Abstract
Study design
Clinical case series.
Objective
To describe the cause, treatment and outcome of 6 cases of perioperative spinal cord injury (SCI) in high-risk adult deformity surgery.
Setting
Adult spinal deformity patients were enrolled in the multi-center Scoli-RISK-1 cohort study.
Methods
A total of 272 patients who underwent complex adult deformity surgery were enrolled in the prospective, multi-center Scoli-RISK-1 cohort study. Clinical follow up data were available up to a maximum of 2 years after index surgery. Cases of perioperative SCI were identified and an extensive case review was performed.
Results
Six individuals with SCI were identified from the Scoli-RISK-1 database (2.2%). Two cases occurred intraoperatively and four cases occurred postoperatively. The first case was an incomplete SCI due to a direct intraoperative insult and was treated postoperatively with Riluzole. The second SCI case was caused by a compression injury due to overcorrection of the deformity. Three cases of incomplete SCI occurred; one case of postoperative hematoma, one case of proximal junctional kyphosis (PJK) and one case of adjacent segment disc herniation. All cases of post-operative incomplete SCI were managed with revision decompression and resulted in excellent clinical recovery. One case of incomplete SCI resulted from infection and PJK. The patient’s treatment was complicated by a delay in revision and the patient suffered persistent neurological deficits up to six weeks following the onset of SCI.
Conclusion
Despite the low incidence in high-risk adult deformity surgeries, perioperative SCI can result in devastating consequences. Thus, appropriate postoperative care, follow up and timely management of SCI are essential.
Similar content being viewed by others
Introduction
Perioperative spinal cord injury (SCI) is an intrinsic risk to any surgery involving the spine at the spinal cord level. The injury can result from any direct, indirect, or ischemic physiologic insult to the spinal cord intraoperatively or immediately postoperatively, leading to temporary or permanent neuronal dysfunction and impairment [1]. In addition to the devastating physical consequences, the psychological effect of this injury as well as the lifetime costs of care for SCI patients pose a significant societal and healthcare burden [2,3,4,5,6].
Adult spinal deformity (ASD) is a heterogenous family of conditions encompassing a broad spectrum of underlying etiology with varying severity, and can involve deformity in either the coronal, sagittal, or axial plane [7,8,9,10,11]. Although not all ASD patients are symptomatic, in severe cases the spinal imbalance as well as compression of neurological elements can cause significant functional limitation and reduced quality of life [7, 12,13,14]. Despite advancements in the field of spine surgery, the reported complication rates in adult deformity surgery remain considerably high, reportedly ranging from 10.5% to 96% [15,16,17]. Previous studies have reported substantial variability in the risk of perioperative neurological complications in spinal deformity surgery, with incidences varying between 0.69% and 5% [18,19,20,21,22,23]. However, due to limitations in the rigor of neurological assessments, the actual prevalence of perioperative SCI is much less certain with rates reportedly <1% [19,20,21,22], although it is recognized that this rate may be under-reported.
To address these knowledge gaps, our group undertook the Scoli-RISK-1 study, a multicenter, prospective cohort study comprised of patients who underwent surgical correction for complex ASD [17, 24,25,26,27]. Previous research based on this database has reported lower extremity motor score (LEMS) decline in 23% of patients at discharge following high-risk deformity corrections [28]. However, to date we have not explicitly focused on an analysis of perioperative SCI. Therefore, the objective of this study was to assess the incidence and spectrum of perioperative SCI in complex high-risk ASD surgeries in the Scoli-RISK-1 cohort, as well as to present the identified cases as a case series to further illustrate the etiological causes, treatment options and eventual outcomes.
Material and methods
Patient population
Scoli-RISK-1 enrolled surgical patients with complex ASD from September 2011 to September 2012. The inclusion criteria limited the recruitment of patients to the age range between 18 and 80 years old with complex cervicothoracic or thoracolumbar deformity, between C7 and L2. In addition, one or more of the following criteria had to be met to be considered a high-risk procedure: spinal curvature with major Cobb angle in the coronal or sagittal plane of ≥80°; the necessity of using corrective osteotomies for congenital deformity or revision procedures, requirement of three-column osteotomies (pedicle subtraction osteotomy (PSO), vertebral column osteotomy (VCR)); diagnosis of myelopathy in the presence of spinal deformity requiring reconstruction, or deformity correction with concurrent ossification of the ligamentum flavum (OLF) or ossification of the posterior longitudinal ligament (OPLL) causing secondary spinal cord compression. Any patients with a history of substance dependency, psychosocial disturbance, active malignancy, active infection, a recent history of trauma/malignancy in the spine, long-term complete paraplegia, active pregnancy, prisoners and other institutionalized individuals were considered ineligible for this study and were thus excluded.
The Scoli-RISK-1 database included patients recruited from 15 specialized centers, nine from North America, three from Europe and three from Asia. Approval for the study was obtained from each site’s ethics board. Informed consent was obtained before enrollment for each patient. All methods were performed in accordance with the relevant guidelines and regulations. The surgical approach, methods of instrumentation, corrective techniques and use of intraoperative neurological monitoring were at the discretion of the primary treating surgeon.
Data collection
Preoperatively, patient demographical data was obtained along with preoperative upright x-ray orthogonal images in both anterior-posterior as well as lateral views. Operative data were collected including levels of instrumentation, osteotomy, interbody insertion, blood loss, and relevant intraoperative events. Neurological outcomes were followed primarily using the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSI). The lower extremity motor score (LEMS) has been previously validated for the assessment of ambulatory capacity in patients who sustained incomplete SCI [29]. With the baseline set within six weeks prior to operation, the postoperative exams were performed at the time of discharge, six weeks, six months and two years follow up. Adverse events were reported by the responsible surgeons and documented along with the known or suspected etiology, course of action, and outcomes.
Retrospective search
An extensive search of the Scoli-RISK-1 database was performed to identify patients with documented perioperative SCI. Our definition of the “perioperative” timeframe begins intraoperatively during the initial surgery and extends up to one year postoperatively. A detailed review of patient data including the type of procedure, the triggering event, the medical or surgical management as well as the final clinical outcome was performed and summarized.
Results
A total of six cases (2.2%) of perioperative SCI were found in the 272 patients enrolled in the Scoli-RISK-1 study (Table 1).
Case 1
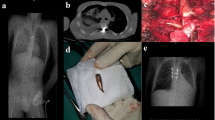
A 48-year-old male with a history of congenital scoliosis and no preoperative neurological deficit underwent a complex T2-L2 posterior spinal fusion with T7 VCR (Fig. 1). Intraoperatively, while working under the spinal cord performing the vertebrectomy, there was a loss of motor evoked potentials bilaterally. After ensuring that there were no compressive elements around the spinal cord, the surgeons went ahead and completed the closure of the vertebrectomy. Postoperative examination revealed 0/5 motor power in bilateral lower limbs with preservation of sensation to pinprick and light touch, in keeping with a T9 incomplete SCI. The patient was treated medically with Riluzole, starting within 12 hours following surgery. For the first 24 hours, 100 mg of Riluzole was given every 12 hours by mouth. Following that Riluzole was continued for 2 weeks at a dosage of 50 mg twice a day. He began experiencing neurological recovery during hospitalization and was discharged to rehabilitation. The patient’s LEMS was 15/50 for the lower limbs bilaterally at the time of discharge. He experienced significant recovery over the ensuing months and his neurological exam improved to his preoperative baseline (50/50) at six months follow up.
A Anterior-posterior (AP) and (B) lateral standing radiographs demonstrated a kyphoscoliotic deformity localized at the main thoracic level, measuring 84° on Cobb angle coronally. Selective computed tomography (C) coronal and (D) sagittal images revealed unsegmented hemivertebrae at the T7 and T8 levels. E, F The patient underwent a T7 vertebral column resection (VCR) and T2-L2 posterior spinal fusion. The procedure was complicated by the intraoperative bilateral loss of motor evoked potentials. Postoperatively, the patient woke with complete loss of power in bilateral lower extremities and preservation of sensation. He was diagnosed with a T9 incomplete SCI and treatment with Riluzole was begun immediately. On the day of discharge, his lower limb power improved to 3/5 motor power bilaterally in all muscle groups. He made a significant recovery in rehabilitation and at 6 months follow up he was ambulatory with grade 5/5 motor strength in bilateral lower extremities.
Case 2
A 57-year-old male with a known history of hypertension, osteoarthritis, and previous spinal fusion procedure elected to undergo a staged corrective operation for ASD. The patient initially presented without any neurological deficit. The staged procedure was carried out with stage 1 including T2-T3 and T3-4 Smith-Peterson osteotomy (SPO) with pedicle screw and temporary rod stabilization from T1 to the pelvis. Given the extent of the surgery, intraoperative blood loss was controlled and kept to an appropriate level. Postoperatively, a slowly progressive loss of motor function in the lower extremities began 12 hours after surgery. Examination revealed an incomplete SCI at the T3 neurological level. Upon investigation, the cause of the neurological deficit was determined to be overcorrection of the deformity, thus the decision was then made by the primary treating surgeon to return to the operating room to release the correction to the proximal thoracic spine. The patient was urgently taken back to the OR within the same day and immediate postoperative improvement was noted in neurological function. The patient returned to the operating room two weeks later for the 2nd stage whereby the pedicle screw and hook construct was then completed along with insertion of interbody cage at L2-3 and an L3 PSO for the final correction. The procedure was uncomplicated, and the patient was discharged to rehabilitation with a LEMS of 23 in the lower extremities and normal sensation. At the six-month follow up visit, persistent lower limb motor and sensory deficits were noted.
Case 3
A 70-year-old female underwent a single-stage T9 to pelvis posterior spinal fusion procedure with T12 VCR and cage reconstruction. The surgical procedure was uncomplicated. However, on postoperative day 1, the patient was found to have weakness bilaterally in the lower extremities associated with decreased sensation. Physical examination revealed 1/5 motor power in hip flexion, knee extension, ankle dorsi and plantar flexion consistent with an incomplete American Spinal Injury Association Impairment Scale (AIS) grade C. Immediate CT scan revealed a compressive epidural hematoma at the T12 level. Revision and evacuation of the hematoma was performed on an urgent basis and the patient made a complete recovery of her motor as well as sensory deficits and was discharged home.
Case 4
A 60-year-old female with a history of non-metastatic cancer, osteoporosis, hypertension and anxiety along with three previous spinal procedures underwent a staged correction for her ASD. The first stage of the operation included T8 to pelvis posterior spinal fusion with VCR of T12 and L1, as well as reconstruction with cage implant. The second stage followed a period of recovery with an extension of the fusion to T3, a PSO at the level of T7 and insertion of an interbody cage at the T6-T7 level. At nine days postoperatively, she experienced electric shock-like sensations in the legs accompanied by the onset of lower extremity weakness. Investigation revealed an incomplete T2 SCI secondary to proximal junctional kyphosis. Emergent surgical revision was performed for deformity correction, decompression, and extension of fusion to C4. The patient recovered with resolution of symptoms and a return of neurological function to baseline.
Case 5
A 52-year-old male presented with a chief complaint of bilateral lower extremity weakness and gait imbalance. He was diagnosed with congenital deformity causing myelopathy. Aside from known hypertension, he was otherwise in good health. However, his history was complicated by two previous spinal decompression and fusion procedures. Preoperatively, he had a mild left lower extremity sensory deficit to light touch and intact LEMS on examination.
The patient underwent T9 to pelvis posterior spinal fusion, with 4 level SPO at T11-T12, T12-L1, L1-L2, L2-L3, 2 level VCR at L3 and L4 with cage reconstruction, and L5-S1 interbody cage insertion. The operation was complicated by excessive bleeding and the development of an intraoperative extensive erythematous rash of unknown etiology, likely an allergic reaction. Additionally, postoperatively the patient developed anemia, thrombocytopenia, and a dental abscess. Despite this, the patient was neurologically stable with no change to the slight sensory deficit present before the operation and was discharged to home.
At five months postoperatively, the patient reported numbness in the right leg with difficulty ambulating and increased urinary frequency. An x-ray confirmed a fracture of the rod. However, given his symptoms were improving, a surgical revision was planned for a later date. At nine months post indexed surgery, the patient fell while getting out of bed. Initially the patient was able to move his legs, however, two hours later he woke up with complete loss of motor function in bilateral lower extremities. Upon arrival at the specialized center, he was found to have a T8 incomplete SCI. Steroids were given and the patient was taken for an urgent MRI which revealed a disc herniation above the fusion construct. He was brought to the operating room for surgical decompression. Postoperatively, despite the complication of postoperative osteomyelitis, neurological status improved, and motor strength improved to 5/5 in assessed muscles of the lower extremities.
Case 6
A 60-year-old male with a medical history of degenerative arthritis complicated by multiple previous spinal operations, underwent a T10 to S1 posterior spinal fusion and L2 PSO. Surgery was uncomplicated and the patient was discharged to home. At five weeks after surgery, he presented with neurological deficits consistent with a T10 incomplete SCI secondary to T9-T10 discitis and epidural abscess. The patient underwent an urgent revision T9 laminectomy with irrigation and debridement of the infection. Postoperatively, due to persistent neurological deficit, a repeat MRI scan was performed revealing a collapse of the T9-T10 disc space causing PJK, as well as the demonstration of an intraosseous abscess and epidural phlegmon resulting in significant compression to the spinal cord. Unfortunately, surgical revision was delayed due to medical reasons. At two weeks following the previous revision procedure, the patient was taken to the operating room for a second repeat decompression with an osteotomy at the level of T9-T10 and extension of the fusion to T4. The patient was discharged to rehabilitation with a persistent neurological deficit. At the six weeks postoperative visit, his motor and sensory loss remained unchanged, with a lower extremity motor score of 22/50 and total sensory scores of 64/100 for both light touch and pin prick. Unfortunately, the patient withdrew consent and no further information on his recovery was available.
Discussion
This study reflects the high risk of complex deformity procedures and provides examples of common causes of perioperative SCI. To address the knowledge gap in this area, this retrospective review provided concrete examples of how perioperative SCI was dealt with by spinal deformity experts.
In this study, using the international, multicentered Scoli-RISK-1 database, we focused on the group of ASD patients with complex spinal deformities. The rate of perioperative SCI in this cohort was found to be 2.2%, comparatively higher than rates which are reported in the literature for typical deformity surgery [19,20,21,22], but not exceeding the reported rate for SCI in advanced posterior column osteotomies [16, 30,31,32]. Although the overall risk for SCI in our cohort is seemingly low, it is essential to note that the patients enrolled in the Scoli-RISK-1 study were treated by spine surgeons with both expertise and experience in spinal deformity procedures, and who worked at specialized institutions. Therefore, the results of this study should not be generalized to all deformity procedures and contexts, and therefore need to be interpreted with caution. Furthermore, we strongly suggest that high-risk complex deformity procedures be performed by surgeons with the proper training and expertise at institutions with adequate resources and experience.
In this series, intraoperative events accounted for one case of SCI, due to direct trauma to the spinal cord. Postoperative adverse events were responsible for the other five, and were comprised of progressive neurological deficit due to overcorrection of deformity, a postoperative compressive hematoma, an adjacent segment disk herniation, a PJK, as well as one case of discitis leading to osteomyelitis, abscess, epidural phlegmon and PJK. Of these six cases, only one case was managed medically, while the others were revised surgically. Five of the six patients demonstrated significant recovery with the prompt treatment of their SCI. In one patient, a delay in surgical revision occurred due to medical reasons. Despite adequate decompression, his motor and sensory deficits remained at the six weeks follow up visit. However, the patient withdrew from the study and no further details concerning his recovery were available. Therefore, this clinical series illustrates the need for clinical vigilance in the perioperative period following complex deformity correction. Additionally, in keeping with the existing literature, this study further demonstrated the urgency for reversal of causative intraoperative maneuvers or revision and decompression of postoperatively identified compressive lesions when appropriate, as determined by the patient’s medical status [1, 19, 22].
As noted in this case series, the administration of corticosteroids in the event of SCI was left to the clinical judgment of the treating surgeon. Due to the previously reported increased risk of infection, the use of methylprednisolone in this context remains inconsistent in the spine community [33,34,35]. However, recent evidence-based guidelines supported by a robust systematic review of the literature demonstrated the benefit of using corticosteroids when administered within 8 hours of acute traumatic SCI with no significant difference in the rate of complications [36, 37].
In the event where no revisable spinal cord compression is identified, the option of medical treatment and observation has been reported [1, 22]. Aside from corticosteroids, a few other agents have been tried and are in various stages of testing or clinical trials [35, 38, 39]. The medically managed case presented here was treated with Riluzole, a voltage-gated sodium channel blocker used previously in the treatment of amyotrophic lateral sclerosis [40]. Riluzole has shown promise in providing neuroprotection and promotes functional recovery after SCI in animal models [41, 42]. Although the application or Riluzole for neuroprotective purposes in SCI is relatively new, early clinical trials in humans are showing promising results [43, 44].
The multicentered randomized controlled trial, Riluzole in Acute Spinal Cord Injury Study (RISCIS), commenced in 2014 [45] with the primary outcome targeted at assessing change in upper and lower extremity motor scores. Due to the global COVID pandemic, the Phase III RISCIS trial was stopped in 2021 prior to reaching the pre-planned patient enrollment [46]. Regardless, with the 193 subjects (54.9% enrollment) randomized to treatment and placebo arm, Fehlings et al., 2023 [46], reported improvement in the gain of 1.76 upper extremity motor and 2.86 total motor score in the Riluzole-treated subjects at 180 days. However, the results did not reach statistical significance. While the primary analysis of the RISCIS trial did not achieve predetermined endpoint efficacy, likely related to insufficient power, secondary analysis of other measures, including SCIM, SF36, EQ5D, GRASSP and neurological levels gained, also showed no statistically significant improvement in the treatment group. However, in the AIS B subgroup, Riluzole-treated patients experienced statistically significant improvements in the SF36 mental component and the SCIM total score, respiratory management and self-care component. Despite these new findings, the usage of Riluzole in the context of SCI is still new and requires further investigation. Although we presented the case of SCI treated non-surgically, we cannot attribute the neurological recovery to Riluzole administration. Hence, we are not advocating for the use of Riluzole in SCI but simply presenting this as an alternative option in cases where surgical intervention may not be possible or indicated.
Finally, there is still a lack of consensus in the prevention and management of intraoperative SCI during spinal surgeries. Recognizing this critical knowledge gap, recently, a focused group of international SCI experts systematically reviewed the current literature and formulated the evidence into clinical practice guidelines [47,48,49]. Using the GRADE protocol, the group recommends prompt identification of patients undergoing surgical procedures at high risk of intraoperative SCI, early involvement of multi-disciplinary care team, and use of intraoperative neuromonitoring during the operation [49]. The AO Spine PRAXIS care pathway was concurrently developed using an evidence-based approach to serve as a reference for the perioperative management of high-risk spine surgery patients. The guideline also includes an extensive checklist, modified from Vitale et al. 2014, to guide and assist surgeons in the event of intraoperative neuromonitoring signal changes [48, 50]. With these novel recommendations and guidelines, we anticipate future prospective multicentered studies to validate and assess their efficacy in reducing intraoperative SCI.
Study limitations
This study is subject to several limitations. Although the strict inclusion criteria allowed for the enrollment of patients with complex ASD, it does not select for a specific underlying diagnosis. Hence, the study cohort is still heterogeneous in terms of the etiology of deformities. Similarly, the surgical procedures for spinal deformities as well as the treatment protocol for perioperative SCI were not standardized, further highlighting the need for evidence-based guidelines to manage perioperative SCI in ASD surgery. Additionally, since the Scoli-RISK-1 cohort included only adult patients who underwent high-risk spinal deformity procedures, the incidence of perioperative SCI reported in this case series needs to be interpreted with caution when being applied to the general spinal deformity patient population. Furthermore, given the rarity of perioperative SCI, the small number of patients precluded the use of statistical analysis to assess for predictors and risk factors. Finally, given the nature of the database, our description of the cases is limited to the available data. Therefore, certain details including exact neurological deficit at the onset of adverse events, the exact nature of the injury and diagnostic imaging performed are not available. However, a future detailed chart review of each case would be of benefit to further dissect and understand each event.
Conclusion
Although the complication rate specific to perioperative SCI remains low in complex high-risk deformity surgeries, this case series highlights the variability in the etiology, operative management, and recovery with medical or surgical treatment. Despite the relatively low incidence, perioperative SCI can result in possible permanent neurological deficits. Thus, it is essential for these procedures to be performed by experienced spine surgeons with skills and expertise in deformity correction. Furthermore, the recovery of these patients varies greatly case by case. We therefore strongly advocate for careful postoperative observation and timely management of SCI to optimize the chance of recovery. Further research, not only with regards to risk factors, but with regards to the course of recovery in these cases is warranted. Finally, this study highlighted the need for standardized clinical practice guidelines for the management of perioperative SCI following complex deformity procedures.
Data availability
The data that supports the findings of this study are available from the corresponding author, MF, upon reasonable request.
References
Ahn H, Fehlings MG. Prevention, identification, and treatment of perioperative spinal cord injury. Neurosurg Focus. 2008;25:E15.
New PW, Jackson T. The costs and adverse events associated with hospitalization of patients with spinal cord injury in Victoria, Australia. Spine. 2010;35:796–802.
French DD, Campbell RR, Sabharwal S, Nelson AL, Palacios PA, Gavin-Dreschnack D. Health care costs for patients with chronic spinal cord injury in the Veterans Health Administration. J Spinal Cord Med. 2007;30:477–81.
Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95:986–95 e1.
Mitchell R, Harvey L, Stanford R, Close J. Health outcomes and costs of acute traumatic spinal injury in New South Wales, Australia. Spine J. 2018;18:1172–9.
Post MW, van Leeuwen CM. Psychosocial issues in spinal cord injury: a review. Spinal Cord. 2012;50:382–9.
Ames CP, Scheer JK, Lafage V, Smith JS, Bess S, Berven SH, et al. Adult spinal deformity: epidemiology, health impact, evaluation, and management. Spine Deform. 2016;4:310–22.
Lu DC, Chou D. Flatback syndrome. Neurosurg Clin N Am. 2007;18:289–94.
Grubb SA, Lipscomb HJ, Coonrad RW. Degenerative adult onset scoliosis. Spine. 1988;13:241–5.
Good CR, Auerbach JD, O’Leary PT, Schuler TC. Adult spine deformity. Curr Rev Musculoskelet Med. 2011;4:159–67.
Protopsaltis TS, Boniello AJ, Schwab FJ. Management of spinal deformity in adult patients with neuromuscular disease. J Am Acad Orthop Surg. 2016;24:634–44.
Glassman SD, Schwab FJ, Bridwell KH, Ondra SL, Berven S, Lenke LG. The selection of operative versus nonoperative treatment in patients with adult scoliosis. Spine. 2007;32:93–7.
Smith JS, Fu KM, Urban P, Shaffrey CI. Neurological symptoms and deficits in adults with scoliosis who present to a surgical clinic: incidence and association with the choice of operative versus nonoperative management. J Neurosurg Spine. 2008;9:326–31.
Pellise F, Vila-Casademunt A, Ferrer M, Domingo-Sabat M, Bago J, Perez-Grueso FJ, et al. Impact on health related quality of life of adult spinal deformity (ASD) compared with other chronic conditions. Eur Spine J. 2015;24:3–11.
Smith JS, Kasliwal MK, Crawford A, Shaffrey CI. Outcomes, expectations, and complications overview for the surgical treatment of adult and pediatric spinal deformity. Spine Deform. 2012;1:4–14.
Yang C, Zheng Z, Liu H, Wang J, Kim YJ, Cho S. Posterior vertebral column resection in spinal deformity: a systematic review. Eur Spine J. 2016;25:2368–75.
Kelly MP, Lenke LG, Shaffrey CI, Ames CP, Carreon LY, Lafage V, et al. Evaluation of complications and neurological deficits with three-column spine reconstructions for complex spinal deformity: a retrospective Scoli-RISK-1 study. Neurosurg Focus. 2014;36:E17.
Girardi FP, Boachie-Adjei O, Rawlins BA. Safety of sublaminar wires with Isola instrumentation for the treatment of idiopathic scoliosis. Spine. 2000;25:691–5.
Diab M, Smith AR, Kuklo TR. Spinal Deformity Study G. Neural complications in the surgical treatment of adolescent idiopathic scoliosis. Spine. 2007;32:2759–63.
Pastorelli F, Di Silvestre M, Plasmati R, Michelucci R, Greggi T, Morigi A, et al. The prevention of neural complications in the surgical treatment of scoliosis: the role of the neurophysiological intraoperative monitoring. Eur Spine J. 2011;20:S105–14.
Smith C, Lamba N, Ou Z, Vo QA, Araujo-Lama L, Lim S, et al. The prevalence of complications associated with lumbar and thoracic spinal deformity surgery in the elderly population: a meta-analysis. J Spine Surg. 2019;5:223–35.
Bridwell KH, Lenke LG, Baldus C, Blanke K. Major intraoperative neurologic deficits in pediatric and adult spinal deformity patients. Incidence and etiology at one institution. Spine. 1998;23:324–31.
Kamerlink JR, Errico T, Xavier S, Patel A, Patel A, Cohen A, et al. Major intraoperative neurologic monitoring deficits in consecutive pediatric and adult spinal deformity patients at one institution. Spine. 2010;35:240–5.
Cerpa M, Lenke LG, Fehlings MG, Shaffrey CI, Cheung KMC, Carreon LY. Evolution and advancement of adult spinal deformity research and clinical care: an overview of the Scoli-RISK-1 study. Global Spine J. 2019;9:8S–14S.
Fehlings MG, Kato S, Lenke LG, Nakashima H, Nagoshi N, Shaffrey CI, et al. Incidence and risk factors of postoperative neurologic decline after complex adult spinal deformity surgery: results of the Scoli-RISK-1 study. Spine J. 2018;18:1733–40.
Lenke LG, Fehlings MG, Shaffrey CI, Cheung KM, Carreon L, Dekutoski MB, et al. Neurologic outcomes of complex adult spinal deformity surgery: results of the prospective, multicenter Scoli-RISK-1 study. Spine. 2016;41:204–12.
Carreon LY, Glassman SD, Shaffrey CI, Fehlings MG, Dahl B, Ames CP, et al. Predictors of health-related quality-of-life after complex adult spinal deformity surgery: a Scoli-RISK-1 secondary analysis. Spine Deform. 2017;5:139–44.
Lenke LG, Shaffrey CI, Carreon LY, Cheung KMC, Dahl BT, Fehlings MG, et al. Lower extremity motor function following complex adult spinal deformity surgery: two-year follow-up in the Scoli-RISK-1 prospective, multicenter, international study. J Bone Joint Surg Am. 2018;100:656–65.
Shin JC, Yoo JH, Jung TH, Goo HR. Comparison of lower extremity motor score parameters for patients with motor incomplete spinal cord injury using gait parameters. Spinal Cord. 2011;49:529–33.
Xie J, Wang Y, Zhao Z, Zhang Y, Si Y, Li T, et al. Posterior vertebral column resection for correction of rigid spinal deformity curves greater than 100 degrees. J Neurosurg Spine. 2012;17:540–51.
Papadopoulos EC, Boachie-Adjei O, Hess WF, Sanchez Perez-Grueso FJ, Pellise F, Gupta M, et al. Early outcomes and complications of posterior vertebral column resection. Spine J. 2015;15:983–91.
Xie JM, Zhang Y, Wang YS, Bi N, Zhao Z, Li T, et al. The risk factors of neurologic deficits of one-stage posterior vertebral column resection for patients with severe and rigid spinal deformities. Eur Spine J. 2014;23:149–56.
Ito Y, Sugimoto Y, Tomioka M, Kai N, Tanaka M. Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury?: a prospective study about neurological recovery and early complications. Spine. 2009;34:2121–4.
Matsumoto T, Tamaki T, Kawakami M, Yoshida M, Ando M, Yamada H. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine. 2001;26:426–30.
Hadley MN, Walters BC, Grabb PA, Oyesiku NM, Przybylski GJ, Resnick DK, et al. Pharmacological therapy after acute cervical spinal cord injury. Neurosurgery. 2002;50:S63–72.
Fehlings MG, Wilson JR, Harrop JS, Kwon BK, Tetreault LA, Arnold PM, et al. Efficacy and safety of methylprednisolone sodium succinate in acute spinal cord injury: a systematic review. Global Spine J. 2017;7:116S–37S.
Fehlings MG, Wilson JR, Tetreault LA, Aarabi B, Anderson P, Arnold PM, et al. A clinical practice guideline for the management of patients with acute spinal cord injury: recommendations on the use of methylprednisolone sodium succinate. Global Spine J. 2017;7:203S–11S.
Ulndreaj A, Badner A, Fehlings MG. Promising neuroprotective strategies for traumatic spinal cord injury with a focus on the differential effects among anatomical levels of injury. F1000Res. 2017;6:1907.
Badhiwala JH, Ahuja CS, Fehlings MG. Time is spine: a review of translational advances in spinal cord injury. J Neurosurg Spine. 2018;30:1–18.
Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2012;3:CD001447.
Martins BC, Torres BBJ, de Oliveira KM, Lavor MS, Osorio CM, Fukushima FB, et al. Association of riluzole and dantrolene improves significant recovery after acute spinal cord injury in rats. Spine J. 2018;18:532–9.
Srinivas S, Wali AR, Pham MH. Efficacy of riluzole in the treatment of spinal cord injury: a systematic review of the literature. Neurosurg Focus. 2019;46:E6.
Grossman RG, Fehlings MG, Frankowski RF, Burau KD, Chow DS, Tator C, et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma. 2014;31:239–55.
Meshkini A, Salehpour F, Aghazadeh J, Mirzaei F, Naseri Alavi SA. Riluzole can improve sensory and motor function in patients with acute spinal cord injury. Asian J Neurosurg. 2018;13:656–9.
Fehlings MG, Nakashima H, Nagoshi N, Chow DS, Grossman RG, Kopjar B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): a randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord. 2016;54:8–15.
Fehlings MG, Moghaddamjou A, Harrop JS, Stanford R, Ball J, Aarabi B, et al. Safety and efficacy of riluzole in acute spinal cord injury study (RISCIS): a multi-center, randomized, placebo-controlled, double-blinded trial. J Neurotrauma. 2023;40:1878–88.
Hejrati N, Srikandarajah N, Alvi MA, Quddusi A, Tetreault LA, Guest JD, et al. The management of intraoperative spinal cord injury—a scoping review. Global Spine J. 2024;14:150S–65S.
Srikandarajah N, Hejrati N, Alvi MA, Quddusi A, Tetreault LA, Evaniew N, et al. Prevention, diagnosis, and management of intraoperative spinal cord injury in the setting of spine surgery: a proposed care pathway. Global Spine J. 2024;14:166S–73S.
Fehlings MG, Alvi MA, Evaniew N, Tetreault LA, Martin AR, McKenna SL, et al. A clinical practice guideline for prevention, diagnosis and management of intraoperative spinal cord injury: recommendations for use of intraoperative Neuromonitoring and for the use of preoperative and intraoperative protocols for patients undergoing spine surgery. Global Spine J. 2024;14:212S–22S.
Vitale MG, Skaggs DL, Pace GI, Wright ML, Matsumoto H, Anderson RC, et al. Best practices in intraoperative neuromonitoring in spine deformity surgery: development of an intraoperative checklist to optimize response. Spine Deform. 2014;2:333–9.
Acknowledgements
This study was organized and funded by AO Spine International through the AO Spine Knowledge Forum Deformity, a focused group of international spine deformity experts acting on behalf of AO Spine. Study support was provided directly through the AO Spine Research Department and AO’s Clinical Investigation and Documentation unit. MF is supported by the Robert Campeau Family Foundation/Dr. C.H. Tator Chair in Brain and Spinal Cord Research at UHN.
Funding
This study was financially supported by the Scoliosis Research Society (SRS), Norton Healthcare, and AO Spine International. AO Spine is a clinical division of the AO Foundation—an independent medically-guided non-profit organization.
Author information
Authors and Affiliations
Contributions
The study’s conceptualization and finalization involved the input of LL, CS, KC, LC, MD, FS, OB, KK, CA, SB, YQ, YM, BD, HM, FP, SL, and MF. The manuscript was authored by FJ, with contributions from HJ, JB, and JW. MF supervised the entire project and provided leadership for the study’s direction. All authors offered critical feedback and played a significant role in shaping the study, conducting the analysis, interpreting the results, and finalizing the manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Fehlings is a co-author of this study and the Editor-in-Chief of Spinal Cord. He has not been involved in handling this manuscript during the submission and/or review processes. BD serves on the K2M and Stryker advisory boards. SB receives royalties from Stryker, is a consultant for Medtronic, Stryker, Globus, Medicrea, owns Green Sun Medical and Providence Medical, and is on the Editorial Boards of Spine Deformity, JNS-Sine, and Spine.
Ethical approval
The Scoli-RISK-1 database included patients recruited from 15 specialized centers, nine from North America, three from Europe and three from Asia. Approval for the study was obtained from each site’s ethics board. Informed consent was obtained before enrollment for each patient.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, F., Joshi, H., Badhiwala, J.H. et al. Spinal cord injury in high-risk complex adult spinal deformity surgery: review of incidence and outcomes from the Scoli-RISK-1 study. Spinal Cord Ser Cases 10, 59 (2024). https://doi.org/10.1038/s41394-024-00673-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-024-00673-y