Abstract
Study design
Systematic review with network meta-analysis.
Objective
We explored the efficacy and safety of different drug treatments in patients with spinal-cord injury (SCI)-related neuropathic pain. We investigated which treatment is most suitable for such patients by judging the efficacy and safety of these drugs.
Methods
We searched the PubMed, Medline, Embase and Cochrane databases from inception to 31 August 2020. The quality of the included studies was assessed. We selected the proportion of patients whose pain was reduced by ≥50% and the prevalence of adverse effects as the outcome indicators of efficacy and safety, respectively.
Results
We included 15 randomized controlled clinical trials involving five interventions (anticonvulsants, antidepressants, anesthetics, opioids and botulinum toxin A). Based on the proportion of patients with pain reduction ≥50%, the order (from highest to lowest) was anticonvulsants > anesthetics > antidepressants > botulinum toxin A > opioids > placebo. With regard to the prevalence of adverse effects, the order of safety (from highest to lowest) was placebo > antidepressants > botulinum toxin A > anticonvulsants > opioids > anesthetics. Analyzes of efficacy and safety revealed that anticonvulsant, antidepressant and botulinum toxin A have good efficacy and safety.
Conclusion
The efficacy of anticonvulsants, anesthetics, antidepressants, opioids and botulinum toxin A was greater than that of placebo for treatment of SCI-related neuropathic pain. However, the prevalence of adverse effects associated with use of these drugs was also higher than that of placebo. Further analyses based on efficacy and safety revealed anticonvulsants to be more suitable for such patients. In addition, antidepressant and botulinum toxin A may be promising treatments for SCI-related neuropathic pain, however, their effects still need to be further explored due to the small sample size.
Similar content being viewed by others
Introduction
Spinal-cord injury (SCI) can cause varying degrees of paralysis, sensory loss, and bladder/intestinal dysfuction [1, 2]. It is estimated that ~30 people suffer from SCI every day in the USA through motor-vehicle accidents, falls, violence, or sports activities [3].
SCI can directly or indirectly cause different degrees of pain, which occurs in the absence of noxious stimuli and may be spontaneous, or may be caused by sensory stimuli [4,5,6]. It is the main complication of SCI, which can affect or slow down the efficacy of rehabilitation for such patients to a certain extent [7, 8]. This not only has a huge impact on quality of life, mental state, and life expectancy, but also affects families and society through a severe economic burden [9, 10]. Therefore, active management of pain and treatment after SCI are necessary.
The treatment options for SCI patients are drug therapy, behavioral therapy, electrical stimulation therapy, and surgery. Among them, drug therapy is the most commonly used option, and includes anticonvulsants, tricyclic antidepressants, antiepileptics, and opioids [11,12,13,14,15]. Although several clinical trials have revealed that these drugs are efficacious in such patients, the evidence for direct comparison between these drugs is lacking, and it is not clear which drug is more efficacious and safer.
A “network meta-analysis” (NMA) is an extension of a traditional meta-analysis. If the aim is to explore the efficacy and safety of multiple different interventions for the same disease but direct comparisons are scarce or absent, the efficacy and safety of multiple interventions can be compared indirectly by establishing a common control group [16, 17]. Rudroju et al. [18] conducted a NMA in 2013 to explore the efficacy and safety of six antidepressants and anticonvulsants in patients with diabetes mellitus-related neuropathic pain.
Here, we undertook a NMA of published studies to explore the efficacy and safety of different drug treatments in patients with SCI-related neuropathic pain. Also, we investigated which treatment was most suitable for such patients by studying the efficacy and safety of these drugs.
Methods
The systematic review and network meta-analysis were performed according to the checklist of the Preferred Reporting Items for Systematic Reviews and Meta-analyses extension statement for network meta-analysis.
Inclusion criteria
The inclusion and exclusion criteria for this study were based on the PICO strategy (P: patient, I: intervention, C: comparison, O: outcome). All included studies met the following criteria: (i) the patients had SCI-related neuropathic pain, were aged ≥18 years, pain impairment score (based on neuropathic pain scale, visual analog scale or Borg’s Category Ratio scale) ≥3; (ii) interventions were drug treatment-related measures (antiepileptics, anticonvulsants, antidepressants, or opioids); (iii) to improve the quality of the evidence, and after a preliminary search of the primary database, we decided that only randomized controlled trials (RCTs) with a study cohort >10 should be included in this NMA; (iv) information on the following outcome indicators in the original text or available from the study authors: the rate of patients with pain reduction ≥50% or a significant improvement in pain, and the prevalence of adverse effects had to be stated.
Exclusion criteria
The exclusion criteria were that: (i) included patients with neuropathic pain caused by other reasons (e.g., stroke); (ii) included patients with a history of severe allergy or severe diseases of the heart, liver or kidney; (iii) included women who were pregnant or lactating; (iv) did not include a control group; (v) were case reports.
Outcome measures
The primary outcome measures were the proportion of patients with pain reduction ≥50% (namely the pain of patients have significantly reduced, undisturbed sleep and can live a normal life based on the standard of pain efficacy score) or significant improvement on the Patient Global Impression of Change scale. The secondary outcome measure was the prevalence of adverse effects related to each drug treatment.
Search strategy
The databases that we used were PubMed, Cochrane Library, Embase, and Medline. Two reviewers searched publications up to 31 August 2020. The search terms used were “spinal cord injury”, “SCI”, “anticonvulsant”, “antidepressant”, “opioids”, “antiepileptic”, “drug therapy”, “pharmacological treatment”, “random”, “randomized controlled trial”, and “RCT”. We searched the references of all identified studies in journals, magazines, and conference abstracts. We also searched the World Health Organization International Clinical Trials Registry Platform to identify RCTs that were ongoing or complete but which had not yet been published. Finally, we searched for RCTs that had been included in relevant systematic reviews or meta-analyses published in the past 2–3 years.
Quality assessment
The inclusion and exclusion criteria were applied by two reviewers. They independently screened the literature-retrieval results and used the Cochrane Quality Evaluation Method to assess all randomized trials included in the present study from randomization (allocation concealment), blinding, selective bias, incomplete data, and other biases. The specific implementation method, taking the implementation of randomization as an example, if randomization was carried out with an appropriate method, the study was classified as having a “low” risk of a bias; if randomization was not done, the study was classified as having a “high” risk of a bias, and if insufficient information regarding the implementation process was available, the study could not be classified as having a high or low risk of a bias and was defined instead as having an “unclear” risk of a bias. The other items were assessed according to this standard. To avoid the risk of a bias, differences were discussed by the two reviewers: if agreement was not reached, a consensus was reached with a third reviewer. For each outcome, we also used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework to assessment of the quality of evidence.
Data extraction
The following data were extracted by two reviewers from all included RCTs: author; year of publication; country; study type; age of patients; intervention type; number of intervention groups; detailed information of drug treatment; proportion of patients with pain reduction ≥50% or significant improvement in pain; prevalence of adverse effects related to each drug treatment. If data were missing, we contacted the corresponding author of the article wherever possible.
Statistical analyses
A pairwise meta-analysis using RevMan 5.3 (Cochrane Collaboration, London, UK), we expressed the efficacy and safety of the different treatments as the relative ratio and 95% confidence interval for dichotomous outcomes. Taking into account the between-study differences, the pooled estimates was using a random-effects model. Heterogeneity was assessed using Cochrane Q test and I2 statistics, I2 < 50% were considered to have low heterogeneity and I2 > 50% were considered to have high heterogeneity.
The Network meta-analysis was performed to compare efficacy and safety among different treatments by using Stata16.0 (StataCorp, College Station, TX USA). Before merging the direct and indirect evidence, we used the node-splitting method to identify whether the network model had inconsistency. And then, the multidimensional scaling method was performed to rank the different interventions for each outcome, the result explained as that the better intervention was closer to the upper right. At last, we combined the efficacy and safety to re-ranked by using the cluster rank, the results also explained as that the better intervention was closer to the upper right.
Results
Literature search
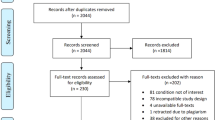
In total, 3181 articles were retrieved. First, 2314 duplicate articles were removed by initial screening of the titles and abstracts. Next, 808 articles were removed by research objective and article type. An additional 44 articles were excluded according to the inclusion and exclusion criteria. Finally, 15 RCTs were included in the NMA (Fig. 1).
Among them, eight articles were based on randomized double-blind crossover trials, one article was based on a randomized double-blind triple-crossover trial, and six articles were based on randomized double-blind parallel trials. The study type, type and detailed information of the intervention, and the number of intervention groups of the included articles (Tables 1 and 2). The total sample size was 977 and included five interventions using anticonvulsants (n = 7), [11,12,13, 15, 19,20,21] anesthetic agents (n = 4) [22,23,24], antidepressants (n = 2) [25, 26], opioids (n = 2) [14, 27], and botulinum toxin A (n = 1) [28].
Quality assessment
An assessment of bias risk using the method described in the Cochrane Collaboration Handbook was undertaken to evaluate the quality of the 15 RCTs with respect to randomization (allocation concealment), blinding, a selective bias, incomplete data, and other biases. In general, the quality of these studies was medium to high (Fig. 2a, b).
The pairwise meta-analysis and GRADE
Based on the different types of interventions, a subgroup analyses were used to test for heterogeneity. In terms of the proportion of patients with pain reduction ≥50% or who had significant improvement in pain, heterogeneity was absent (P > 0.1) (Supplementary Fig. 1a). A fixed-effect model revealed that anticonvulsants, anesthetics and antidepressants increased the efficacy of pain relief in patients with SCI-related neuropathic pain compared with that proffered by placebo, and the differences were significant (P < 0.05 for all). However, the efficacy of opioids and botulinum toxin A was not better than that elicited by placebo for pain relief in these patients, and the differences were not significant (P > 0.05 for all).
In the included studies, the common adverse events of drug treatments are dizziness and somnolence, compared with the placebo, there were significant differences in the group of anticonvulsant and anesthetic (P < 0.05 for all). Moreover, the adverse events related to the digestive system are also common (such as nausea and dry mouth), however, there was significant difference only in the intervention of anticonvulsant (P < 0.05). Subsequently, based on the prevalence of adverse effects of each intervention, a subgroup analyses (Supplementary Fig. 1b) demonstrated that there was an absence of heterogeneity (P > 0.1). A fixed-effect model revealed that use of anticonvulsants or anesthetics increased the prevalence of adverse effects in patients with SCI-related neuropathic pain, and the differences were significant (P < 0.05 for all). The prevalence of adverse effects after use of antidepressants, opioids or botulinum toxin A was not increased compared with that using placebo, and the differences were not significant (P > 0.05 for all).
The credibility of the evidence for the efficacy and the adverse effects was rated low. The reason for the low quality of evidence ratings were mainly driven by the indirectness of several comparisons and the imprecision and this implies that further research is needed to provide more evidence on which to base judgments (Table 3).
NMA
Network chart of different interventions
In Fig. 3, a direct network connection between any two intervention groups indicates a direct comparison, whereas no connection indicates a lack of direct comparison. The size of the dots in the figure represents the sample size, and the thickness of the line represents the number of studies. As the Fig. 3A, B showed that Each drug type (anticonvulsants, anesthetics, antidepressants, opioids, and botulinum toxin A) was compared directly with placebo (control), but there was no direct comparison between each drug type. That is to say there was no direct or indirect evidence of merging, so consistency analysis was not required.
NMA ranking
In terms of the proportion of patients with pain reduction ≥50% and the prevalence of adverse effects, we conducted a NMA of the five interventions (Fig. 4a, b). In the drug treatment of patients with SCI-related neuropathic pain, anticonvulsants, anesthetics, antidepressants, opioids and botulinum toxin A were better than placebo for improving the proportion of patients with efficacious pain relief. The order (from highest to lowest) was anticonvulsants > anesthetics > antidepressants > botulinum toxin A > opioids > placebo.
In terms of the prevalence of adverse effects, the safety of these drug treatments was lower than that elicited by placebo. Among them, the prevalence of adverse effects of anesthetics was the highest. The order of safety (from the highest to the lowest) was placebo > antidepressants > botulinum toxin A > anticonvulsants > opioids > anesthetics.
Because talk about the effect of one drug, it is not only need to consider the aspect of efficacy but also need to consider its safety. Based on this, we further analyzed the efficacy and safety of each drug in patients with SCI-related neuropathic pain (Fig. 5), the X-axis is showed the rank of different drug types based on safety and the Y-axis is showed the rank of different drug types based on the efficacy. From this figure, we can know that placebo has the best safety, but also the worst efficacy, the opioid has the less efficacy and less safety. However, the anesthetics has the best efficacy, but also the worst safety. Therefore, the better drugs were in the upper right corner (antidepressant, botulinum toxin A and anticonvulsants), these both had good efficacy and safety.
Discussion
Pain is felt in about two-thirds of patients with SCI, and can divided into nociceptive pain and neuropathic pain [29]. The latter occurs frequently in such patients and treatment is extremely challenging [9, 30]. Clinical evidence recommends antidepressants and anticonvulsants as first-line treatments for patients with SCI-related neuropathic pain [31, 32]. However, a systematic review published in 2016 [33] showed that most antidepressants did not reduce the neuropathic pain of patients with SCI. That observation contradicts the evidence recommended by guidelines, and there is very limited evidence for the efficacy of drug treatment in such patients.
In recent years, some studies have suggested that capsaicin and endodermal botulinum toxin may be promising to alleviate SCI-related neuropathic pain [34, 35]. However, recent studies focusing on the efficacy of drug treatments in patients with SCI-related neuropathic pain have compared drugs with placebo, and direct comparisons between drugs have not been made.
We identified RCTs focusing on SCI-related neuropathic pain by setting strict criteria for inclusion and exclusion. After retrieval, 15 RCTs were included. The total sample size was 977 and included five interventions. The NMA revealed that, in the drug treatment of patients with SCI-related neuropathic pain, anticonvulsants, anesthetics, antidepressants, opioids and botulinum toxin A were better than placebo for increasing the proportion of patients with efficacious pain relief. The order of efficacy (from highest to lowest) was anticonvulsants > anesthetics > antidepressants > botulinum toxin A > opioids > placebo. However, in terms of the prevalence of adverse effects, the safety of these drugs was lower than that for placebo. Among them, the prevalence of adverse effects caused by anesthetics was the highest. The order of safety (from highest to lowest) was placebo > antidepressants > botulinum toxin A > anticonvulsants > opioids > anesthetics. Combining these two sets of results we found that, although active drug treatment improved the proportion of patients with efficacious pain relief, a high prevalence of adverse effects was documented.
Adverse effects can reduce the efficacy of drug treatments. Therefore, we further analyzed the efficacy and safety of each drug in patients with SCI-related neuropathic pain: antidepressant, botulinum toxin A, and anticonvulsants had good efficacy and safety. However, the sample size of studies related to treatment using botulinum toxin A included in the NMA was small, so further analyses will be needed to confirm our results. Although antidepressants were very safe, their efficacy was slightly inferior to that of anesthetic and anticonvulsants. Adverse effects offset the efficacy of opioids and anesthetics, so the effects of these two drugs in patients with SCI-related neuropathic pain must be studied further.
Limitations
Our study had two main limitations. First, the drugs used in the included studies, such as antidepressants (amitriptyline and venlafaxine-XR), anticonvulsants (pregabalin, gabapentin, levetiracetam, Lamotrigine, and valproate), anesthetics (lidocaine and ketamine) and opioids (tramadol and morphine), had different treatment cycles, mechanism and follow-up times, which hampered comparisons. Second, most studies had small sample sizes; only studies using anticonvulsants had large study cohorts, which may have reduced the power of our findings. Therefore, we will update this NMA when large and high quality RCTs are published.
Conclusion
The efficacy of anticonvulsants, anesthetics, antidepressants, opioids and botulinum toxin A was greater than that of placebo for treatment of SCI-related neuropathic pain. However, the prevalence of adverse effects associated with use of these drugs was also higher than that of placebo. Further analyses based on efficacy and safety revealed anticonvulsants to be more suitable for treatment of SCI-related neuropathic pain. In addition, antidepressant and botulinum toxin A may be promising treatments for SCI-related neuropathic pain, however, their effects still need to be further explored due to the small sample size.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Awai L, Bolliger M, Ferguson AR, Courtine G, Curt A. Influence of spinal cord integrity on gait control in human spinal cord injury. Neurorehabil Neural Repair. 2016;30:562–72. https://doi.org/10.1177/1545968315600524
Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–43. https://doi.org/10.1038/nrn1955
Kjell J, Olson L. Rat models of spinal cord injury: from pathology to potential therapies. Dis Model Mech. 2016;9:1125–37. https://doi.org/10.1242/dmm.025833
Demant DT, Lund K, Vollert J, Majer C, Segerdahl M, Finnerup NB, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014;155:2263–73. https://doi.org/10.1016/j.pain.2014.08.014
Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA, et al. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:CD003726 https://doi.org/10.1002/14651858.CD003726.pub4
Helfert SM, Reimer M, Hper J, Baron R. Individualized pharmacological treatment of neuropathic pain. Clin Pharmacol Ther. 2015;97:135–42. https://doi.org/10.1038/s41393-020-00595-0
Budh CN, Osteraker AL. Life satisfaction in individuals with a spinal cord injury and pain. Clin Rehabil. 2007;21:89–96. https://doi.org/10.1177/0269215506070313
John DP, Scott JR, Bret LH, Michael JD. Interference due to pain following spinal cord injury: important predictors and impact on quality of life. Pain. 2002;100:231–42. https://doi.org/10.1016/S0304-3959(02)00069-6
Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–57. https://doi.org/10.1016/s0304-3959(02)00452-9
Vranken JH. Mechanisms and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem. 2009;9:71–78. https://doi.org/10.2174/187152409787601932
Finnerup NB, Grydehoj J, Bing J, Johannesen IL, Biering-Sorensen F, Sindrup SH, et al. Levetiracetam in spinal cord injury pain: a randomized controlled trial. Spinal Cord. 2009;47:861–7. https://doi.org/10.1038/sc.2009.55
Finnerup NB, Sindrup SH, Bach FW, Johannesen IL, Jensen TS. Lamotrigine in spinal cord injury pain: a randomized controlled trial. Pain. 2002;96:375–83. https://doi.org/10.1016/s0304-3959(01)00484-5
Levendoglu F, Ogün C, Ozerbil O, Ogün TC, Ugurlu H. Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine. 2004;29:743–51. https://doi.org/10.1097/01.brs.0000112068.16108.3a
Norrbrink C, Lundeberg T. Tramadol in neuropathic pain after spinal cord injury: a randomized, double-blind, placebo-controlled trial. Clin J Pain. 2009;25:177–84. https://doi.org/10.1097/AJP.0b013e31818a744
Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006;67:1792–1800. https://doi.org/10.1212/01.wnl.0000244422.45278.ff
Catala-Lopez F, Tobias A, Cameron C, Moher D, Hutton B. Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int. 2014;34:1489–96. https://doi.org/10.1007/s00296-014-2994-2
Hassan S, Ravishankar N, Nair NS. Methodological considerations in network meta-analysis. Int J Med Sci Public Health. 2015;4:588–94. https://doi.org/10.5455/ijmsph.2015.21012015131
Rudroju N, Bansal D, Talakokkula ST, Gudala K, Hota D, Bhansali A, et al. Comparative efficacy and safety of six antidepressants and anticonvulsants in painful diabetic neuropathy: a network meta-analysis. Pain Physician. 2013;16:E705–714.
Cardenas DD, Nieshoff EC, Suda K, Goto S, Sanin L, Knapp LE, et al. A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology. 2013;80:533–9. https://doi.org/10.1212/WNL.0b013e318281546b
Drewes AM, Andreasen A, Poulsen LH. Valproate for treatment of chronic central pain after spinal cord injury. A double-blind cross-over study. Paraplegia. 1994;32:565–9. https://doi.org/10.1038/sc.1994.89
Tai Q, Kirshblum S, Chen B, Millis S, Johnston M, DeLisa JA. Gabapentin in the treatment of neuropathic pain after spinal cord injury: a prospective, randomized, double-blind, crossover trial. J Spinal Cord Med. 2002;25:100–5. https://doi.org/10.1080/10790268.2002.11753609
Finnerup NB, Biering-Sorensen F, Johannesen IL, Terkelsen AJ, Juhl GI, Kristensen AD, et al. Intravenous lidocaine relieves spinal cord injury pain: a randomized controlled trial. Anesthesiology. 2005;102:1023–30. https://doi.org/10.1097/00000542-200505000-00023
Kvarnstrom A, Karlsten R, Quiding H, Gordh T. The analgesic effect of intravenous ketamine and lidocaine on pain after spinal cord injury. Acta Anaesthesiol Scand. 2004;48:498–506. https://doi.org/10.1111/j.1399-6576.2003.00330.x
Loubser PG, Donovan WH. Diagnostic spinal anaesthesia in chronic spinal cord injury pain. Paraplegia. 1991;29:25–36. https://doi.org/10.1038/sc.1991.4
Cardenas DD, Warns CA, Turner JA, Marshall H, Brooke MM, Loeser JD, et al. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain. 2002;96:365–73. https://doi.org/10.1016/s0304-3959(01)00483-3
Richards JS, Bombardier CH, Wilson CS, Chiodo AE, Brooks L, Tate DG, et al. Efficacy of venlafaxine XR for the treatment of pain in patients with spinal cord injury and major depression: a randomized, controlled trial. Arch Phys Med Rehabil. 2015;96:680–9. https://doi.org/10.1016/j.apmr.2014.11.024
Siddall PJ, Molloy AR, Walker S, Mather LE, Rutkowski SB, Cousins MJ. The efficacy of intrathecal morphine and clonidine in the treatment of pain after spinal cord injury. Anesth Analg. 2000;91:1493–8. https://doi.org/10.1097/00000539-200012000-00037
Han ZA, Song DH, Oh HM, Chung ME. Botulinum toxin type A for neuropathic pain in patients with spinal cord injury. Ann Neurol. 2016;79:569–78. https://doi.org/10.1002/ana.24605
Bryce TN, Biering-Sorensen F, Finnerup NB, Cardenas DD, Defrin R, Lundeberg T, et al. International spinal cord injury pain classification: part I. Background and description. Spinal Cord. 2012;50:413–7. https://doi.org/10.1038/sc.2011.156. March 6-7, 2009.
Hagen EM, Rekand T. Management of neuropathic pain associated with spinal cord injury. Pain Ther. 2015;4:51–65. https://doi.org/10.1007/s40122-015-0033-y
Moulin D, Boulanger A, Clark AJ, Clarke H, Dao T, Finley GA, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19:328–35. https://doi.org/10.1155/2014/754693
Tan T, Barry P, Reken S, Baker M, Guideline Development G. Pharmacological management of neuropathic pain in non-specialist settings: summary of NICE guidance. BMJ. 2010;340:c1079. https://doi.org/10.1136/bmj.c1079
Mehta S, Mcintyre A, Janzen S, Loh E, Teasell R. A systematic review of pharmacological treatments of pain after spinal cord injury: an update. Arch Phys Med Rehabil. 2016;97:1381–91. https://doi.org/10.1016/j.apmr.2015.12.023
Chun A, Levy I, Yang A, Delgado A, Tsai CY, Leung E, et al. Treatment of at-level spinal cord injury pain with botulinum toxin A. Spinal Cord Ser Cases. 2019;5:77 https://doi.org/10.1038/s41394-019-0221-9
Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol. 2009;22:467–74. https://doi.org/10.1097/WCO.0b013e3283311e13
Author information
Authors and Affiliations
Contributions
CT performed the study subject and design, data extraction, statistical analysis, interpretation of data and manuscript drafting. LM contributed to the study design, data extraction, statistical analysis and manuscript revising. MF contributed to the study design, data extraction, statistical analysis and interpretation of data. ZZ and FM performed study design, statistical analysis, and critical revision of manuscript. WQ and LX extracted the data. SD and HQ was involved in critical revision of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mei, L., Fengqun, M., Zhengyao, Z. et al. Efficacy and safety of different drug treatments in patients with spinal-cord injury-related neuropathic pain: a network meta-analysis. Spinal Cord 60, 943–953 (2022). https://doi.org/10.1038/s41393-022-00804-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-022-00804-y
This article is cited by
-
Spinal cord tethering and syringomyelia after trauma: impact of age and surgical outcome
Scientific Reports (2023)