treatment of interstitial cystitis [43]. One of the adverse effects of this drug is maculopathy in a wide spectrum including cystoid macular oedema with and without leakage in fluorescein angiography [43]. In a study on 70 eyes of patients taking pentosan polysulfate sodium, 9 eyes had macular oedema and fluorescein angiography in one of the patients showed dye leakage [43]. In a case report of a patient receiving PPS for 10 years, a fluorescein angiogram showed CMO without leakage in both eyes which responded well to intravitreal bevacizumab injections [44].
Hydroxychloroquine
Hydroxychloroquine (HCQ) is used to treat many autoimmune disorders [45]. One of the known adverse effects of this drug is macular toxicity [45]. Macular oedema with [46] and without leakage in FA is also reported in patients taking this drug. Parikh and colleagues described two cases of non-leaking CMO due to HCQ [47]. The first one was a case of HCQ toxicity due to using a total dose of 292 grams of HCQ in 2 years which the drug was stopped 3 years earlier. OCT showed ellipsoid zone and external limiting membrane loss in parafovea and intraretinal cystoid spaces with no late-phase leakage in fluorescein angiography. CMO resolved 4 years after drug discontinuation [47]. The second case used a total dose of 1308 grams of HCQ in 9 years. She had a CMO which did not respond to intravitreal bevacizumab, nepafenac eye drop, steroids, mycophenolate mofetil, or intravitreal triamcinolone. OCT showed diffuse outer retinal loss and inner retinal thinning and CMO with no leakage in fluorescein angiography. HCQ was discontinued and the CMO was resolved 22 months later [47]. Chang and Sheu described a case using HCQ 6.90 mg/kg daily for 18 months who developed anterior uveitis and macular oedema and the macular oedema was persistent after resolution of uveitis and improved after drug cessation. Fluorescein angiography was not performed on this patient due to allergy [48].In another case, the patient had received 8.5 mg/kg daily for 20 years with a total dose of 2920 grams and presented with HCQ retinal toxicity and bilateral CMO. HCQ was stopped but CMO did not resolve 3 months after that. At that point, 2% dorzolamide -eye-drop was started BD which led to resolution of CMO after 3 months [49]. Another patient received 7.7 mg/kg HCQ daily for 18 years with retinal toxicity and CMO responded well to dorzolamide 2% eye drops in 2 months and recurrence of CMO also improved with the same treatment [49].
In a study on 11 patients with CQ/HCQ retinopathy who were followed after drug cessation, bilateral cystoid macular oedema was detected in 3 patients and FA performed in 1 patient showed mild leakage. Treatment with dorzolamide or acetazolamide did not show any benefit in 2 patients and had a mild effect in 1 of the patients [45].
B. Retinal dystrophies
X-linked juvenile retinoschisis (XLRS)
XLRS is an X-linked recessive vitreoretinal dystrophy manifesting with cystic maculopathy, peripheral schisis of the retina, and electronegative ERG [50, 51]. Non-leaking CMO is also a feature of this disorder [52]. In this dystrophy, an RS1 gene mutation causes abnormal expression of retinoschisin which is important in cell-to-cell interactions. This can cause abnormalities in retinal structure and lead to intraretinal cystoid spaces [53].
These Cystic lesions in the macula of patients with XLRS may resolve without treatment [51] but some treatment options are reported in the literature. One of the most commonly used treatment options is using carbonic anhydrase inhibitors. In a study, 30.8% of eyes receiving dorzolamide eye drops for macular schisis showed a decrease in central foveal thickness [51]. Ghajarnia and Gorin reported an 8-year-old boy with XLRS and schisis in the central macula who responded well to acetazolamide 250 mg daily. About 2 months after discontinuation of the drug, recurrence of schisis occurred, and restarting acetazolamide led to improvement in macular morphology [54].In a study by Khandhadia et al 7 eyes of patients with XLRS were treated with dorzolamide 2% eye drop three times daily. This treatment led to an improvement of central macular thickness in OCT but without improvement in visual acuity [55].In a study by Verbakel and colleagues on children with XLRS, treatment with carbonic anhydrase inhibitors led to a significant reduction of foveal thickness in 55.6% of patients, and in most of them, this was apparent 1 month after starting treatment. An improvement in vision was also observed in 27.8% of the eyes [56]. Galantuomo et al described a marked rebound effect after cessation of acetazolamide in a patient who did not receive any maintenance treatment in contrast to his brother who received topical dorzolamide after acetazolamide discontinuation [57].
Ansari and colleagues reported a case of XLRS who developed unilateral non-leaking CMO after scleral buckle and pars plana vitrectomy of one eye. CMO worsened after a 1-month follow-up of the patient and then triamcinolone acetonide was injected intravitreally. Recurrences of CMO were treated with intravitreal triamcinolone, multiple intravitreal dexamethasone implants,s and intravitreal fluocinolone implantation [58].
Figure 1 shows macular oct scans of a 12-year-old male case of XLRS with CMO. Hyporeflective spaces mostly in the inner nuclear layer are visible in both eyes (Fig. 1).
NR2E3-related retinopathies
Enhanced S-cone syndrome (ESCS) and Goldmann Favre Syndrome (GFS) are phenotypes of an autosomal recessive retinal degenerative disease which occur due to mutations in NR2E3 [59]. Fundus examination typically reveals degenerative nummular pigmentary changes along vascular arcades, foveal schisis, and macular pigmentary changes [60]. Macular cysts and oedema without leakage in FA can also exist [61]. Iannaccone and colleagues described a 48-year-old male case of ESCS with acute vision loss and macular cystic changes without any pooling or leakage in FA who was successfully treated with oral acetazolamide [62]. Kiszkielis et al reported a 28-year-old man with ESCS and macular cysts without leakage in FA in both eyes who was treated with topical dorzolamide 3 times a day. This treatment resulted in improved VA, foveal thickness, and function in one of the eyes in 6 months [60]. Also in another report by Genead et al, dorzolamide eye drop improved VA, macular thickness, microperimetry, and contrast sensitivity after 4 months in a patient with ESCS and unilateral non-leaking CMO [63]. In contrast, a seven-year-old boy with ESCS and cystoid macular lesions with no leakage in fluorescein angiography was treated with dorzolamide drops for 7 months but no improvement was noted in macular morphology [64]. The authors hypothesized that the breakdown of the blood-retinal barrier could be of more importance in older patients, leading to this difference in response to CAI drugs between children and adults [64]. In addition to medical management of these cystic macular lesions, surgical management is also reported in the literature. Bechet et al performed pars plana vitrectomy with silicone oil in a case of ESCS with macular schisis which was unresponsive to topical and oral CAIs. After surgery improvement in visual acuity and a decrease in foveoschisis were noted [59].
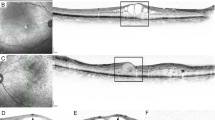
Figure 2 demonstrates fundus photo and macular OCT scans of a patient with ESCS and CMO (figure 2).
Fundus photo of right (A) and left (C) eyes of a patient with enhanced-S-cone syndrome demonstrating nummular pigmentary changes along vascular arcades, schisis and pigmentary changes in fovea. Macular OCT scans of right (B) and left (D) eyes show large hyporeflective spaces mostly in outer nuclear layers and schisis in inner nuclear layers.
Retinitis pigmentosa (RP)
Retinitis pigmentosa is a heterogeneous group of hereditary retinal diseases that cause loss of RPE cells and photoreceptors [65]. Difficulty in night vision and loss of peripheral vision are characteristic symptoms and the occurrence of CMO can affect central vision in these patients [65]. Some of the proposed mechanisms for CMO in RP patients include fluid leakage through RPE, vitreoretinal interface abnormalities, degeneration of Muller cells, and disruption of the blood-retinal barrier [66, 67]. Iovino and colleagues reported that subfoveal choroidal thickness was significantly higher and choroidal vascularity index was significantly lower in RP patients with CMO compared to those without CMO and these findings can demonstrate the role of the choroid in the pathogenesis of CMO in these patients [67].
Up to 49% of RP patients can have cystoid macular lesions with different mechanisms which might show no leakage in fluorescein angiography. Non-leaking intraretinal cystoid spaces are reported to account for less than 30% of overall cystoid macular lesions in these patients [68]. Cystoid macular lesions occur most commonly in autosomal dominant forms of RP [68].
Lai and colleagues reported a case of RP with non-leaking CMO due to MAK gene mutation [68]. In a study by YEO et al, in 20 RP eyes with CMO, 6 eyes showed mild leakage in the late phase of fluorescein angiography and the degree of leakage did not correlate with the amount of CMO [69]. The authors hypothesized that this mild leakage in fluorescein angiography may be due to capillary barrier breakdown induced by Müller cells and not a vasculogenic CMO [69].
The most common drugs used for treating CMO in RP patients are different types of CAIs that can reduce leakage in fluorescein angiography [70]. However, the exact effect of these agents or other treatments in non-leaking CMO has not been identified in the current literature.
Figure 3 shows FAF, macular OCT, and FA images of a 30-year-old patient with RP sine pigmento and CMO. The absence of dye leakage in FA is noted in this patient (Fig. 3).
A, B fundus autofluroscence of both eyes showing a ring of hyperautofluroscence in the centre of macula. C, D. macular OCT scans of both eyes demonstrate hyporeflective spaces mostly in inner and outer nuclear layers in both eyes compatible with cystoid macular oedema. In flurosceine angiography of both eyes (E, F) absence of dye leakage is visible.
Gyrate atrophy
Gyrate atrophy is a type of chorioretinal degeneration due to OAT gene mutation with autosomal recessive inheritance. CMO without leakage in FA can occur in this disorder probably due to RPE and Müller cells dysfunction because of the toxic effect of a high concentration of ornithine [71]. Foveoschisis without leakage in FA has been reported [72]. Tripathy et al reported 3 siblings with gyrate atrophy and 2 of them had cystic macular changes and foveoschisis in OCT without leakage in FA [73]. Kim and colleagues also report a twin with gyrate atrophy and CMO in OCT with minimal dye leakage in fluorescein angiography [74]. Some treatment options have been mentioned in the literature. Çavdarlı et al reported a case of gyrate atrophy with non-leaking CMO that was treated with brinzolamide and nepafenac eye drops which led to a decrease in macular thickness and improved visual acuity [75]. In a case report by Piozzi and colleagues combination of topical indomethacin and systemic acetazolamide in a patient with gyrate atrophy and CMO led to a decrease in macular thickness [76]. In another study, the authors used posterior subtenon triamcinolone acetonide injection with a theory that chronic exposure of the retina to high levels of ornithine could cause retinal inflammation and this might be a reason for CMO in these patients. This treatment led to a decrease in CMO in OCT imaging [77]. In a case report by Elnahry et al monthly intravitreal bevacizumab injection caused improvement in intraretinal cystic spaces without leakage in FA in a patient with gyrate atrophy [78]. Intravitreal bevacizumab injection can improve visual acuity and decrease central macular thickness [79]. A diet with low protein and vitamin B6 supplementation has been shown to improve CMO in these patients [80, 81]. However, Doguizi et al reported a 9-year-old boy case of gyrate atrophy with bilateral CMO which did not show any improvement after 2 years of an arginine-restricted diet [82].
Figure 4 shows fundus photography and macular oct scans of a 14-year-old boy with gyrate atrophy. Macular oedema is visible in oct of both eyes.
Choroideremia
Choroideremia is progressive chorioretinal dystrophy that has X-linked inheritance [83]. Cystoid macular oedema is a feature of this disorder. In a study by Genead and Fishmann, 62.5% of patients with choroideremia had CMO on spectral-domain OCT in at least one eye due to malfunction of the blood-retinal barrier and intraretinal fluid accumulation [83]. Murro and colleagues detected intraretinal cysts in 38% of choroideremia patients on swept-source OCT in the inner nuclear layer and ganglion cell layer. The authors concluded that since all patients had cysts in the inner nuclear layer, dysfunction of Muller cells could be the responsible mechanism [84]. In a study by Iovino et al there was no significant correlation between choroidal parameters and the presence of cystoid spaces in choroideremia patients, so choroid is probably not involved in development of cystoid spaces in these patients [85]. There is limited data about treatment options for CMO in patients with choroideremia. Genead and colleagues reported that topical dorzolamide 2% three times daily caused a decrease in macular thickness and improvement of visual acuity in 2 choroideremia patients with CMO [86].
C. Systemic disorders
Cohen syndrome
Cohen syndrome is a rare disease with autosomal recessive inheritance. Characteristic features include hypotonia, developmental delay, microcephaly, obesity, neutropenia, hypermobility, psychomotor retardation, and facial features [87]. Many ophthalmological findings are reported; the most common are high myopia and retinal dystrophy [88]. Since this disorder is a syndromic form of rod-cone dystrophy, one of the most consistent OCT findings includes peripheral loss of photoreceptor layers [89]. Cystoid macular oedema without leakage in fluorescein angiography can also occur [88, 90]. In a study, 80 percent of patients at the time of first OCT examination had bilateral cystoid maculopathy [89]. CMO may resolve without treatment in time as dystrophy of photoreceptors evolves [89]. Beck and colleagues reported an 11-year-old girl with Cohen syndrome and bilateral non-leaking CMO who was treated with dorzolamide eye drops which led to stabilization of VA and macular features [87]. Brinzolamide eye drops are also reported to cause resolution of CMO in these patients [91]. In some studies, however, no significant improvement is observed with CAIs [89, 92].
Maternally inherited diabetes and deafness (MIDD)
MIDD is caused by a mitochondrial DNA mutation and presents with maternally inherited early adult-onset diabetes, sensorineural hearing loss, and maculopathy. Non-leaking cystoid macular oedema has been reported in this disorder. Marco-Campmany et al reported a case of MIDD who had bilateral hyperpigmented perifoveal dots which evolved to fovea-sparing macular atrophy. About twenty years after the first visit the patient had a unilateral decline in VA and cystic macular changes in OCT without leakage in FA. The patient had a good response to intravitreal anti-VEGF injections with a resolution of macular cysts and improvement in VA[93]. Qian and colleagues reported a 30-year-old MIDD patient with macular cystoid and pigmentary changes in both eyes without leakage in FA. After starting immunosuppressives and dorzolamide drops there was a reduction of macular cysts and an improvement in visual acuity. The patient continued on dorzolamide but macular cysts recurred in both eyes and resolved after intravitreal triamcinolone injection. During follow-ups, RPE atrophy progressed without recurring macular cysts [94].
Multiple myeloma
Multiple myeloma is a malignancy of plasma cells in which excess immunoglobulins are produced. Manifestations in fundus examination include macular oedema, retinal haemorrhages, microaneurysms, dilation, tortuosity, and occlusions of vessels due to hyperviscosity and anaemia [95]. These changes can look like diabetic retinopathy but the absence of leakage in FA and lack of response to usual therapies for diabetic macular oedema can help differentiate these two conditions [95]. Non-leaking CMO could even be the presenting sign of multiple myeloma [96].
Macular oedema without leakage in FA in these patients can occur due to immunoglobulins deposition in outer retinal layers and subretinal space and accumulation of water by osmosis (99). The absence of leakage in FA indicates that blood-retina-barrier disruption is not the main mechanism for macular oedema [97]. The oedema usually resolves after systemic treatment and resorption of paraproteins from the subretinal space [98]. Georgakopoulos et al report a case of multiple myeloma with immunogammopathy maculopathy who was treated with intravitreal dexamethasone implant in addition to systemic treatment of multiple myeloma which led to complete resolution of bilateral macular oedema [97].
Waldenstrom’s macroglobulinemia
Waldenstrom macroglobulinemia is characterized by monoclonal IgM immunogammopathy which causes elevated serum viscosity and hyperviscosity-related retinopathy. Deposition of IgM molecules in subretinal space in these patients can induce an osmotic gradient and cause an accumulation of fluid in subretinal space [99]. Baker et al reported 4 cases of Waldenstrom’s Macroglobulinemia with serous macular detachment and cystoid macular oedema and 3 of these patients had no leakage in fluorescein angiography [99]. They hypothesized that outer retinal disruption can allow immunoglobulins and intraretinal fluid to flow into subretinal space and cause a serous retinal detachment [99]. Besirli and Johnson report a case of non-leaking CMO in a patient with Waldenström macroglobulinemia which did not show a response to intravitreal bevacizumab, peripheral laser photocoagulation, or intravitreal triamcinolone and improved after systemic therapy of multiple myeloma [100].
Conclusion
Cystoid macular oedema without leakage in fluorescein angiography can occur in a variety of disorders like maculopathy due to side effects of drugs, retinal dystrophies, and systemic disorders. The exact underlying mechanisms of non-leaking CMO are not yet clear but the absence of dye leakage in most cases points to a mechanism other than vasogenic oedema and dysfunction of capillaries.
Conventional treatment options may not show benefit in some of these disorders and diagnosis of these diseases may prompt using specific ways of treatment that are discussed in each section of the article. In addition, some of these disorders can mimic common retinal problems, and the absence of dye leakage in FA can help differentiate these disorders which may even be fatal in some cases.
References
Holló G, Aung T, Cantor LB, Aihara M. Cystoid macular edema related to cataract surgery and topical prostaglandin analogs: Mechanism, diagnosis, and management. Surv Ophthalmol. 2020;65:496–512.
Chetrit M, Bonnin S, Mané V, Erginay A, Tadayoni R, Gaudric A, et al. Acute pseudophakic cystoid macular edema imaged by optical coherence tomography angiography. Retina 2018;38:2073–80.
Bringmann A, Reichenbach A, Wiedemann P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004;36:241–9.
Lingao MD, Ganesh A, Karthikeyan AS, Al Zuhaibi S, Al-Hosni A, Al Khayat A, et al. Macular cystoid spaces in patients with retinal dystrophy. Ophthalmic Genet. 2016;37:377–83.
Mavrikakis E, Lam WC. Macular schisis and detachment secondary to large optic nerve head cup: a newly recognized syndrome amenable to vitrectomy. Acta ophthalmologica. 2011;89:95–6.
Gohil R, Sivaprasad S, Han L, Mathew R, Kiousis G, Yang Y. Myopic foveoschisis: a clinical review. Eye 2015;29:593–601.
Dajani HM, Lauer AK. Optical coherence tomography findings in niacin maculopathy. Can J Ophthalmol. 2006;41:197–200.
Domanico D, Carnevale C, Fragiotta S, Verboschi F, Altimari S, Vingolo EM. Cystoid macular edema induced by low doses of nicotinic Acid. Case reports in ophthalmological medicine. 2013;2013:713061.
Courtney R, Singh R. Spectral domain optical coherence tomography features in niacin maculopathy. Eye 2014;28:629–32.
Cai S, Liu TA, Arevalo JF. Evolution of ellipsoid zone abnormalities on optical coherence tomography associated with niacin maculopathy. JAMA Ophthalmol. 2019;137:849–51.
Freisberg L, Rolle TJ, Ip MS. Diffuse macular edema in niacin-induced maculopathy may resolve with dosage decrease. Retinal Cases Brief Rep. 2011;5:227–8.
Padrón Pérez N, Rubio Caso MJ, Arias Barquet L, Caminal, Mitjana JM. Bilateral cystoid macular edema in a patient with taxane-based chemotherapy. Can J Ophthalmol. 2013;48:e3–4.
Teitelbaum BA, Tresley DJ. Cystic maculopathy with normal capillary permeability secondary to docetaxel. Optom Vis Sci. 2003;80:277–9.
Arora S, Surakiatchanukul T, Arora T, Errera MH, Agrawal H, Lupidi M, et al. Retinal toxicities of systemic anticancer drugs. Surv Ophthalmol. 2022;67:97–148.
Fortes BH, Liou H, Dalvin LA. Ophthalmic adverse effects of taxanes: The Mayo Clinic experience. European Journal of Ophthalmology. 2022;32:602–11.
Kuznetcova TI, Cech P, Herbort CP. The mystery of angiographically silent macular oedema due to taxanes. Int Ophthalmol. 2012;32:299–304.
Shih CH, Lee YC. Impaired retinal pigment epithelium in paclitaxel-induced macular edema: a case report. Med (Baltim). 2018;97:e11229.
Nomi N, Ota M, Fukumura M, Nuno Y, Hatano M, Wakuta M, et al. Indocyanine green angiography findings of cystoid macular edema secondary to paclitaxel therapy. Jpn J Ophthalmol. 2018;62:163–7.
Lee J, Ra H, Baek J. Ultra-widefield angiographic imaging of albumin-bound paclitaxel-induced cystoid macular edema. Indian J Ophthalmol. 2019;67:2058–9.
Nakao S, Ikeda Y, Emi Y, Ishibashi T. Possibility of Müller cell dysfunction as the pathogenesis of paclitaxel maculopathy. ophthalmic surg lasers imaging. Retina 2016;47:81–4.
Perez JM, Teo K, Ong R, Maruyama-Inoue M, Freund KB, Tan ACS. Optical coherence tomography characteristics of taxane-induced macular edema and other multimodal imaging findings. Graefes Arch Clin Exp Ophthalmol. 2020;258:1607–15.
Kaya M, Atas F, Gulsum Guc Z, Oztop I, Durak I, Saatci AO. A cross-sectional optical coherence tomography study in patients on taxane-based therapy and a case report with the literature review. Cutan Ocul Toxicol. 2020;39:287–93.
Chelala E, Arej N, Antoun J, Kourie HR, Zaarour K, Haddad FG, et al. Central macular thickness monitoring after a taxane-based therapy in visually asymptomatic patients. Chemotherapy 2017;62:199–204.
Torrado LA, Fivgas GD. Unilateral cystoid macular edema and bilateral subfoveal hyperreflectivity following docetaxel chemotherapy: A case report. Am J Ophthalmol Case Rep. 2020;20:100995.
Haider A, Bababeygy SR, Lu SY. Cystoid macular edema and macular pigmentation associated with nab-Paclitaxel therapy. Retin Cases Brief Rep. 2015;9:220–2.
Nghiem-Buffet S, Cohen SY, Giocanti-Auregan A. Docetaxel retinopathy: a case report. Case Rep. Ophthalmol. 2017;8:21–5.
Elhusseiny AM, Relhan N, Smiddy WE. Docetaxel-induced maculopathy possibly potentiated by concurrent hydroxychloroquine use. Am J Ophthalmol Case Rep. 2019;16:100560.
Enzsoly A, Kammerer K, Nemeth J, Schneider M. Bilateral cystoid macular edema following docetaxel chemotherapy in a patient with retinitis pigmentosa: a case report. BMC Ophthalmol. 2015;15:32.
Ehlers J, Rayess H, Steinle N. Topical dorzolamide therapy for taxane-related macular oedema. Eye 2013;27:102–4.
Dwivedi R, Tiroumal S. Possible efficacy of topical dorzolamide in the treatment of paclitaxel-related cystoid macular edema. Retin Cases Brief Rep. 2018;12:75–9.
Yokoe T, Fukada I, Kobayashi K, Shibayama T, Miyagi Y, Yoshida A, et al. Cystoid macular edema during treatment with paclitaxel and bevacizumab in a patient with metastatic breast cancer: a case report and literature review. Case Rep. Oncol. 2017;10:605–12.
Telander DG, Sarraf D. Cystoid macular edema with docetaxel chemotherapy and the fluid retention syndrome. Semin Ophthalmol. 2007;22:151–3.
Koo NK, Kim YC. A case of paclitaxel-induced maculopathy treated with methazolamide. Korean J Ophthalmol. 2012;26:394–7.
Meyer KM, Klink T, Ugurel S, Bröcker EB. Regression of paclitaxel-induced maculopathy with oral acetazolamide. Graefes Arch Clin Exp Ophthalmol. 2012;250:463–4.
Georgakopoulos CD, Makri OE, Vasilakis P, Exarchou A. Angiographically silent cystoid macular oedema secondary to paclitaxel therapy. Clin Exp Optom. 2012;95:233–6.
Rahman HT, Yeh S, Bergstrom CS. Cystoid macular edema without leakage secondary to nab-Paclitaxel (Abraxane): clinical experience with intravitreal bevacizumab. J Ocul Pharm Ther. 2013;29:360–2.
Hassall MM, Andrew NH. Single-eye trial of a topical carbonic anhydrase inhibitor versus intravitreal bevacizumab for the treatment of taxane drug-induced cystoid macula oedema. BMJ Case Rep. 2016;2016:https://doi.org/10.1136/bcr-2015-212733.
Baskin DE, Garg SJ. Abraxane-induced cystoid macular edema refractory to concomitant intravenous bevacizumab. Can J Ophthalmol. 2011;46:200–1.
Matsuoka N, Hasebe H, Mayama T, Fukuchi T. Sub-Tenon Injections of Triamcinolone Acetonide Had Limited Effect on Cystoid Macular Edema Secondary to Nanoparticle Albumin-Bound-Paclitaxel (Abraxane). Case Rep. Ophthalmol Med. 2015;2015:181269.
Burgos-Blasco B, Hernandez-Ruiz S, Lopez-Guajardo L, Donate-Lopez J. Dexamethasone intravitreal implant in cystoid macular edema secondary to paclitaxel therapy. Am J Ophthalmol Case Rep. 2020;18:100653.
Murphy CG, Walsh JB, Hudis CA, Lake D, Theodoulou M. Cystoid macular edema secondary to nab-paclitaxel therapy. J Clin Oncol. 2010;28:e684–7.
Tapia Quijada HE, Quijada Fumero E, Mesa Lugo FI, Serrano García M, Betancor Caro N. Nepafenac for cystoid macular oedema secondary to paclitaxel. Arch Soc Esp Oftalmol (Engl Ed). 2021;96:434–7.
Hanif AM, Armenti ST, Taylor SC, Shah RA, Igelman AD, Jayasundera KT, et al. Phenotypic spectrum of pentosan polysulfate sodium-associated maculopathy: a multicenter study. JAMA Ophthalmol. 2019;137:1275–82.
De Larochellière E, Bourgault S. Pentosan polysulfate sodium-induced pigmentary maculopathy with nonleaking cystoid macular edema successfully treated with anti–vascular endothelial growth factor therapy. Retinal Cases & Brief Reports. 2022;16:482–5.
Kellner S, Weinitz S, Farmand G, Kellner U. Cystoid macular oedema and epiretinal membrane formation during progression of chloroquine retinopathy after drug cessation. Br J Ophthalmol. 2014;98:200–6.
Hong EH, Ahn SJ, Lim HW, Lee BR. The effect of oral acetazolamide on cystoid macular edema in hydroxychloroquine retinopathy: a case report. BMC Ophthalmol. 2017;17:124.
Parikh VS, Modi YS, Au A, Ehlers JP, Srivastava SK, Schachat AP, et al. Nonleaking cystoid macular edema as a presentation of hydroxychloroquine retinal toxicity. Ophthalmology 2016;123:664–6.
Chang CY, Sheu SJ. Macular edema might be a rare presentation of hydroxychloroquine-induced retinal toxicity. Taiwan J Ophthalmol. 2017;7:56–8.
Kim DG, Yoon CK, Kim HW, Lee SJ. Effect of topical dorzolamide therapy on cystoid macular edema in hydroxychloroquine retinopathy. Can J Ophthalmol. 2018;53:e103–e7.
Ali S, Seth R. X-linked juvenile retinoschisis in females and response to carbonic anhydrase inhibitors: case report and review of the literature. Semin Ophthalmol. 2013;28:50–4.
Wang NK, Liu L, Chen HM, Tsai S, Chang TC, Tsai TH, et al. Clinical presentations of X-linked retinoschisis in Taiwanese patients confirmed with genetic sequencing. Mol Vis. 2015;21:487–501.
Önen M, Zor K, Küçük E, Yıldırım G. X-linked retinoschisis in females in a consanguineous family: a rare entity. Turk J Ophthalmol. 2020;50:252–4.
Guimaraes TAC, Capasso JE, Levin AV. Paradoxical response to carbonic anhydrase inhibitors in patients with intraretinal cystoid spaces. Ophthalmic Genet. 2019;40:213–8.
Ghajarnia M, Gorin MB. Acetazolamide in the treatment of X-linked retinoschisis maculopathy. Arch Ophthalmol. 2007;125:571–3.
Khandhadia S, Trump D, Menon G, Lotery AJ. X-linked retinoschisis maculopathy treated with topical dorzolamide, and relationship to genotype. Eye (Lond). 2011;25:922–8.
Verbakel SK, van de Ven JP, Le Blanc LM, Groenewoud JM, de Jong EK, Klevering BJ, et al. Carbonic anhydrase inhibitors for the treatment of cystic macular lesions in children with X-linked juvenile retinoschisis. Invest Ophthalmol Vis Sci. 2016;57:5143–7.
Galantuomo MS, Fossarello M, Cuccu A, Farci R, Preising MN, Lorenz B, et al. Rebound macular edema following oral acetazolamide therapy for juvenile X-linked retinoschisis in an Italian family. Clin Ophthalmol. 2016;10:2377–82.
Ansari WH, Browne AW, Singh RP. Juvenile X-linked retinoschisis responsive to intravitreal corticosteroids. Am J Ophthalmol Case Rep. 2017;5:48–51.
Bechet L, Atia R, Zeitz C, Mohand-Saïd S, Sahel JA, Barale PO, et al. Management of a case of Enhanced S-cone syndrome with massive foveoschisis treated with pars plana vitrectomy with silicone oil tamponade. Ophthalmic Genetics. 2021;42:615–8.
Kiszkielis M, Lubiński W, Penkala K. Topical dorzolamide treatment of macular cysts in the enhanced S-cone syndrome patient. Doc Ophthalmologica. 2013;126:241–6.
Audo I, Michaelides M, Robson AG, Hawlina M, Vaclavik V, Sandbach JM, et al. Phenotypic variation in enhanced S-cone syndrome. Invest Ophthalmol Vis Sci. 2008;49:2082–93.
Iannaccone A, Fung KH, Eyestone ME, Stone EM. Treatment of adult-onset acute macular retinoschisis in enhanced s-cone syndrome with oral acetazolamide. Am J Ophthalmol. 2009;147:307–12. e2
Genead MA, Fishman GA, McAnany JJ. Efficacy of topical dorzolamide for treatment of cystic macular lesions in a patient with enhanced S-cone syndrome. Doc ophthalmologica. 2010;121:231–40.
Bušić M, Bjeloš M, Bosnar D, Ramić S, Bušić I. Cystoid macular lesions are resistant to topical dorzolamide treatment in enhanced S-cone syndrome child. Doc Ophthalmologica. 2016;132:67–73.
Salvatore S, Fishman GA, Genead MA. Treatment of cystic macular lesions in hereditary retinal dystrophies. Surv Ophthalmol. 2013;58:560–84.
Ganesh A, Stroh E, Manayath GJ, Al-Zuhaibi S, Levin AV. Macular cysts in retinal dystrophy. Curr Opin Ophthalmol. 2011;22:332–9.
Iovino C, Au A, Hilely A, Violanti S, Peiretti E, Gorin MB, et al. Evaluation of the choroid in eyes with retinitis pigmentosa and cystoid macular edema. Invest Ophthalmol Vis Sci. 2019;60:5000–6.
Lai YH, Capasso JE, Kaiser R, Levin AV. Intraretinal cystoid spaces in a patient with retinitis pigmentosa due to mutation in the MAK gene. Ophthalmic Genet. 2016;37:424–6.
Yeo JH, Kim YJ, Yoon YH. Optical coherence tomography angiography in patients with retinitis pigmentosa–associated cystoid macular edema. Retina 2020;40:2385–95.
Liew G, Moore AT, Webster AR, Michaelides M. Efficacy and prognostic factors of response to carbonic anhydrase inhibitors in management of cystoid macular edema in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2015;56:1531–6.
Mansour AM, Elnahry AG, Tripathy K, Foster RE, Mehanna CJ, Vishal R, et al. Analysis of optical coherence angiography in cystoid macular oedema associated with gyrate atrophy. Eye (Lond). 2021;35:1766–74.
Zhioua Braham I, Ammous I, Maalej R, Boukari M, Mili Boussen I, Errais K, et al. Multimodal imaging of foveoschisis and macular pseudohole associated with gyrate atrophy: a family report. BMC Ophthalmol. 2018;18:89.
Tripathy K, Chawla R, Sharma YR, Gogia V. Ultrawide field fluorescein angiogram in a family with gyrate atrophy and foveoschisis. Oman J Ophthalmol. 2016;9:104–6.
Kim SJ, Lim DH, Kim JH, Kang SW. Gyrate atrophy of the choroid and retina diagnosed by ornithine-δ-aminotransferase gene analysis: a case report. Korean J Ophthalmol. 2013;27:388–91.
Çavdarlı C, Şahlı E, Çavdarlı B, Alp MN. Regression of macular edema with topical brinzolamide and nepafenac alone and identification of a novel gyrate atrophy mutation. Arquivos Brasileiros de Oftalmologia. 2020;83:149–52.
Piozzi E, Alessi S, Santambrogio S, Cillino G, Mazza M, Iggui A, et al. Carbonic anhydrase inhibitor with topical NSAID therapy to manage cystoid macular edema in a case of gyrate atrophy. Eur J Ophthalmol. 2017;27:e179–e83.
Alparslan Ş, Fatih MT, Muhammed Ş, Adnan Y. Cystoid macular edema secondary to gyrate atrophy in a child treated with sub-tenon injection of triamcinolone acetonide. Rom J Ophthalmol. 2018;62:246.
Elnahry AG, Hassan FK, Abdel-Kader AA. Bevacizumab for the treatment of intraretinal cystic spaces in a patient with gyrate atrophy of the choroid and retina. Ophthalmic Genet. 2018;39:759–62.
Elnahry AG, Aboulfotouh MR, Nassar GA. Treatment of intraretinal cystic spaces associated with gyrate atrophy of the choroid and retina with intravitreal bevacizumab. J Pediatr Ophthalmol Strabismus. 2020;57:400–6.
Casalino G, Pierro L, Manitto MP, Michaelides M, Bandello F. Resolution of cystoid macular edema following arginine-restricted diet and vitamin B6 supplementation in a case of gyrate atrophy. J aapos. 2018;22:321–3.
Heller D, Weiner C, Nasie I, Anikster Y, Landau Y, Koren T, et al. Reversal of cystoid macular edema in gyrate atrophy patients. Ophthalmic Genet. 2017;38:549–54.
Doguizi S, Sekeroglu MA, Anayol MA, Yilmazbas P. Arginine-restricted therapy resistant bilateral macular edema associated with gyrate atrophy. Case Rep. Ophthalmol Med. 2015;2015:137270.
Genead MA, Fishman GA. Cystic macular oedema on spectral-domain optical coherence tomography in choroideremia patients without cystic changes on fundus examination. Eye (Lond). 2011;25:84–90.
Murro V, Mucciolo DP, Giorgio D, Sodi A, Passerini I, Bacci G, et al. Optical coherence tomography (OCT) features of cystoid spaces in choroideremia (CHM). Graefes Arch Clin Exp Ophthalmol. 2019;257:2655–63.
Iovino C, Di Iorio V, Testa F, Bombace V, Melillo P, Vupparaboina KK, et al. Choroidal vascularity features in patients with choroideremia and cystoid spaces. Diagnostics. 2021;11:382.
Genead MA, McAnany JJ, Fishman GA. Topical dorzolamide for treatment of cystoid macular edema in patients with choroideremia. Retina 2012;32:826–33.
Beck KD, Wong RW, Gibson JB, Harper CA III. Nonleaking cystoid macular edema in Cohen syndrome. J Am Assoc Pediatr Ophthalmol Strabismus. 2019;23:38–9. e1
Liles CA, Tensmeyer MS, York JM, Ekanayake LS, Lew J. Cystoid macular edema in a 10-year-old boy with Cohen syndrome. Cureus. 2020;12:e8443.
Gabrielle P-H, Faivre L, Audo I, Zanlonghi X, Dollfus H, Thiadens AA, et al. Cystoid maculopathy is a frequent feature of Cohen syndrome-associated retinopathy. Sci Rep. 2021;11:1–12.
Rakusiewicz K, Kanigowska K, Hautz W, Wicher D, Młynek M, Wyszyńska M, et al. Coexistence of bilateral macular edema and pale optic disc in the patient with Cohen syndrome. Open. Medicine 2021;16:156–60.
Sevik MO, Aykut A, Şahin Ö. Resolution of cystoid macular edema with topical carbonic anhydrase inhibitor in a patient with retinal dystrophy associated with Cohen syndrome. Ophthalmic Genetics. 2021;42:619–23.
Nasser F, Kurtenbach A, Biskup S, Weidensee S, Kohl S, Zrenner E. Ophthalmic features of retinitis pigmentosa in Cohen syndrome caused by pathogenic variants in the VPS13B gene. Acta Ophthalmol. 2020;98:e316–e21.
Marco-Campmany A, Pacheco-Cervera J, Navarrete-Sanchis J, Tomás-Torrent JM, García-Canet S, Cuadrado-Gómez T, et al. Intravitreal bevacizumab in cystoid macular edema associated to maternally inherited diabetes and deafness’s macular dystrophy. European Journal of Ophthalmology. 2022;32:NP34–9.
Qian CX, Branham K, Khan N, Lundy SK, Heckenlively JR, Jayasundera T. Cystoid macular changes on optical coherence tomography in a patient with maternally inherited diabetes and deafness (MIDD)-associated macular dystrophy. Ophthalmic Genet. 2017;38:467–72.
Rao K, Murthy H, Muralidhar NS, Rani PK. Multiple myeloma masquerading as diabetic macular oedema. Case Reports. 2018;2018:bcr-2017.
da Cruz NFS, Milhomens Filho JAP, Ferraro DMN, Polizelli MU, de Moraes Ambrogini NSB. Hyperviscosity retinopathy and immunogammopathy maculopahy as new onset of multiple myeloma. Case Rep Ophthalmol. 2021;12:578–84.
Georgakopoulos CD, Plotas P, Angelakis A, Kagkelaris K, Tzouvara E, Makri OE. Dexamethasone implant for immunogammopathy maculopathy associated with IgA multiple myeloma. Ther Adv Ophthalmol. 2019;11:2515841418820441.
Reddy SV, Payne S, Schaal S. Angiographically silent macular edema. JAMA Ophthalmol. 2016;134:453–4.
Baker PS, Garg SJ, Fineman MS, Chiang A, Alshareef RA, Belmont J, et al. Serous macular detachment in Waldenström macroglobulinemia: a report of four cases. Am J Ophthalmol. 2013;155:448–55.
Besirli CG, Johnson MW. Immunogammopathy maculopathy associated with Waldenström macroglobulinemia is refractory to conventional interventions for macular edema. Retin Cases Brief Rep. 2013;7:319–24.
Author information
Authors and Affiliations
Contributions
MN: 1. Design of the work. 2. Data collection. 3. Drafting the article. 4. Critical revision of the article. 5. Final approval of the last version. SH:. 1. Data collection. 2. Drafting the article. 3. Critical revision of the article. 4. Final approval of the last version. SC: 1. Design of the work. 2. Drafting the article. 3. Critical revision of the article. 4. Final approval of the last version. AG: 1. Design of the work. 2. Drafting the article. 3. Critical revision of the article. 4. Final approval of the last version. LM:. 1. Data collection. 2. Drafting the article. 3. Critical revision of the article. 4. Final approval of the last version. FA: 1. Design of the work. 2. Data collection. 3. Drafting the article. 4. Critical revision of the article. 5. Final approval of the last version
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naseripour, M., Hemmati, S., Chaibakhsh, S. et al. Cystoid macular oedema without leakage in fluorescein angiography: a literature review. Eye 37, 1519–1526 (2023). https://doi.org/10.1038/s41433-022-02230-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02230-z