Abstract
Endothelins and their receptors, ETA and ETB, play vital roles in maintaining vascular homeostasis. Therapeutically targeting endothelin receptors, particularly through ETA antagonists, has shown efficacy in treating pulmonary arterial hypertension (PAH) and other cardiovascular- and renal-related diseases. Here we present cryo-electron microscopy structures of ETA in complex with two PAH drugs, macitentan and ambrisentan, along with zibotentan, a selective ETA antagonist, respectively. Notably, a specialized anti-ETA antibody facilitated the structural elucidation. These structures, together with the active-state structures of ET-1-bound ETA and ETB, and the agonist BQ3020-bound ETB, in complex with Gq, unveil the molecular basis of agonist/antagonist binding modes in endothelin receptors. Key residues that confer antagonist selectivity to endothelin receptors were identified along with the activation mechanism of ETA. Furthermore, our results suggest that ECL2 in ETA can serve as an epitope for antibody-mediated receptor antagonism. Collectively, these insights establish a robust theoretical framework for the rational design of small-molecule drugs and antibodies with selective activity against endothelin receptors.
Similar content being viewed by others
Introduction
Endothelins are pivotal regulators of cardiovascular functions, essential for maintaining vascular tone and overall cardiovascular homeostasis1. Three endothelin peptides, namely ET-1, ET-2, and ET-3, are characterized by two unique cysteine–cysteine crosslinks2 and activate the endothelin receptor (ETR) subtypes ETA and ETB3,4. Notably, human ETA and ETB receptors share 63% sequence homology3 but differ significantly in their ligand affinity and function. For instance, ETA preferentially binds ET-1 and ET-2 over ET-35, mediating strong vasoconstriction, whereas ETB exhibits equal affinity for all three isoforms, primarily inducing vasorelaxation through nitric oxide and facilitating ET-1 clearance6,7. Consequently, ETRs are crucial targets for the treatment of cardiovascular diseases8.
Given the complex role of ETRs, the development of therapeutic drugs has primarily focused on vasodilatory antagonists for treating pulmonary arterial hypertension (PAH) and autoimmune diseases9,10. Notable selective ETA antagonists or dual ETA/ETB antagonists include bosentan, macitentan, and ambrisentan11,12,13,14,15. Additionally, ETR antagonists like sparsentan, aprocitentan, and zibotentan are currently under investigation for their potential efficacy in treating refractory hypertension and various kidney diseases16,17,18,19. Conversely, ETB agonists are being explored for therapeutic benefits such as vasodilation and neuroprotection20,21. Furthermore, the development of therapeutic vaccines and monoclonal antibodies targeting ETA represents an exciting frontier in PAH treatment, combining high specificity with a reduced risk of side effects22. Preclinical studies have shown promise in these approaches to decrease pulmonary arterial pressure23,24.
Structural studies on ETRs have elucidated the mechanisms of endothelin ligand recognition and activation. Extensive X-ray crystallography work has revealed the interaction patterns within the ETB receptor when bound with various ligands, including ET-1, ET-3, and several antagonists5,25,26,27,28,29. Complementarily, recent cryo-electron microscopy (cryo-EM) studies on ETA and ETB in complex with G protein in active states have provided insights into the conserved recognition mechanisms for endogenous agonists and the selectivity for synthetic agonists between ETA and ETB30. Despite these advances, the structural basis for ETA antagonism remains less understood, a gap that limits the design of selective antagonists. In addition to small-molecule antagonists, a monoclonal antibody (Fab301) specifically targeting ETA is currently in phase Ib clinical trials23. The specificity of antibodies may address the selectivity issues associated with small-molecule antagonists for ETRs22,24. However, there is currently no structural information on antibody–receptor complexes24,31.
This study aims to address these questions by presenting cryo-EM structures of human ETA in complex with key small-molecule antagonists and Fab301 and by detailing the activation mechanisms of both ETA and ETB receptors. These molecular insights are crucial for advancing the development of therapeutics for conditions such as PAH and are part of broader efforts to enhance the specificity and efficacy of ETR-targeted drugs.
Results
Structure determination of antagonist-bound ETA structures
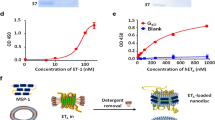
To understand the structure basis for the antagonist binding modes in ETA, we analyzed three distinct compounds — macitentan, ambrisentan and zibotentan — measuring their activities on ETA in calcium mobilization assay (Fig. 1a; Supplementary Fig. S1). Determining the structure of inactive-state G protein-coupled receptors (GPCRs) via cryo-EM is inherently challenging due to the absence of the heterotrimeric G protein, which is essential for particle alignment in active GPCR structure determination. Here, we developed an optimized strategy to solve the inactive ETA structures. Initially, to overcome the low surface expression of ETA, endoglucanase H was fused to the N-terminus of ETA, along with N- and C-terminal truncations (Materials and methods). Then, to compensate for the absence of the G protein, a thermostabilized apocytochrome b562 RIL (BRIL)32 protein was fused between TM5 and TM6, replacing ETA’s third intracellular loop (ICL3) (Supplementary Fig. S1a). The design of the BRIL fusion sites for uninterrupted helicity was informed by AlphaFold233 predictions.
a The inhibition activity of the four different antagonists on ETA in calcium mobilization assay. The IC50 values of macitentan, ambrisentan, zibotentan and bosentan are 1.3 nM, 0.6 nM, 8.6 nM and 9.9 nM, respectively. Data are presented as means ± SEM (n = 5). b Cryo-EM density maps of Fab301–ETA–anti-BRIL Fab-Nb complex (left panel); zoomed-in view of the junction site and the surface presentation of Fab301 (right panel). c–e Cartoon representation of Fab301–ETA–anti-BRIL Fab-Nb complexes with different antagonists: macitentan (c), ambrisentan (d), zibotentan (e). Components of ETA complexes are colored as indicated. The EM density map for each ligand is shown as colored mesh.
Subsequent stabilization of the modified ETA construct involved the introduction of a BRIL-binding Fab and a nanobody (Nb) that reinforces the hinge region, as previously described34,35. Despite these modifications, we encountered stability and orientation preferences during cryo-EM grid preparation. To address these issues, we introduced an ETA-specific antibody, Fab30123, which not only conferred additional stability to the complex but also exhibited antagonistic effects against ET-1-induced ETA signaling. These strategic modifications enabled us to determine the cryo-EM structures of Fab301-bound ETA in complex with macitentan, ambrisentan and zibotentan, at nominal resolutions of 3.1 Å, 3.2 Å and 3.2 Å, respectively (Fig. 1b−e; Supplementary Fig. S2 and Table S1). The high-resolution cryo-EM maps provided detailed electron density in the orthosteric ligand-binding pocket, enabling accurate placement of each antagonist (Supplementary Fig. S2). It is noteworthy that the interaction interface between Fab301 and ETA is quite limited, and within the cryo-EM particles, Fab301 appears to exhibit a degree of movement.
Comparative analysis of the macitentan–ETA, ambrisentan–ETA and zibotentan–ETA complexes reveals a conserved conformation among the three structures, with Cα root mean square deviation (RMSD) values between 0.7 Å and 1.0 Å (Supplementary Fig. S3a, b). Compared to the crystal structures of antagonist-bound ETB, both receptors adopt a similar inactive conformation, with an RMSD of 1.5 Å (Supplementary Fig. S3c).
Binding of macitentan to ETA
Macitentan, designed as an ETA-selective antagonist, is a derivative of the dual-acting antagonist bosentan. In our calcium mobilization assay, macitentan shows a high affinity to ETA, with an IC50 of 1.3 nM (Fig. 1a; Supplementary Table S2), contrasting to the lower affinity to ETB at 14.5 μM (Supplementary Fig. S4b), which is also consistent with the previous research results36. Structurally, macitentan retains the pyrimidine core characteristic of bosentan but distinguishes itself with a sulfonamide substitution at the fourth position37 (Supplementary Fig. S1b, c). The binding of macitentan within the ETA pocket involves several distinct interactions (Fig. 2a). The sulfonamide moiety in macitentan establishes a hydrogen bond with R3266.55 (Fig. 2b), and forms ionic interactions with residues K1663.33, K2555.38 and R3266.55 from ETA (Fig. 2b). The bromophenyl group is embedded in the receptor’s hydrophobic core, forming hydrophobic interactions with residues W3196.48, V1693.36, Y2635.46, H3236.52, and L2595.42 from TMs 3, 5, and 6 (Fig. 2c), and is further stabilized by a cation–π interaction with K1663.33 (Fig. 2b). The 2-(5-bromopyrimidin-2-yl)oxyethoxy component, linked to the sixth position of the pyrimidine, allows the oxygen atom in the bromopyrimidine to form a hydrogen bond with Q1653.32 (Fig. 2d). Importantly, this moiety inserts deeper into the orthosteric pocket than the similar groups in bosentan or K-8794 in ETB, positioned to form a halogen bond with D1262.50 (Fig. 2d). Furthermore, bromopyrimidine engages in π–π interactions with Y1292.53 and the “toggle switch” residue W3196.48 (Fig. 2d). The ethyl tail attached to the sulfonamide moiety is nestled within a hydrophobic pocket formed by F1613.28, P1623.29, and F2244.64 from TM3 and TM4 of ETA. Prior research has demonstrated that substituting the sulfonamide moiety with sulfamide significantly boosts ETR antagonists’ receptor affinity11. Our structural analysis reveals that the addition of the -NH- group in macitentan contributes to a more stable electrostatic network with K1663.33 and K2555.38, enhancing macitentan’s binding affinity (Fig. 2b). Notably, the R3266.55A mutation showed minimal influence on macitentan’s activity on ETA, aligning with macitentan’s stronger electrostatic network at this location in ETA (Fig. 2m).
a–d Schematic of macitentan’s interactions with key residues in ETA. e–h Schematic of ambrisentan’s interactions with key residues in ETA. i–l Schematic of zibotentan’s interactions with key residues in ETA. The selectivity of zibotentan towards ETA may be attributed to Y1292.53 in ETA but H1502.53 in ETB (l). m–o The antagonistic effects of four different antagonists on the ETA R3266.55A variant (m); effects of mutations in ETA (n) and ETB (o) on zibotentan’s antagonistic activity. ΔpIC50 represents the difference in pIC50 values between the wild type (WT) and the mutants of ETA. Data are presented as means ± SEM (n ≥ 3). Hydrogen bonds are highlighted with gray dashed lines.
Binding of ambrisentan to ETA
Ambrisentan, a propionic acid derivative discovered through high-throughput screening, differentiates itself from macitentan by featuring a carboxylic acid group instead of a sulfonamide moiety15 (Supplementary Fig. S1d). Despite its smaller molecular weight relative to macitentan and zibotentan, ambrisentan exhibits a remarkably high affinity to ETA, befitting its role as an ETA-selective antagonist (Fig. 1a). The structural elucidation of its binding to ETA uncovers essential interactions. The carboxylic acid group forms ionic interactions with the positively charged residues K1663.33 and R3266.55 (Fig. 2f), while a network of hydrogen bonds with residues R3266.55, K1663.33, and Q1653.32 firmly anchors ambrisentan in the binding site (Fig. 2e, f). Moreover, the hydrophobic interaction of one benzene ring from the symmetric pair in ambrisentan with residues F1613.28, P1623.29, and F2244.64 creates a snug fit within a subpocket (Fig. 2f). The counterpart benzene ring engages with residues L3226.51, W3196.48, I3557.39, and A3587.42 spanning TM6 and TM7 (Fig. 2g). Additionally, the methyl groups on the pyrimidine ring of ambrisentan adapt to the hydrophobic cores formed by residues V1693.36, L2595.42, Y2635.46, and H3236.52 from TMs 3, 5 and 6 (Fig. 2h), further stabilizing the antagonist binding within the receptor. Furthermore, our molecular dynamics (MD) simulations indicate the stable binding of ambrisentan within the pocket (Supplementary Fig. S5).
Binding of zibotentan to ETA
Zibotentan, a sulfonamide-based compound, exhibits selective antagonism towards ETA with an IC50 of 8.6 nM in our calcium mobilization assay (Fig. 1a). Structurally distinguished by a central pyridine ring flanked by two bulky substituents (Fig. 2i), zibotentan adopts a chair-like conformation within the orthosteric binding pocket, contrasting with the spatial arrangement of macitentan (Supplementary Fig. S1c, e). The sulfonamide group forms electrostatic interactions with Q1653.32, K1663.33 and R3266.55 (Fig. 2j), whereas the pyrazinyl rings, modified with methoxy and methyl groups, engage in hydrophobic contacts with residues around W3196.48 from TMs 3, 5 6 (Fig. 2k). On the opposite end, the 1,3,4-oxadiazol-2-yl segment of zibotentan extends towards ECL2, forming a π–π interaction with F1613.28 and a hydrogen bond with K1402.64 (Fig. 2l). Furthermore, Y1292.53 appears to participate in polar interactions or hydrogen bonds with the pyridine ring’s nitrogen (Fig. 2l).
Subsequent structural investigations suggest that zibotentan’s selectivity for ETA may be significantly influenced by the residue F1613.28, which is a valine (V1773.28) in ETB. This phenylalanine F1613.28 acts as a “tray” that stabilizes one end of the zibotentan molecule (Fig. 2l). Concordantly, mutation of F1613.28A or F1613.28V significantly reduces zibotentan’s activity on ETA (Fig. 2n). Additionally, the interaction with Y1292.53 is also crucial; its alteration to phenylalanine or histidine (Y1292.53F or Y1292.53H) markedly reduces zibotentan’s activity on ETA, highlighting the importance of this residue for antagonist specificity (Supplementary Fig. S4c−e). Intriguingly, introducing corresponding mutations into ETB (V1773.28F and H1502.53Y) partially impairs the activity of zibotentan on ETB, which underscores the critical role of these residues in the determination of antagonist selectivity in ETA or ETB subtype (Fig. 2o).
Structure basis of antagonist selectivity in ETA and ETB
Structural elucidation of ETA in complex with various antagonists, each featuring distinct scaffold architectures, advances our understanding of the antagonist binding modes in ETA and delineates commonalities critical for their antagonistic function. An important aspect of their activity is the interaction with the positively charged region in ETA’s orthosteric pocket. Functionally important groups, such as the sulfonylamide in bosentan, macitentan, and zibotentan, along with the carboxylic acid group in ambrisentan, are essential. These functional groups occupy the position corresponding to W21, the C-terminal end of the endogenous agonist ET-1 (Fig. 3a), a key engagement site with ETA30. Another shared trait of these antagonists is their interaction in close proximity to TM5 and TM6, which helps stabilize ETA conformation in this region (Fig. 3b). The three antagonist-bound structures display a hydrophobic moiety in this vicinity, forming hydrophobic contacts with residues from TMs 3, 5 and 6 (Fig. 3b). These groups also hinder the inward movement of the side chain of W3196.48 through hydrophobic or π–π interactions (Fig. 3b). Mutagenesis data, such as L2595.42A, Y2635.46A or L3226.51A, further confirm the critical role of these hydrophobic contacts in sustaining antagonist efficacy (Supplementary Fig. S4f−h).
a The sulfonylamide in macitentan and zibotentan, along with the carboxylic acid group in ambrisentan, engage with the positively charged area (blue surface) in ETA’s orthosteric pocket. b Three antagonists hinder the inward movement of W3196.48’s side chain through analogous functional groups at the similar positions. c The conformation of F1613.28 in different states. d In ETB’s corresponding position, V1773.28, offers a larger antagonist-binding pocket. e Different spatial accommodation of antagonists in ETA and ETB. The bulky hydrophobic group (4-t-butylphenyl) of bosentan is better adapted to ETB, whereas the smaller hydrophobic group of macitentan shows a preference for ETA. f Effects of V1773.28F in ETB on the antagonistic activities of ambrisentan and macitentan (n = 3). g Difference in pIC50 values between ETA and ETB variants and WT in antagonist experiments. Data are presented as means ± SEM (n ≥ 3). All data were analyzed by one-way ANOVA by Dunnett’s multiple test compared with WT. For mutants, *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 were considered statistically significant.
Regarding the selectivity differences, we observed that, in ETA, F1613.28 rotates inwards to the orthosteric pocket in the antagonist-bound state, which results in a compact antagonist-binding pocket in ETA (Fig. 3c). In contrast, in the corresponding site in ETB, a smaller V1773.28 yields a more expansive antagonist-binding pocket (Fig. 3d). This spatial variation accounts for the more effective accommodation of the larger bulky hydrophobic group (4-t-butylphenyl) of bosentan within ETB, while the smaller hydrophobic groups of macitentan and ambrisentan show a preference for ETA (Fig. 3e, f). Regarding zibotentan’s ETA selectivity, as previously discussed, this may be attributed to the different residue Y1292.53 in ETA compared to H1502.53 in ETB (Figs. 2l, 3g). Together, Y1292.53 and F1613.28 in ETA may partially account for the observed selectivity of antagonists toward ETB.
Active and inactive conformation features of ETRs
To delve deeper into the activation mechanisms of ETRs, we determined the structures of ETA and ETB in their active states, at global resolutions of 3.3 Å for ET-1-bound ETA–miniGs/q–Nb35 complex and 3.2 Å for ETB–miniGs/q–Nb35 complex (Fig. 4a, b; Supplementary Fig. S6 and Table S1). In addition, a structure of ETB complexed with the selective agonist BQ302038, was determined at a resolution of 3.0 Å (Fig. 4c; Supplementary Fig. S6a and Table S1). The overall conformations of ET-1-bound ETA–miniGs/q and ETB–miniGs/q complex structures, including the ET-1 binding poses, are consistent with previously reported ETR structures30 (Supplementary Fig. S7a, b). Notably, the binding pose of BQ3020 in ETB closely resembles that of ET-1, with a Cα RMSD of 0.8 Å for the receptor (Fig. 4d, f). BQ3020, which differs from the ETB-selective agonist IRL1620 by a single residue (Fig. 4e), exhibits an overall structural similarity, as the BQ3020–ETB and IRL1620–ETB complex structures display an RMSD of 0.9 Å. This indicates the aligned positioning of the agonists within the binding pocket (Fig. 4d). In the BQ3020–ETB complex, BQ3020’s C-terminal configuration closely resembles that of ET-1, albeit with a slightly downward shift in the α-helix within the binding pocket, due to the absence of disulfide-bond constraints (Fig. 4d).
a–c Cryo-EM density maps of ET1–ETA–miniGs/q–Nb35 (a), ET1–ETB–miniGs/q–Nb35 (b), and BQ3020–ETB–miniGs/q–Nb35 (c) complexes. d Structural comparison of ETB bound to BQ3020, IRL1620, and ET-1. Conformational comparison between BQ3020 and IRL1620 in the binding pocket (upper right), and between BQ3020 and ET-1 (bottom right). The arrow indicates that the α-helix in the binding pocket shifts slightly downward. e Sequence alignment of peptides including ET-1, BQ3020 and IRL1620. f Cross-section of the BQ3020-binding pocket in ETB. BQ3020 is displayed as spheres (left panel). Detailed interactions between BQ3020 and ETB are shown (right panel).
Additionally, the mutation W146ECL1A in ETA does not significantly affect receptor activation, whereas the analogous mutation W167ECL1A in ETB results in a substantial reduction in ETB activation (Supplementary Fig. S7c and Table S3). This suggests that ETB requires interactions with larger hydrophobic groups at this site for activation. Within ETB’s ECL1, F169ECL1 is posited to engage in a π–π interaction with W167ECL1 (Supplementary Fig. S7d), a key conformation for receptor function. Alanine mutation of F169ECL1 hampers ET-1’s capability to activate ETB (Supplementary Fig. S7c).
Employing the Residue–Residue Contact Score (RRCS) tool39, we analyzed structures bound by three antagonists, confirming that the residue contacts are characteristics of the inactive-state class A GPCRs (Supplementary Fig. S8). A comparative analysis of the macitentan-bound ETA structure against the ET-1-bound ETA structure reveals significant conformational changes when ETA transits from the inactive to active states. ET-1 binding promotes an inward movement of the extracellular portions of TM2, TM6 and TM7, resulting in a more compact receptor core (Fig. 5a). Concurrently, ECL2 moves inwards substantially, acting as a “lid” that secures ET-1 in place, facilitated by a π–π interaction between ET-1’s Y13 and Y231ECL2 in ETA (Fig. 5a). However, we cannot exclude the influence of Fab301 on the conformational changes of ECL2 when compared to the state bound alone by the small-molecular antagonist. The EM density map allowed the modeling of extended N-terminal residues, revealing a tighter packing with ECL2 and ECL3 in the ET-1-bound ETA structure compared to the macitentan-bound state (Fig. 5a). These structural rearrangements lead to a more compact orthosteric pocket for ET-1 interaction. The characteristic outward displacement of the cytoplasmic part of TM6 by 9.7 Å in the ET-1-bound ETA–miniGs/q structure, reflects the conformational changes, indicative of class A GPCR activation (Fig. 5b). Moreover, the intracellular portion of TM7 undergoes a displacement of ~2.9 Å (measured by the Cα atom of L3697.53), in the active state (Fig. 5c).
The purple cartoon represents ET1-bound ETA, whereas cyan cartoon represents macitentan-bound ETA. a Conformational changes in loop regions and helical rearrangement during ETA activation (top view). b, c Structural comparison reveals the outward extension of TM6 (b) and the movement of the cytoplasmic portion of TM7 (c). d The downward movement of the side chain of I19 in ET-1-bound ETA structure. e–i Conformational changes of key motifs related to ETA activation. NPxxY (c), PIF motif (e, f), DRY (i).
Mechanism for ETA activation
The antagonist-bound ETA structures illuminate the underlying activation mechanism. Upon ET-1 binding, the side chain of I19 residue exerts pressure on I3557.39, prompting a downward shift of this residue and the associated intracellular half of TM7 (Fig. 5d). Concurrently, ET-1’s W21 residue engages with the “toggle switch” residue W3196.48 (Fig. 5e). This interaction induces a downward rotation of W3196.48, facilitating a hydrogen bond formation between its nitrogen atom and N3617.45 (Fig. 5e). The downward motions of W3196.48 and N3617.45 promote an inward-to-outward rotation of F3156.44 within the P5.50I/V3.40F6.44 motif (Fig. 5e, f), triggering the outward movement of TM6 at the cytoplasmic end (Fig. 5e). These sequential events are accompanied by the disruption of interactions between L1763.43, L3116.40 and V3126.41, which results in the release of the stacking of TM3 and TM6, further promoting the outward swing of TM6 (Fig. 5g). Additionally, the downward movement of N3617.45 leads to the collapse of the Na+ pocket, previously stabilized by D1262.50, T1723.39, and N3617.45 in the inactive state, and triggers subsequent rearrangements between TM7, TM3 and TM2 (Fig. 5h).
Regarding the highly conserved N7.49P7.50xxY7.53 motif in class A GPCRs, ETA and ETB feature a variant of N7.49P7.50xxL7.53Y7.54, wherein Y7.53 is replaced by L7.53. During ETA activation, L1793.46 does not engage in a polar inter-helix interaction, which permits L3697.53 to move downward, enhancing its coupling with the C-terminus of the G protein’s α5 helix (Fig. 5c). Concerning the D3.49R3.50Y3.51 motif, ETA activation disrupts the conserved ion lock between D1823.49 and R1833.50. The released R1833.50 then forms a polar interaction with Y2755.58 and establishes interactions with the α5 helix of the G protein, anchoring the receptor in its active state (Fig. 5i).
Insights into antibody design to antagonize ETA
In our structural analysis of the antagonists-bound ETA, the employed Fab301 shows antagonistic effect and facilitates the structure determination. The EM density maps clearly reveal the binding of Fab301 to ETA’s ECL2 region (Fig. 6a). However, due to the dynamic nature of the interaction between Fab301 and ETA’s ECL2, pinpointing their precise binding interface is challenging. We addressed this by using AlphaFold2 multimer33,40 prediction to model the Fab301–ETA complex interface (Supplementary Fig. S9a). Ultimately the epitope of Fab301 was roughly located based on the fitting of the predicted model with the experimental EM densities (Fig. 6c).
a Cryo-EM density maps showing the Fab301 binding to the ECL2 region of ETA. b Differences between the retention time of each mutant–Fab301 complex and the WT–Fab301 complex. The letter “Δt” represents the retention time of the mutant sample minus the retention time of the WT sample. c The Fab301–ETA complex predicted by AlphaFold2 multimer was modeled, which fits well with the experimental electron density of Fab301. Zoomed-in view of the antibody-binding interface is shown (right panel). d Response of ET-1 on WT ETA and ETA mutants in ECL2. Data are presented as means ± SEM (n = 4). All data were analyzed by one-way ANOVA by Dunnett’s multiple test compared with WT. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 were considered statistically significant. e Sequence alignment of the ECL2 regions between ETA and ETB. Amino acids are classified according to their properties. f Conformational change of ECL2 between ET-1-bound ETA and Fab301-bound ETA. The gray area represents the Fab301 density, and the arrow indicates that the ECL2 expands outward in the inactive structure.
To pinpoint the exact ECL2 residues involved in binding, we performed an extensive alanine scanning mutagenesis. Our size exclusion chromatography analysis identified that the region spanning residues 230ECL2–235ECL2 in ETA, and in particularly, residues R232ECL2 and G233ECL2, are crucial for Fab301 binding. Mutations of R232ECL2A or G233ECL2A resulted in a near-complete loss of Fab301’s binding to ETA (Fig. 6b; Supplementary Fig. S10). These results complement our predicted binding mode, where R232ECL2 of ETA forms an important polar interaction network with Fab301 (Fig. 6c). Moreover, the hydrophobic interactions at this interface are crucial for antibody binding, as confirmed by our calcium mobilization assay on Fab301 (Supplementary Fig. S9b). Within this model, E230ECL2 establishes hydrogen bonds with the CDRL1 and CDRL2 regions of Fab301, whereas Y231ECL2 and E234ECL2, together with R232ECL2, participate in a complex hydrogen bond network within the CDRH3 region of Fab301 (Fig. 6c). Alanine mutations on E230ECL2, Y231ECL2, and R232ECL2 also affected ET-1’s activity on ETA. Notably, Y231ECL2 mutation showed the most significant effect in calcium mobilization assay (Fig. 6d). These data underscore the essential role of ECL2 in the recognition of ligands and Fab301 by ETA.
Comparative structural analysis of ETA bound by ET-1 and Fab301 illustrates how ECL2 residues E230ECL2, Y231ECL2, and R232ECL2, which directly interact with ET-1, undergo a conformational change upon Fab301 binding. This change fosters a polar interaction network with Fab301, effectively inhibiting ET-1 binding to ETA (Fig. 6f). Additionally, an ECL2 sequence alignment between ETA and ETB uncovers the basis for Fab301’s selectivity for ETA over ETB (Fig. 6e). The distinct sequence variation, specifically at residues E230–Q235 of ETA, is pronounced between the two receptors. This divergence provides a valuable template for the design of selective antibodies targeting the ETR subtypes.
Discussion
The structural elucidation of antagonist-bound ETA in this study provides critical insights into the mechanisms underpinning receptor selectivity and activity modulation. The cryo-EM structures of ETA in complex with macitentan, ambrisentan, and zibotentan underscore the intricate molecular interactions that govern antagonist binding.
The analysis of antagonist-bound ETA structures reveals conserved features aligning with the inactive state observed in antagonist-bound ETB structures, suggesting common structural themes in ETR antagonism. Macitentan’s high affinity to ETA is attributed to several key interactions, including hydrogen bonds and ionic interactions within the orthosteric pocket. The unique sulfonamide moiety enhances these interactions, resulting in a stable electrostatic network crucial for high binding affinity. Similarly, ambrisentan’s high affinity and selectivity are due to its carboxylic acid group forming robust ionic and hydrogen bonds, coupled with hydrophobic interactions that create a snug fit within the binding pocket. Zibotentan’s unique chair-like conformation and its interactions with residues around TM3, TM5, and TM6 further highlight the structural adaptations that facilitate selective binding.
The selectivity of antagonists for ETA over ETB is primarily influenced by specific residues within the binding pocket. F1613.28 in ETA, which rotates inward in the antagonist-bound state, creates a compact binding pocket that favors smaller hydrophobic groups, whereas the corresponding V1773.28 in ETB accommodates larger groups. The residue Y1292.53 in ETA, compared to H1502.53 in ETB, further contributes to this selectivity by engaging in critical polar interactions.
The antagonistic effect of Fab301 and its ability to stabilize the ETA structure were utilized to gain further insights into antibody–ETA interactions. The binding of Fab301 to the ECL2 region of ETA involves critical residues, notably R232ECL2 and G233ECL2, essential for Fab301’s binding affinity. The detailed mapping of the Fab301–ETA interface provides a template for designing selective antibodies that target specific ETR subtypes.
This study enhances our understanding of the structural basis for antagonist selectivity and activation mechanisms of ETRs. The high-resolution structures of antagonist-bound ETA reveal conserved features essential for selective binding and receptor stabilization. Key residues within the orthosteric pocket play crucial roles in determining antagonist affinity and selectivity, offering valuable insights into the design of selective therapeutic agents.
Materials and methods
Cell lines
Spodoptera frugiperda (Sf9, expression systems) and Trichoplusia ni (High Five, Thermo Fisher Scientific) cells were gown in ESF medium at 27 °C and 120 rpm. CHO-K1 cells were cultured in Ham’s F-12K (Kaighn’s) Medium (Gibco-Thermo Fisher Scientific) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco-Thermo Fisher Scientific), and 100 U/mL Penicillin-Streptomycin (Gibco-Thermo Fisher Scientific) in a humidified incubator at 37 °C with 5% CO2.
ETA and ETB construct design
For the complex structures of G protein-coupled ETA and ETB, the human ETA or ETB genes were cloned into pFastBac1 vector. This vector was modified to include a hemagglutinin (HA) signal peptide, a Flag tag at the N-terminus of the receptor, an HRV3C protease recognition site, and a 10× His tag at the C-terminus. To improve the protein yield of ETA, we fused an endoglucanase H (PDB: 2CIT) at the N-terminus of ETA, simultaneously truncating residues 1–49 at the N-terminus and residues 406–427 at the C-terminus. Similarly, for ETB, we truncated N-terminal residues 1–66 and C-terminal residues 407–442 and fused a sialidase H (PDB: 2VK5) to the N-terminus.
For the antagonist-bound ETA structure determination, the cytochrome b562RIL (BRIL) protein was inserted into the ICL3, specifically replacing residues 282–298 of ETA. Additionally, we appended sequences ARRQL and ERARSTL from A2A adenosine receptor to the N-terminus and C-terminus of BRIL, respectively41. The truncations for ETA were consistent with those described above.
ETA–miniGs/q–Nb35 and ETB–miniGs/q–Nb35 complex formation and purification
The ETA–miniGs/q–Nb35 and ETB–miniGs/q–Nb35 complexes were assembled in vitro using modified ETA and ETB, miniGαs/q, Gβ1γ2 and Nb35. The expression and purification of miniGαs/q, Gβ1γ2 and Nb35 were performed as previously described42. ETA and ETB were expressed in Sf9 insect cells using the Bac-to-Bac baculovirus expression system (Invitrogen). The cells were cultured at 27 °C and collected 48 h after infection. Subsequently, 2 L cell pellets were sequentially washed and centrifuged with hypotonic buffer (10 mM HEPES, pH 7.4, 20 mM KCl, 10 mM MgCl2, EDTA-free protease inhibitor cocktail tablets), followed by a high osmotic buffer (10 mM HEPES, pH 7.4, 1 M NaCl. 20 mM KCl, 10 mM MgCl2, EDTA-free protease inhibitor cocktail tablets). The purified membrane was solubilized with a buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 1% (w/v) lauryl maltose neopentyl glycol (LMNG, Anatrace), 0.2% (w/v) cholesterol hemisucinate (CHS, Sigma-Aldrich)) for 2 h at 4 °C. The supernatant was separated by ultracentrifugation for 30 min, and then incubated with TALON resin (Takara) overnight at 4 °C. The resin was washed with a 15-column volume (CV) wash buffer I (25 mM HEPES, pH 7.4,100 mM NaCl, 10% (v/v) glycerol, 0.05% (w/v) LMNG, 0.01% (w/v) CHS, 20 mM imidazole) and a 15-CV wash buffer II (25 mM HEPES, pH 7.4,100 mM NaCl, 10% (v/v) glycerol, 0.01% (w/v) LMNG, 0.002% (w/v) CHS, 30 mM imidazole). Then elution was carried out with a 3-CV buffer (25 mM HEPES, pH 7.4, 100 mM NaCl, 10% (v/v) glycerol, 0.01% (w/v) LMNG, 0.002% (w/v) CHS, 300 mM imidazole). After the protein was eluted, the imidazole was removed by PD MiniTrap G-25 column (GE Health Care). ETA or ETB, miniGαs/q, Gβ1γ2 and Nb35 were mixed at a molar mass ratio of 1:1.2:1.2:1.5, incubated at 24 °C for 1 h; and then 1 μL apyrase (0.5 U/μL) were added, followed by an additional 1-h incubation at 25 °C. The mixture samples were loaded onto the Superdex 200 10/300 column (GE Healthcare) in an equilibration buffer (20 mM HEPES, pH 7.4, 100 mM NaCl, 0.00075% (w/v) LMNG, 0.00015% (w/v) CHS, 0.00025% (w/v) GDN, 100 mM TCEP). The peak fractions containing ETA– or ETB–miniGs/q–β1γ2–Nb35 complex were collected and concentrated to 2.0–4.0 mg/mL for cryo-EM sample preparation. 10 μM ET-1 or BQ3020 were added to the buffer during the purification process.
Expression and purification of Fab301
As previously reported, Getagozumab23 was used to produce the Fab301 fragment. The Fab301 fragment was codon-optimized and synthesized by GenScript. The corresponding light and heavy chain genes were then subcloned into the pFastBac Dual vector for expression using the Bac-to-Bac baculovirus expression system. Hi5 insect cells were infected with baculovirus at a density of 2 × 106 cells per mL and cultured at 27 °C. Cells were harvested 72 h post infection by centrifugation at 2000 rpm for 30 min, and the clear supernatant was collected. The pH of this supernatant was adjusted to 7.0 before the supernatant was applied to a 2 mL Ni-NTA resin and incubated at 4 °C for 2 h. The column was subsequently washed with a 15-CV buffer containing 20 mM HEPES, pH7.0, 500 mM NaCl, and 20 mM imidazole to remove nonspecifically bound proteins. The protein of interest was eluted from the column using an elution buffer containing 20 mM HEPES, pH 7.0, 100 mM NaCl, and 400 mM imidazole. The eluted protein fractions were then further purified on a Superdex 200 10/300 column, which was equilibrated in a buffer containing 20 mM HEPES, pH7.0, 100 mM NaCl, and 10% glycerol. Monomeric fractions were pooled, concentrated to 8.3 mg/mL, flash-frozen in liquid nitrogen, and stored at –80 °C for future use.
Fab301–ETA–anti-BRIL Fab-Nb complex formation and purification
The purification of the ETA receptor was performed analogously to the method described above, with the addition of one of the antagonists — ambrisentan, macitentan, or zibotentan during purification. The anti-Bril Fab was expressed in mammalian cells and purified following the protocol described previously34. The anti-Fab Nb was expressed in E. coli BL21(DE3) strain and purified according to the previously established methods35.
For complex formation, the ETA, Fab301, anti-BRIL Fab, and anti-Fab Nb were mixed at a molar mass ratio of 1:1.2:1.2:1.5. This mixture was incubated at 4 °C for 4 h, and then concentrated and applied to a Superdex 200 10/300 GL column preequilibrated with the buffer containing 20 mM HEPES, pH 7.4, 100 mM NaCl, 0.00075% (w/v) LMNG, 0.00015% (w/v) CHS, 0.00025% (w/v) GDN, 100 mM TCEP, and 50 μM of the chosen antagonist — either ambrisentan, macitentan or zibotentan. Fractions containing the peak of interest were concentrated to an approximate concentration of 12 mg/mL for cryo-EM specimen preparation.
Cryo-EM sample preparation and data collection
A total of 3 μL of each complex sample, BQ3020–ETB–miniGs/q, ET1–ETB–miniGs/q, ET1–ETA–miniGs/q, Fab301–macitentan–ETA, Fab301–ambrisentan–ETA or Fab301–zibotentan–ETA, was applied to glow-discharged 300 mesh alloy grids (CryoMatrix Amorphous alloy film R1.2/1.3), and vitrified by Vitrobot Mark IV (Thermo Fisher Scientific). Excess sample was blotted by a filter paper for 3 s with a blot force of 2 before plunge-freezing in liquid ethane with a FEI Vitrobot Mark IV at 100% humidity and 4 °C. The frozen grids were transferred to liquid nitrogen and stored for data acquisition. Cryo-EM data collection was conducted with the Krios G4 cryo-transmission electron microscope (Thermo Fisher Scientific) operating at 300 kV, equipped with the Falcon 4 Direct Electron Detector (Thermo Fisher Scientific). Movies were recorded at a calibrated magnification of 130,000×, yielding a pixel size of 0.96 Å. A total dose of 60 electrons per square angstrom (e–/Å2) was administered. Automated data collection was facilitated by the EPU software, utilizing a defocus range spanning from –1.0 μm to –2.0 μm.
Cryo-EM data processing
The overall cryo-EM data processing workflows for the BQ3020–ETB–miniGs/q, ET1–ETB–miniGs/q, ET1–ETA–miniGs/q, Fab301–macitentan–ETA, Fab301–ambrisentan–ETA and Fab301–zibotentan–ETA are shown in Supplementary Figs. S2 and S6. Cryo-EM movie stacks were corrected for beam-induced shifts utilizing the dose-weighting approach in Patch Motion Correction43. The contrast transfer function (CTF) parameters were calculated by employing the patch CTF estimation in CryoSPARC44.
For the BQ3020–ETB–miniGs/q–Nb35 complex, a total of 7685 images were imported in CryoSPARC v.4.0.1. A conventional neural network-based method Topaz45 implemented in CryoSPARC, was used for particle picking. 2,662,705 particles were extracted and then subjected to iterative 2D classification and ab initio reconstruction. Subsequently, 199,360 particles were selected for heterogeneous refinement. The best class was selected for homogeneous refinement, non-uniform refinement, and local refinement, generating a high-quality density map at a resolution of 3.0 Å. DeepEMhancer was applied to enhance local density. The processing steps for the ET1–ETB–miniGs/q–Nb35 and ET1–ETA–miniGs/q–Nb35 complexes, mirrored this approach, with specific details provided in Supplementary Fig. S6.
For Fab301–macitentan–ETA, Fab301–ambrisentan–ETA and Fab301–zibotentan–ETA complexes, the initial data processing steps were consistent with those of the G protein complexes. However, additional post-processing strategies were implemented. The Fab301–macitentan–ETA complex achieved high-quality density after the standard local refinement; thus, no further optimization was performed. In contrast, for the ambrisentan and zibotentan complexes, masks were generated post local refinement to omit the dynamic regions of Fab301. Detailed statistics on the number of images and particles at each processing stage are available in Supplementary Fig. S2.
Model building and refinement
For Fab301–macitentan–ETA, Fab301–ambrisentan–ETA and Fab301–zibotentan–ETA complexes, the AlphaFold2-predicted ETA was used for the receptor modeling. The initial models for BRIL, anti-BRIL Fab and anti-Fab Nb were based on the structures derived from the GPR183 complex structure (PDB: 7TUY)46. For the BQ3020–ETB–miniGs/q–Nb35, ET1–ETB–miniGs/q–Nb35, and ET1–ETA–miniGs/q–Nb35 complexes, model building and refinement of the miniGs/q and Nb35 began with the miniGs/q structure from the GPR139–miniGs/q complex structure (PDB: 7VUH)42. Model docking into the EM density maps was carried out using Chimera47, followed by iterative manual adjustments and rebuilding in Coot48. Subsequent refinement was performed using phenix.real_space_refine in PHENIX49. Statistical validation of the model was conducted through MolProbity50. Structural figures were prepared using ChimeraX51. The complete refinement statistics are documented in Supplementary Table S1.
Intracellular calcium mobilization assay
CHO-K1 cells were cultured in Ham’s F-12K (Kaighn’s) Medium (Gibco-Thermo Fisher Scientific) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco-Thermo Fisher Scientific), and 100 U/mL Penicillin-Streptomycin (Gibco-Thermo Fisher Scientific) in a humidified incubator at 37 °C with 5% CO2. Cells were seeded in 6-cm dishes overnight; when the density reached 60%–80%, the cells were transferred to F12K Medium supplemented with 10% FBS and transfected with 1.5 μg DNA encoding the ETA WT or mutants or 3 μg DNA encoding the ETB WT or mutants using TransIT2020 (Mirus Biosciences). The next day, transfected cells were harvested from the plate using Versene buffer (Gibco-Thermo Fisher Scientific) and seeded into black-sided, clear-bottomed 384-well plates (Agilent) at a density of 1,5000 cells per well. After 20 h, the 1% dFBS medium was removed, and cells were loaded with 20 μL/well of 1× Calcium 6 dye (Molecular Devices) and incubated at 37 °C for 1 h in the dark. 10 μL/well of 3× ET-1 was added and the plates were read using the FLIPR Tetra High Throughput Cellular Screening System (Molecular Devices). To measure antagonist or Fab potency, 10 μL/well of 3× antagonist or Fab was added and incubated with the cells for 30 min at room temperature. The fluorescence intensity was recorded for 2 min after 10 μL/well of 4× ET-1 addition. All data were analyzed using GraphPad Prism 8 and the data are from at least three independent replicate experiments. All plots are shown as means ± SEM. Data were determined using one-way ANOVA followed by Dunnett’s multiple test compared with WT. The top value was normalized to 100% and the bottom value was normalized to 0% for the final presentation. Nonlinear curve fit was performed using a four-parameter logistic equation (log (agonist vs response) or log (inhibitor vs response)).
MD simulation
MD simulations were performed using the GROMACS2021 software, employing the CHARMM36m force field52 and incorporating TIP3 water molecules. Parameters for ambrisentan were derived using the CGenFF force field53. To compensate for the missing ICL3 region in the ambrisentan complex structure, a segment from ETA in the G protein complex was used. The complete structure was prepared with the Protein Preparation Wizard in Maestro (2023-1, Schrödinger), which included the determination of the protonation states of residues at pH 7.4 using PROPKA. The protein was embedded into a lipid bilayer composing 150 POPC molecules using CHARMM-GUI54, which also facilitated the addition of 0.15 M sodium and chloride ions to neutralize the system’s charge. Energy minimization and equilibration processes were performed following the default protocol of CHARMM-GUI, using a cutoff distance of 12 Å for nonbonded contacts and the Particle Mesh Ewald (PME) method55 for long-range van der Waals interactions. Subsequent MD production runs of 500 ns were conducted at a temperature of 310 K and 1 bar using a semi-isotropic Parrinello-Rahman barostat. The final MD trajectories were analyzed and visualized using VMD56 software, where the ligand RMSD calculations were also performed.
Data availability
The atomic coordinates for BQ3020–ETB–miniGs/q, ET1–ETB–miniGs/q, ET1–ETA–miniGs/q, Fab301–macitentan–ETA, Fab301–ambrisentan–ETA and Fab301–zibotentan–ETA complexes have been deposited in the Protein Data Bank (PDB) with the accession codes 8XVE, 8XVH, 8XVI, 8XVJ, 8XVK and 8XVL, respectively. The EM maps for BQ3020–ETB–miniGs/q, ET1–ETB–miniGs/q, ET1–ETA–miniGs/q, Fab301–macitentan–ETA, Fab301–ambrisentan–ETA and Fab301–zibotentan–ETA complexes have been deposited in the Electron Microscopy Data Bank (EMDB) with the codes EMD-38702, EMD-38704, EMD-38705, EMD-38706, EMD-38707 and EMD-38708, respectively.
References
Dhaun, N. & Webb, D. J. Endothelins in cardiovascular biology and therapeutics. Nat. Rev. Cardiol. 16, 491–502 (2019).
Barton, M. & Yanagisawa, M. Endothelin: 30 years from discovery to therapy. Hypertension 74, 1232–1265 (2019).
Davenport, A. P. et al. Endothelin. Pharmacol. Rev. 68, 357–418 (2016).
Houde, M., Desbiens, L. & D’Orleans-Juste, P. Endothelin-1: Biosynthesis, signaling and vasoreactivity. Adv. Pharmacol. 77, 143–175 (2016).
Shihoya, W. et al. Crystal structures of human ETB receptor provide mechanistic insight into receptor activation and partial activation. Nat. Commun. 9, 4711 (2018).
Haryono, A., Ramadhiani, R., Ryanto, G. R. T. & Emoto, N. Endothelin and the cardiovascular system: the long journey and where we are going. Biology 11, 759 (2022).
Rodriguez-Pascual, F., Busnadiego, O., Lagares, D. & Lamas, S. Role of endothelin in the cardiovascular system. Pharmacol. Res. 63, 463–472 (2011).
Hassoun, P. M. Pulmonary arterial hypertension. N. Engl. J. Med. 385, 2361–2376 (2021).
Liu, C., Chen, J., Gao, Y., Deng, B. & Liu, K. Endothelin receptor antagonists for pulmonary arterial hypertension. Cochrane Database Syst. Rev. 3, CD004434 (2021).
Nunes, J. P. L. et al. Prevalence of auto-antibodies associated to pulmonary arterial hypertension in scleroderma - A review. Autoimmun. Rev. 17, 1186–1201 (2018).
Bolli, M. H. et al. The discovery of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N’-propylsulfamide (Macitentan), an orally active, potent dual endothelin receptor antagonist. J. Med. Chem. 55, 7849–7861 (2012).
Patel, T. & McKeage, K. Macitentan: first global approval. Drugs 74, 127–133 (2014).
Croxtall, J. D. & Keam, S. J. Ambrisentan. Drugs 68, 2195–2204 (2008).
Galie, N. et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 117, 3010–3019 (2008).
Newman, J. H., Kar, S. & Kirkpatrick, P. Ambrisentan. Nat. Rev. Drug Discov. 6, 697–698 (2007).
Martinez-Diaz, I. et al. Endothelin receptor antagonists in kidney disease. Int. J. Mol. Sci. 24, 3427 (2023).
Stern, E. P. et al. Zibotentan in systemic sclerosis-associated chronic kidney disease: a phase II randomised placebo-controlled trial. Arthritis Res. Ther. 24, 130 (2022).
Schlaich, M. P. et al. Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial. Lancet 400, 1927–1937 (2022).
Trachtman, H. et al. DUET: a phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J. Am. Soc. Nephrol. 29, 2745–2754 (2018).
Koyama, Y. Endothelin ET(B) receptor-mediated astrocytic activation: pathological roles in brain disorders. Int. J. Mol. Sci. 22, 4333 (2021).
Vatter, H. et al. Characterization of the endothelin-B receptor expression and vasomotor function during experimental cerebral vasospasm. Neurosurgery 60, 1100–1108 (2007).
Shah, P. K. Active and passive vaccination for pulmonary arterial hypertension: a novel therapeutic paradigm. J. Am. Coll. Cardiol. 73, 2581–2583 (2019).
Zhang, C. et al. Therapeutic monoclonal antibody antagonizing endothelin receptor A for pulmonary arterial hypertension. J. Pharmacol. Exp. Ther. 370, 54–61 (2019).
Dai, Y. et al. Immunotherapy of endothelin-1 receptor type A for pulmonary arterial hypertension. J. Am. Coll. Cardiol. 73, 2567–2580 (2019).
Shihoya, W. et al. Activation mechanism of endothelin ETB receptor by endothelin-1. Nature 537, 363–368 (2016).
Shihoya, W. et al. X-ray structures of endothelin ETB receptor bound to clinical antagonist bosentan and its analog. Nat. Struct. Mol. Biol. 24, 758–764 (2017).
Nagiri, C. et al. Crystal structure of human endothelin ET(B) receptor in complex with peptide inverse agonist IRL2500. Commun. Biol. 2, 236 (2019).
Izume, T., Miyauchi, H., Shihoya, W. & Nureki, O. Crystal structure of human endothelin ET(B) receptor in complex with sarafotoxin S6b. Biochem. Biophys. Res. Commun. 528, 383–388 (2020).
Sano, F. K., Akasaka, H., Shihoya, W. & Nureki, O. Cryo-EM structure of the endothelin-1-ET(B)-G(i) complex. Elife 12, e85821 (2023).
Ji, Y. et al. Structural basis of peptide recognition and activation of endothelin receptors. Nat. Commun. 14, 1268 (2023).
Dai, Y. et al. Long-term effect of a vaccine targeting endothelin-1 receptor type A in pulmonary arterial hypertension. Front. Cardiovasc. Med. 8, 683436 (2021).
Chun, E. et al. Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. Structure 20, 967–976 (2012).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Mukherjee, S. et al. Synthetic antibodies against BRIL as universal fiducial marks for single-particle cryoEM structure determination of membrane proteins. Nat. Commun. 11, 1598 (2020).
Ereno-Orbea, J. et al. Structural basis of enhanced crystallizability induced by a molecular chaperone for antibody antigen-binding fragments. J. Mol. Biol. 430, 322–336 (2018).
Iglarz, M. et al. Pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist. J. Pharmacol. Exp. Ther. 327, 736–745 (2008).
Boss, C., Bolli, M. H. & Gatfield, J. From bosentan (Tracleer(R)) to macitentan (Opsumit(R)): the medicinal chemistry perspective. Bioorg. Med. Chem. Lett. 26, 3381–3394 (2016).
Ihara, M. et al. A novel radioligand [125I]BQ-3020 selective for endothelin (ETB) receptors. Life Sci. 51, PL47–PL52 (1992).
Zhou, Q. et al. Common activation mechanism of class A GPCRs. Elife 8, e50279 (2019).
Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. bioRxiv https://doi.org/10.1101/2021.10.04.463034 (2022).
Tsutsumi, N. et al. Structure of human Frizzled5 by fiducial-assisted cryo-EM supports a heterodimeric mechanism of canonical Wnt signaling. Elife 9, e58464 (2020).
Zhou, Y. et al. Molecular insights into ligand recognition and G protein coupling of the neuromodulatory orphan receptor GPR139. Cell Res. 32, 210–213 (2022).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Chen, H., Huang, W. & Li, X. Structures of oxysterol sensor EBI2/GPR183, a key regulator of the immune response. Structure 30, 1016–1024.e5 (2022).
Pettersen, E. F. et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010).
Wu, E. L. et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 35, 1997–2004 (2014).
Darden, T., York, D. & Pedersen, L. Particle mesh ewald - an N.log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Acknowledgements
This work was supported by the National Key R&D Program of China (2022YFA1302902 to T.H.), the National Natural Science Foundation of China (32230026 to Z. Liu, 32271262 to T.H. and 82070085 to G.T.), the CAS Strategic Priority Research Program (XDB37030104 to Z. Liu), the Natural Science Foundation of Shanghai (21ZR1412900 to Y.S.), the Research Project of Shanghai Municipal Health Commission (20234Z0010 to G.T.). Shanghai Frontiers Science Center for Biomacromolecules and Precision Medicine at ShanghaiTech University. We thank the Shanghai Municipal Government and ShanghaiTech University for financial support. The cryo-EM data were collected at the Bio-Electron Microscopy Facility of ShanghaiTech University, with the assistance from Qianqian Sun, Dandan Liu. We also thank the staff at the Assay, Cell Expression, Cloning and Purification Core Facilities of iHuman Institute for their support.
Author information
Authors and Affiliations
Contributions
Z. Luo, T.H., Z. Liu and C.M. conceived and supervised the overall project. J.H. and S.L. purified the protein complex and prepared the samples for cryo-EM analysis. S.L., J.H., and L.W. collected cryo-EM data and performed cryo-EM map calculation, model building and the structure refinement under the supervision of Z. Liu and T.H. X.Z. performed the functional studies supervised by T.H. Y.Z. assisted with the functional studies. J.H. and Q.T. generated the ET insect expression constructs. H.Y. and X.L. performed the AlphaFold2 multimer prediction for Fab301 supervised by F.B. J.L. and L.J. performed protein expression. Z. Liu, T.H., Z. Luo, C.M. S.L., J.H., and X.Z. analyzed the complex structures and designed structure-function experiments. T.H., G.T., J.H., and S.L. drafted the manuscript and designed the figures and tables. T.H. wrote the manuscript with the input from Z. Liu, Z. Luo, C.M. and all other authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hou, J., Liu, S., Zhang, X. et al. Structural basis of antagonist selectivity in endothelin receptors. Cell Discov 10, 79 (2024). https://doi.org/10.1038/s41421-024-00705-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41421-024-00705-9