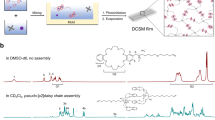
Abstract
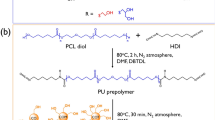
In this study, polymers with different copolymerization composition ratios of α-chloro-ε-caprolactone (α-ClCL) and ε-caprolactone (ε-CL) were prepared using α-ClCL, which can polymerize on its own. The copolymers were prepared by using trimethylolpropane as the initiator, and acryloyl groups were added to the polymer ends to form macromonomers capable of cross-linking reactions. The functionalized macromonomers were confirmed to possess shape-memory properties when cross-flinked in film form by heat. The composition of the functional groups in the macromonomer could be adjusted by changing the ratio of α-ClCL to ε-CL used in the copolymerization. In addition, the chloro group introduced by α-ClCL was converted into an azide group. Both the cross-linked film with chloro groups and the film converted to azide groups prepared in this study exhibited shape-memory according to the softening point of the film. Through fluorescence microscopy, it was confirmed that the converted azide groups were modified with alkylated rhodamine B based on the click reaction. Furthermore, azide-assisted films are expected to add various functions through click reactions in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Satti SM, Shah AA. Polyester‐based biodegradable plastics: an approach towards sustainable development. Lett Appl Microbiol 2020;70:413–30. https://doi.org/10.1111/lam.13287
Becker G, Wurm FR. Functional biodegradable polymers via ring-opening polymerization of monomers without protective groups. Chem Soc Rev 2018;47:7739–82. https://doi.org/10.1039/c8cs00531a
Nakamura A, Kobayashi N, Koga N, Iino R. Positive charge introduction on the surface of thermostabilized PET hydrolase facilitates PET binding and degradation. ACS Catal. 2021;11:8550–64. https://doi.org/10.1021/acscatal.1c01204
Lu H, Diaz DJ, Czarnecki N-J, Zhu C, Kim W, Shroff R, et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature. 2022;604:662–7. https://doi.org/10.1038/s41586-022-04599-z
Yamashita T, Matsumoto T, Yamada R, Ogino H. Display of PETase on the cell surface of Escherichia coli using the anchor protein PgsA. Appl Biochem Biotechnol 2024 https://doi.org/10.1007/s12010-023-04837-8
Mecerreyes D, Humes J, Miller R-D, Hedrick J-L, Detrembleur C, Lecomte P, et al. First example of an unsymmetrical difunctional monomer polymerizable by two living/controlled methods. Macromol Rapid Commun 2000;21:779–84.
Groner MD, Fabreguette FH, Elam JW, George SM. Low-temperature Al2O3 atomic layer deposition. Chem Mater 2004;16:639–45. https://doi.org/10.1021/cm0304546
Alteheld A, Feng Y, Kelch S, Lendlein A. Biodegradable, amorphous copolyester‐urethane networks having shape‐memory properties. Angew Chem Int Ed 2005;44:1188–92. https://doi.org/10.1002/anie.200461360
Ebara M, Uto K, Idota N, Hoffman J, Aoyagi T. The taming of the cell: shape-memory nanopatterns direct cell orientation. Int J Nanomed 2014;9:117–26. https://doi.org/10.2147/ijn.s50677
Uto K, Aoyagi T, DeForest CA, Hoffman AS, Ebara M. A combinational effect of “bulk” and “surface” shape‐memory transitions on the regulation of cell alignment. Adv Healthcare Mater. 2017;6 https://doi.org/10.1002/adhm.201601439
Iwamatsu K, Uto K, Takeuchi Y, Hoshi T, Aoyagi T. Preparation of temperature-responsive, cationized, poly(ε-caprolactone)-based, cross-linked materials by a macromonomer design and positive charge control on the surface. Polym J 2018;50:447–54. https://doi.org/10.1038/s41428-018-0030-1
Makiguchi K, Satoh T, Kakuchi T. Diphenyl phosphate as an efficient cationic organocatalyst for controlled/living ring-opening polymerization of δ-valerolactone and ε-caprolactone. Macromolecules. 2011;44:1999–2005. https://doi.org/10.1021/ma200043x
Takao A, Fusae M, Yu N. Preparation of cross-linked aliphatic polyester and application to thermo-responsive material. J Controlled Release 1994;32:87–96. https://doi.org/10.1016/0168-3659(94)90228-3
Uto K, Yamamoto K, Hirase S, Aoyagi T. Temperature-responsive cross-linked poly(ε-caprolactone) membrane that functions near body temperature. J Controlled Release 2006;110:408–13. https://doi.org/10.1016/j.jconrel.2005.10.024
Zako T, Matsushita S, Hoshi T, Aoyagi T. Direct surface modification of polycaprolactone-based shape memory materials to introduce positive charge aiming to enhance cell affinity. Materials. 2021;14:5797 https://doi.org/10.3390/ma14195797
Houk K-N, Jabbari A, Hall H-K, Alemán C. Why δ-valerolactone polymerizes and γ-butyrolactone does not. J Org Chem 2008;73:2674–8. https://doi.org/10.1021/jo702567v
Moore T, Adhikari R, Gunatillake P. Chemosynthesis of bioresorbable poly(γ-butyrolactone) by ring-opening polymerisation: a review. Biomaterials. 2005;26:3771–82. https://doi.org/10.1016/j.biomaterials.2004.10.002
Lenoir S, Riva R, Lou X, Detrembleur CH, Jérôme R, Lecomte PH. Ring-opening polymerization of α-chloro-ε-caprolactone and chemical modification of poly(α-chloro-ε-caprolactone) by atom transfer radical processes. Macromolecules. 2004;37:4055–61. https://doi.org/10.1021/ma035003l
Wang SW, Lin YK, Fang JY, Lee R-S. Photo-responsive polymeric micelles and prodrugs: synthesis and characterization. RSC Adv. 2018;8:29321–37. https://doi.org/10.1039/c8ra04580a
Bolley A, Mameri S, Dagorne S. Controlled and highly effective ring‐opening polymerization of α‐chloro‐ε‐caprolactone using Zn‐ and Al‐based catalysts. J Polym Sci 2020;58:1197–206. https://doi.org/10.1002/pol.20190214
Yin G, Chen G, Zhou Z, Li Q. Modification of PEG-b-PCL block copolymer with high melting temperature by the enhancement of POSS crystal and ordered phase structure. RSC Adv. 2015;5:33356–63. https://doi.org/10.1039/c5ra01971k
Bexis P, Thomas AW, Bell CA, Dove A-P. Synthesis of degradable poly(ε-caprolactone)-based graft copolymers via a “grafting-from” approach. Polym Chem 2016;7:7126–34. https://doi.org/10.1039/c6py01674j
Liu M, Vladimirov N, Fréchet J-M-J. A new approach to hyperbranched polymers by ring-opening polymerization of an ab monomer: 4-(2-hydroxyethyl)-ε-caprolactone. Macromolecules. 1999;32:6881–4. https://doi.org/10.1021/ma990785x
Tian D, Dubois PH, Jérôme R. Macromolecular engineering of polylactones and polylactides. 23. synthesis and characterization of biodegradable and biocompatible homopolymers and block copolymers based on 1,4,8-Trioxa[4.6]Spiro-9-Undecanone. Macromolecules. 1997;30:1947–54. https://doi.org/10.1021/ma961614k
Taniguchi I, Lovell N-G. Low-temperature processable degradable polyesters. Macromolecules. 2012;45:7420–8. https://doi.org/10.1021/ma301230y
Zhou, Z, Meng, Y, Wei, C, Bai, Y, Wang, X, Quan, D, et al. Linear shape memory polyester with programmable splitting of crystals. Macromol Mater Eng. 2021;306 https://doi.org/10.1002/mame.202100254
Van Horn BA, Wooley KL. Toward cross-linked degradable polyester materials: investigations into the compatibility and use of reductive amination chemistry for cross-linking. Macromolecules. 2007;40:1480–8. https://doi.org/10.1021/ma061654g
Mecerreyes D, Miller R-D, Hedrick J-L, Detrembleur C, Jérôme R. Ring-opening polymerization of 6-hydroxynon-8-enoic acid lactone: novel biodegradable copolymers containing allyl pendent groups. J Polym Sci Part A: Polym Chem 2000;38:870–5.
Lou X, Detrembleur C, Lecomte PH, Jérôme R. Living ring-opening (CO)polymerization of 6,7-Dihydro-2(5H)-Oxepinone into unsaturated aliphatic polyesters. Macromolecules. 2001;34:5806–11.
El Jundi A, Buwalda S, Bethry A, Hunger S, Coudane J, Bakkour Y, et al. Double-hydrophilic block copolymers based on functional poly(ε-Caprolactone)s for pH-dependent controlled drug delivery. Biomacromolecules. 2019;21:397–407. https://doi.org/10.1021/acs.biomac.9b01006
Wang G, Shi Y, Fu Z, Yang W, Huang Q, Zhang Y. Controlled synthesis of poly(ε-Caprolactone)-graft-polystyrene by atom transfer radical polymerization with poly(ε-Caprolactone-Co-α-Bromo-ε-Caprolactone) copolymer as macroinitiator. Polymer. 2005;46:10601–6. https://doi.org/10.1016/j.polymer.2005.06.105
Gao C, Tsou CH, Zeng CY, Yuan L, Peng R, Zhang XM. Organocatalyzed ring-opening copolymerization of α-Bromo-γ-butyrolactone with ε-caprolactone for the synthesis of functional aliphatic polyesters – pre-polymers for graft copolymerization. Des Monomers Polym 2018;21:193–201. https://doi.org/10.1080/15685551.2018.1550288
Lee R, Huang Y. Synthesis and characterization of amphiphilic block–graft MPEG‐b‐(PαN3CL‐g‐alkyne) degradable copolymers by ring‐opening polymerization and click chemistry. J Polym Sci, Part A Polym Chem 2008;46:4320–31. https://doi.org/10.1002/pola.22741
Suksiriworapong J, Sripha K, Junyaprasert VB. Synthesis and characterization of bioactive molecules grafted on poly(ɛ-Caprolactone) by “click” chemistry. Polymer. 2010;51:2286–95. https://doi.org/10.1016/j.polymer.2010.03.034
Conte C, Costabile G, d’Angelo I, Pannico M, Musto P, Grassia G, et al. Skin transport of PEGylated poly(ε-Caprolactone) nanoparticles assisted by (2-Hydroxypropyl)-β-cyclodextrin. J Colloid Interface Sci 2015;454:112–20. https://doi.org/10.1016/j.jcis.2015.05.010
Riva R, Lussis P, Lenoir S, Jérôme C, Jérôme R, Lecomte P. Contribution of “click chemistry” to the synthesis of antimicrobial aliphatic copolyester. Polymer. 2008;49:2023–8. https://doi.org/10.1016/j.polymer.2008.03.008
Ebara M, Kotsuchibashi Y, Uto K, Aoyagi T, Kim YJ, Narain R, et al. Smart Biomaterials, NIMS Monographs, Springer Tokyo, 2014, p. 321-36.
Leroux F, Campagne C, Perwuelz A, Gengembre L. Polypropylene film chemical and physical modifications by dielectric barrier discharge plasma treatment at atmospheric pressure. J Colloid Interface Sci 2008;328:412–20. https://doi.org/10.1016/j.jcis.2008.09.062
Maegawa K, Tanimoto H, Onishi S, Tomohiro T, Morimoto T, Kakiuchi K. Taming the reactivity of alkyl azides by intramolecular hydrogen bonding: site-selective conjugation of unhindered diazides. Org Chem Front. 2021;8:5793–803. https://doi.org/10.1039/D1QO01088C
Wang J, Horwitz MA, Dürr AB, Ibba F, Pupo G, Gao Y, et al. Asymmetric azidation under hydrogen bonding phase-transfer catalysis: a combined experimental and computational study. J Am Chem Soc. 2022;144:4572–84. https://doi.org/10.1021/jacs.1c13434
Uto K, Matsushita Y, Ebara M. Multiphase PCL semi-interpenetrating networks exhibiting the triple- and stress-free two-way shape memory effect. Polym Chem 2023;14:1478–87. https://doi.org/10.1039/d2py01607a
Acknowledgements
Scanning electron microscopy and energy dispersive X-ray spectroscopy were performed at the Joint Research Center for Environmentally Conscious Technologies in Materials Science at ZAIKEN, Waseda University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoshida, T., Hoshi, T. & Aoyagi, T. Molecular design of reactive polycaprolactone that can be induced into shape-memory materials promotes further functionalization. Polym J (2024). https://doi.org/10.1038/s41428-024-00948-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41428-024-00948-z