Abstract
Study design
Systematic review.
Objectives
To synthetise the available scientific literature reporting early interventions to prevent neurogenic lower urinary tract dysfunction (NLUTD) after acute supra-sacral spinal cord injury (SCI).
Methods
The present systematic review is reported according to the PRISMA guidelines and identified articles published through April 2021 in the PubMed, Embase, ScienceDirect and Scopus databases with terms for early interventions to prevent NLUTD after SCI. Abstract and full-text screenings were performed by three reviewers independently, while two reviewers performed data extraction independently. An article was considered relevant if it assessed: an in-vivo model of supra-sacral SCI, including a group undergoing an early intervention compared with at least one control group, and reporting clinical, urodynamic, biological and/or histological data.
Results
Of the 30 studies included in the final synthesis, 9 focused on neurotransmission, 2 on the inflammatory response, 10 on neurotrophicity, 9 on electrical nerve modulation and 1 on multi-system neuroprosthetic training. Overall, 29/30 studies reported significant improvement in urodynamic parameters, for both the storage and the voiding phase. These findings were often associated with substantial modifications at the bladder and spinal cord level, including up/downregulation of neurotransmitters and receptors expression, neural proliferation or axonal sprouting and a reduction of inflammatory response and apoptosis.
Conclusions
The present review supports the concept of early interventions to prevent NLUTD after supra-sacral SCI, allowing for the emergence of a potential preventive approach in the coming decades.
Similar content being viewed by others
Spinal cord injury (SCI) incidence is estimated to be between 40 and 80 new cases per million population, meaning that every year, between 250,000 and 500,000 new people become para- or tetra paretic worldwide [1]. Spinal cord injury is associated with mechanical insults and secondary processes, including local hypoxia, ischaemia, oxidative stress, reactive gliosis, excitotoxicity and scarring [2, 3]. Endogenous repair mechanisms in the central nervous system (CNS), especially at the spinal cord level, are negligible, with innate plasticity unable to re-establish the original connectivity [4]. Reorganising pathways by sprouting, unmasking, or other compensatory mechanisms may contribute to the dysfunction that develops following SCI. Since several and complex neural circuits localised at the spinal cord level contribute to the coordinated activity of the bladder and the urethral sphincters, SCI often leads to neurogenic lower urinary tract dysfunction (NLUTD).
This dysfunction emerges as follow: acute supra-sacral SCI starts with an initial phase of “spinal shock” resulting in detrusor and sphincter areflexia, followed by the emergence of a spinal reflex occurring after several weeks, resulting in both storage and voiding phase dysfunction, including detrusor overactivity (DO), bladder compliance disorders (BCD) and detrusor-sphincter dyssynergia (DSD) [5]. The emergence of urinary incontinence and recurrent urinary tract infections are responsible for significant loss of quality of life [6]. Despite many advances regarding their prevention and management over the last three decades, the associated complications are still considered the leading cause of hospitalisation and the fifth cause of mortality in this population [5, 7]. For storage dysfunction, several treatments are available in a therapeutic escalation strategy in order to restore a low-pressure bladder reservoir, including antimuscarinics, β3-adrenergic agonists, intra-vesical botulinum toxin injections, as well as tibial nerve stimulation (TNS) and sacral nerve modulation (SNM), before considering augmentation cystoplasty or other urinary diversions [8, 9]. With respect to voiding dysfunction, even if α-blockers can be proposed, clean intermittent self-catheterisation (CISC) constitutes the standard of care and is often the only way to ensure regular, complete, and low-pressure bladder emptying [8, 9].
To our knowledge, current international recommendations only focus on the management of NLUTD without addressing any prevention steps [8, 9]. Several groups have recently reported various early interventions, using neurotransmission, inflammatory response, neurotrophicity, electrical nerve modulation or neuroprosthetic training, to prevent or attenuate NLUTD after SCI, allowing for the emergence of a potential preventive approach in the coming decades.
The purpose of the present systematic review was to synthesise the scientific literature focusing on early interventions to prevent the emergence of NLUTD after acute supra-sacral SCI.
Methods
Information source and search strategy
The review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [10]. We identified articles published through April 2021 in the PubMed, Embase, ScienceDirect and Scopus databases with MeSH and non-MeSH terms for early intervention to prevent or attenuate NLUTD after SCI (Supplementary Table 1). In order to be exhaustive, publications identified from references were added to the final synthesis if they were considered relevant, and an updated search was performed in July 2021.
Eligibility criteria
Eligibility criteria were defined per PICOS: Patient (P), Intervention (I), Comparator (C), Outcome (O), Study Design (S). An article was deemed appropriate if it assessed: an in-vivo model of supra-sacral SCI (human or animal) (P), including a group undergoing an early intervention—before DO, BCD or DSD has emerged—to prevent and/or attenuate storage and/or voiding phase dysfunction (I), compared with at least one control group not undergoing any early intervention (C), and reporting clinical, urodynamic, biological and/or histological data (O). No restriction on study type was established (S).
Study selection and data extraction
Abstract and full-text screenings were performed by three reviewers independently (NV, DS, PLD). Disagreement was resolved by discussion with the help of a fourth reviewer (XB). Two reviewers performed data extraction independently (NV, XB), and a standardised form was used to extract data on study methodology, characteristics of in vivo model, early intervention performed, and clinical, urodynamic, biological as well as histological outcomes. Any discrepancy concerning data extraction was resolved by discussion.
Risk of bias assessment
To assess the risk of bias (RoB), reports were reviewed by two reviewers independently (NV, XB) using the SYRCLE (SYstematic Review Center for Laboratory animal Experimentation [11]) tool to assess studies focusing on animal models, and the ROBINS-I (Risk of Bias in Non-Randomised Studies—of Interventions [12]) tool to assess studies focusing on humans. Any disagreement concerning the RoB assessment was resolved by discussion.
Data synthesis
Data synthesis was performed through a structured presentation detailing step by step the main results associated with early interventions focusing on (1) neurotransmission, (2) inflammatory response, (3) neurotrophicity, (4) electrical nerve modulation, and (5) neuroprosthetic training.
Results
Study selection
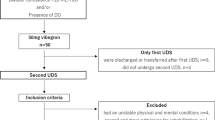
A total of 3792 abstracts were retrieved. After removal of duplicates and abstract screening, 48 articles were deemed eligible for full-text screening, of which 30 were included in the final synthesis (Fig. 1, Supplementary Table 2).
Risk of bias of included studies
Of the 29 studies focusing on animals, 17 had an unclear RoB, and only 7 had an overall low RoB (Fig. 2). For the one human study, overall RoB was low (Fig. 3).
Study characteristics
Of the 30 studies, 9 focused on neurotransmission (Table 1), 2 on inflammatory response, 10 on neurotrophicity (Table 2), 9 on electrical nerve modulation and 1 on multi-system neuroprosthetic training (Table 3).
Models included rats in 20 studies, mice in 4, dogs in 4, minipigs in 1 and humans in 1. SCI was performed between T2 and T12 by spinal cord transection (SCT) in 17 studies, by Hemi-transection (SCHT) in 1, by spinal cord compression (SCC) in 10 and both by transection and compression in 1. The study focusing on human SCI exclusively included AIS A traumatic lesions. Of the clinical outcomes of interest, 28 studies reported on urodynamic parameters, 5 on metabolic cages or bladder diary data, and 3 on contractility tests of ex-vivo bladder strips. Tissues were analysed in 25 studies, including bladder in 18, spinal cord in 12, and dorsal root ganglia in 2.
Neurotransmission
Muscarinic pathway
Biardeau et al. [13] and Temeltas et al. [14] assessed the effect of early pre [13]- and post-synaptic [14] inhibitions of the muscarinic pathway in SCT rats. Evaluating subcutaneous fesoterodine fumarate [13] and bladder wall injections of botulinum toxin A [14], they both reported a significant improvement in cystometric pressure parameters [13, 14], associated with a significant decrease in bladder fibrosis and hyperplasia [14]. Interestingly, Biardeau et al. [13] showed an effect persistent after a 72h wash-out period and hypothesised that the early administration of an antimuscarinic drug could act not only through an acute pharmacological effect but also by countering pathological modifications of muscarinic pathways, mainly at the M2 and M3 receptor level. Similarly, Temeltas et al. [14] reported better histological and cystometric outcomes after early injections (day 7) when compared with late injections (day 28), but the differences were not statistically significant.
Adrenergic pathway
Lee et al. [15] and Kadewaka et al. [16] assessed the effect of early α-adrenergic inhibition in SCT rats through intraperitoneal tamsulosin [15] or oral naftopidil [16]. Arguing that an α1D-adrenergic receptor localised in the detrusor can promote acetylcholine secretion [15] and facilitate vasoconstriction [16] at the bladder level, the authors reasoned that inhibition of such receptors could improve the SCI-induced bladder remodelling. Even if Lee et al. [15] reported no significant modification in cystometric pressure parameters—with a urodynamic study performed only 1 week after SCI—they reported bladder strips contractility in the presence of M3 antagonist to be significantly decreased in tamsulosin-treated SCI rats. Furthermore, in tamsulosin-treated SCI rats, pERK1/2 and Rho-kinase expressions—associated with the activation of muscarinic receptors, particularly M2 receptors—were reported to be increased and decreased, respectively. Kadewaka et al. [16] reported an increase in bladder compliance and voiding efficiency associated with a decrease in urethral pressure in naftopidil-treated rats. They also reported a decrease early after SCI in upregulation of ischaemia and fibrosis markers and collagen concentration at the bladder level.
Nitrergic pathway
Kadewaka et al. [16] also assessed the effect of early oral tadalafil administration, a phosphodiesterase type 5 inhibitor, thought to improve bladder tissue oxygenation. When comparing treated SCT rats with non-treated SCT rats, the authors found a decrease in upregulation fibrosis markers and collagen concentration late after SCI, associated with an increase in bladder compliance.
Purinergic pathway
Munoz et al. [17] assessed the effect of early intrathecal instillation of P2X7R inhibitor in SCT rats. P2X7R is a purinergic receptor expressed in microglia that downplays proper tissue regeneration after SCI by enhancing inflammatory response and contributing to long-term scar formation. The authors showed a decrease in the number of non-voiding contractions (NVCs) associated with a decrease in microglial activation at the spinal cord level, and a concomitant decrease of urothelial P2X3—an ionotropic purinergic receptor involved in the transmission of bladder afferent activity—at the bladder level.
Glutamatergic pathway
Wang et al. [18] assessed the effect of a replication-defective herpes simplex virus vector of kynurenine aminotransferase (HSVrd KAT II) administered through an early bladder wall injection in SCT rats. The N-methyl-D-aspartate receptor (NMDAr), found in the lumbosacral spinal cord, is an ionotropic glutamatergic receptor that has been reported to play an essential role in the micturition reflex pathway. In parallel, kynurenic acid, synthesis of which is catalysed by KAT II, acts as an endogenous non-competitive antagonist at the glycine site of the glutamate NMDAr and has been reported to directly influence the micturition reflex. With KAT II protein and mRNA levels significantly increased at the L6-S1 spinal cord level, the authors found a significant decrease in maximum voiding pressure and maximum urethral closure pressure, associated with an increase in voiding efficiency in treated SCT rats when compared with non-treated SCT rats. They hypothesised that transport of KAT II to Onuf’s nucleus and then to L6-S1 parasympathetic preganglionic neurons could reduce the urethral pressure by blocking NMDAr, influencing neurotransmitter levels in the L6-S1 spinal cord and even desensitising C-fibre afferents.
GABAergic pathway
Miyazato et al. [19] assessed the effect of HSVrd Glutamic acid decarboxylase (GAD) administered through an early bladder wall injection in SCT rats. GAD is an enzyme that catalyses the decarboxylation of glutamate to GABA and contributes to maintaining the significant physiological supply of GABA. Hypofunction of inhibitory GABAergic neuronal activity in the spinal cord after SCI has been suspected of contributing to the genesis of DSD and DO. The authors reported a significant association between GAD mRNA increased expression in L6-S1 dorsal root ganglia (DRG) and a voiding urethral pressure decrease. Meanwhile, they did not identify associated differences regarding bladder activity and baseline urethral pressure. The intrathecal administration of a GABA antagonist almost completely reversed the decrease in the voiding urethral pressure. The authors hypothesised that GABA synthesis in bladder afferent pathways could inhibit Onuf’s nucleus that innervates the external urethral sphincter (EUS) via the suppression of C-fibre bladder afferent activity to decrease DSD.
TRPV1 desensitisation
Two studies assessed the effect of early administration of resiniferatoxin [20] or capsaicin [21] in SCT rats, using subcutaneous administration [21] or bladder [20] instillation. Capsaicin and resiniferatoxin, one of its derivatives, are ultrapotent desensitising agonists of the transient receptor potential vanilloid‐1 (TRPV1) known to be increased in urothelial cells and nerve fibres in the case of neurogenic DO. The authors reported an increased bladder capacity [20] and a decrease in the number of NVCs [21], with some of the rats presenting with complete suppression of DO [21]. However, Thomas et al. reported no significant improvement in the intercontraction interval, voiding pressure or voiding efficiency, or in EUS activity during both NVCs and voiding bladder contractions. Oliveira et al [20] showed after bladder instillation of resiniferatoxin decreased expressions of TRPV1, calcitonin gene‐related peptide (CGRP)—two markers of peptidergic fibres—and growth‐associated protein 43 (GAP43)—a marker of sprouting nerve fibres—at the bladder level, with no modification of CGRP and GAP43 expression or in the number of activating transcription factor 3 (ATF3) positive nuclei—a marker of neuronal stress—in the L5-S1 DRG. The authors hypothesised that the early administration of vanilloid therapy might mitigate the development of high intra-vesical pressures, in a long‐lasting manner. It might prevent C-fibre afferents becoming hyperexcitable [21] by inhibiting peptidergic fibres at the bladder level [20], without inducing damage to the DRG neurons [20].
Inflammatory response
Shunmugavel et al. [22] assessed the effect of early oral administration of S-Nitrosoglutathione (GSNO) in SCC rats. GSNO, an endogenous nitrosylating agent, has anti-inflammatory properties. Since it has been reported to ameliorate inflammatory sequelae observed in the bladder and renal tissues after SCI, the authors postulated that GSNO would improve the recovery of micturition dysfunction by reducing the bladder tissue inflammation associated with SCI. They reported that GSNO-treated SCI rats regained significant micturition control compared to vehicle-only SCI rats. They also found a significant decrease in bladder weight, proteinuria, urine osmolality, and immune cell infiltration and collagen deposition at the bladder level. They also reported a decrease in iNOS and ICAM-1 (mediator of inflammation expression) at the bladder and kidney level, associated with a decrease of TUNEL-positive cells at the bladder level, indicating a decrease in the apoptotic process.
David et al. [23] assessed the effect of early intrathecal instillation of the Toll-like Receptor 9 (TR9L) inhibitor in SCC mice. TLR9 is a receptor expressed in the immune system cells that triggers signalling cascades, leading to a pro-inflammatory cytokine response. The authors reported a significant decrease in urinary retention associated with decreased bladder weight, bladder volume and bladder wall thickness. At the spinal cord level, they reported the white matter to be significantly more spared.
Neurotrophicity
Axonal growth inhibitors antagonists
Mothe et al. [24] and Schneider et al. [25] assessed the effect of early antagonization of axonal growth inhibitors using intravenous administration of elazanumab [24], a human monoclonal antibody targeting Repulsive Guidance Molecule (an RGMa), and intrathecal administration of anti-Nogo A [25] in SCC and SCT rats, respectively. RGMa is a potent inhibitor of axonal growth that has been reported to be rapidly upregulated after injury of the central nervous system. In contrast, myelin-enriched membrane protein Nogo-A, a potent nerve fibre growth inhibitory protein, has been reported to be implicated in the low level of spontaneous neuronal regeneration after SCI. The authors reported an earlier spontaneous voiding ability [24] associated with a decrease in bladder wall hypertrophy [24], a decrease in EUS EMG activity [24], as well as a significant decrease of maximum bladder pressure during voiding [25]. Mothe et al. [24] also found more remarkable tissue preservation at the spinal cord level, characterised by reduced lesion areas associated with increased perilesional neuronal sparing as well as serotonergic and corticospinal axonal plasticity. Schneider et al. [25] found higher densities of fibres originating from the pontine micturition centre in the lumbosacral grey matter, and a decreasing number of inhibitory interneurons in lamina X. The authors hypothesised that early and temporary neutralisation of the neurite growth inhibitory factor Nogo-A might contribute to the reconfiguration of bladder control at spinal and supraspinal levels.
Neurotrophic factors
Two studies assessed the effect of early inhibition of neurotrophic factors in SCT mice, using oral administration of brain‐derived neurotrophic factor (BDNF) [26] and nerve growth factor (NGF) [27] inhibitors. Although both reported a significant decrease in cystometric pressure parameters [26, 27], BDNF inhibition only reduced NVCs in a late phase following SCI, while NGF inhibition acted earlier after SCI. Furthermore, while BDNF inhibitors improved voiding dysfunction [26], NGF inhibitors did not modify EUS EMG activity [27]. After NGF-inhibitor treatment, NGF expression was decreased at the bladder and the spinal cord level, while TRPA1 and TRPV1 expressions—predominantly found in C-fibre afferent pathways—were significantly decreased in L6/S1 dorsal root ganglia (DRG) [27]. The authors concluded that short- to long-term BNDF inhibition could improve voiding dysfunction associated with DSD, while a long‐term BNDF inhibition was required to reduce the later‐phase development of C-fibres-dependent DO. On the other hand, NGF inhibition could precociously slow down TRPV1 upregulation, attenuate C-fibres activation and thus prevent the early emergence of DO.
Mitsui et al. [28] assessed the effect of early intrathecal administration of fibroblasts (Fb) expressing BDNF and Neurotrophin 3 (NT3) in SCC rats. According to the authors, BDNF and NT3 act as neurotrophic factors on specific neurons of the central and peripheral nervous system, helping to support the survival of existing neurons, and encouraging growth and differentiation of new neurons and synapses. The authors reported a significant decrease in voiding pressure and in the number of NVCs. The density of small dorsal root axons increased in the superficial layers of the dorsal horn in non-treated SCI rats but not in Fb-BDNF/NT3-treated SCI rats, suggesting inhibition of sprouting of primary afferents by Fb-BDNF/NT3. Synaptophysin immunoreactivity in the lumbosacral dorsal horn was similar in treated and non-treated SCI rats, consistent with restoring synaptic density after SCI in both groups—probably through different pathways. The authors concluded that Fb-BDNF/NT3 transplants could contribute to organisation of spinal circuitry after SCI.
Mure et al. [29] and Chung et al. [30] assessed the effect of early administration of neurotrophic facilitators, including administration of dehydroepiandrosterone (DHEA) [29] in SCC mice and inosine [30] in SCC/SCT rats. DHEA as a neuroactive steroid could act as a modulator of neurotrophic factor receptors and has previously been shown to promote neurological recovery after SCI. As a purine nucleoside with neurotrophic properties, inosine has been shown to promote corticospinal tract fibres sprouting and improve motor function in pre-clinical models of SCI. Mure et al. [29] reported a faster recovery of autonomic bladder control in DHEA-treated SCI mice while Chung et al [30] reported a significant decrease in the frequency of NVC in inosine-treated SCC/SCT rats and a significant decrease in the amplitude of NVCs in inosine-treated SCT rats. After DHEA administration [29], change in function was associated with a of collagen type3-type1 ratio similar to that seen in sham-operated animals. At the same time, after inosine administration [30], bladder analysis showed an increased expression of the pan-neuronal marker SYP and Aδ-fibre marker NF200 and a decreased expression of the C-fibre marker TRPV1. Mure et al. [29] concluded that DHEA could prevent NLUTD after SCI through its neuroprotective and neuroactive properties, including glucocorticoid and cyclooxygenase-2,12 inhibition or even act directly on the bladder tissue through the androgen and estrogen metabolism. In addition, Chung et al. [30] concluded that inosine could prevent DO through modulation of sensory neurotransmission.
Neural precursor cells
Mitsui et al. assessed in three distinct studies [31,32,33]. the effect of early intrathecal implantation—at the lesion site in SCC rats—of restricted neuronal precursor (NRP) and glial restricted precursor (GRP) alone [33] or combined with 2,3-dihydroxy-6-nitro-7 sulfamoylbenzo(f)quinoxaline (NBQX) [31] as well as early intrathecal implantation of EG6 immortalised neural stem cells [32]. Microinjection of NBQX has been shown to significantly decrease the amount of tissue loss following SCI through inhibition of AMPA/Kainite receptors contributing to the excitotoxicity mediated tissue damage that ensues within minutes following traumatic SCI. NRP/GRP alone [33] or in combination with NBQX [31] was reported to increase voided volume per micturition and improve cystometric pressure parameters, including a decrease in micturition pressure and the number of NVCs. On the other hand, EG6 cells [32] were reported to decrease the post-void residual volume and increase voiding efficiency without impacting voided volume or DO. NRP/GRP-treated and NBQX-treated SCI rats [31,32,33] presented with increased sprouting, regeneration or sparing of descending projections to the lumbosacral cord associated with a decrease in the size of the lesion.
Similarly, these rats [31, 33] showed a decrease in the sprouting of primary afferents in the lumbosacral cord. Furthermore, in NBQX + NRP/GRP-treated SCI rats [33], the density of serotonergic, noradrenergic, and corticotrophin-releasing factor-positive fibres was increased, while the density of axons in the dorsal horn appeared normal. The authors concluded that neural stem cell transplantation could prevent NLUTD by providing local protection consisting of increased sparing/sprouting of descending pathways, thus preventing sprouting by dorsal root axons.
Electrical nerve modulation
Pudendal nerve modulation
Three studies assessed the effect of early 5–10 Hz pudendal nerve modulation (PNM) in SCI minipigs [34] and dogs [35, 36]. They all reported a significant decrease in the number of NVCs and a significant increase in bladder capacity and compliance. Interestingly, Chen et al. [36] assessed this effect at different time points (early: day 30, delayed: day 180) and found?? significant changes after early PNM treatment compared to baseline, while no significant change was found after delayed PNM treatment. They also noted the presence of fewer collagen fibres associated with more elastin fibres in early PNM-treated compared with delayed PNM-treated SCI dogs. Keller et al. [34] directly compared 10 Hz PNM to 10 Hz SNM and noted a significant increase in bladder capacity associated with a significant decrease in voiding pressure and DSD in SNM-treated minipigs compared to PNM-treated and non-treated SCI minipigs. Although structural results revealed SCI‐typical fibrotic alterations in both SNM and PNM-treated SCI minipigs, SNM-treated SCI minipigs showed a better‐balanced distribution of smooth muscle to connective tissue, with a trend towards reduced progression of bladder wall scarring.
Sacral nerve modulation
Five studies assessed the effect of early 10–60 Hz SNM in animals [34, 37,38,39,40]. For SCI minipigs [34] and rats [37, 38], the authors found a significant improvement in DO, including a decrease in the frequency and maximum pressure of NVCs, associated with an increase in the time between contractions and the duration of contraction. In the Shi et al. [38] study, SNM was proven to be able to decrease DO regardless of the delay between SCI and implantation. However, the authors reported SNM to be particularly effective when implanted 2–4 weeks after SCI, at the end of the spinal shock phase and before the development of DO. For SCI dogs, Hassouna et al. [39] and Li et al. [40], on the basis of particularly elaborate studies, reported the delay before the return of DO after spinal shock to be significantly decreased in NMS-treated SCI dogs, when compared to SCI dogs managed with indwelling or intermittent catheterization, with SNM reported to ensure complete voiding up to 8 months [40].
Sievert et al. [41] assessed the effect of early SNM in supra-sacral post-traumatic AIS A SCI humans. In early SNM-treated SCI patients, the authors noted the persistence of low bladder pressure (<30 cmH2O) without any NVCs or bladder compliance disorders throughout the filling phase, without any pelvic activity, during a mean follow-up period of 26 (range 5.4–38.9) months. The bladder diaries revealed a mean catheterised volume of 582 ml (range 480–650 ml). The participants, who did not receive an antimuscarinic or botulinum toxin before their last evaluation, did not report involuntary urine leakage. On the contrary, despite taking antimuscarinics, the non-treated SCI patients were reported to present with lower bladder capacity (mean 208 ml; range 57–314 ml) and higher bladder pressure (>30 cmH2O). Furthermore, they reported mean catheterised volumes of 294 ml (range, 105–390 ml), with more frequent CISC and a more frequent use of urinary condoms because of urinary incontinence. The authors concluded that early SNM implantation in SCI patients might dramatically change NLUTD management, as it could prevent DO and urinary incontinence and provide standard bladder capacity.
Multi-system neuroprosthetic training
Horst et al. [42] assessed the effect of a multi-system neuroprosthetic training (MSNT) program in bilateral T7 + T11 SCHT rats. The MSNT program included an electrochemical stimulation (epidural S2-L1 electrical stimulation associated with serotonergic and dopamine agonists administration) and locomotor treadmill-based training. MSNT has been previously reported to trigger a massive reorganisation of descending and intra-spinal pathways in SCI rats. The authors reported a significant decrease in the number of NVCs while bladder capacity increased 3-fold in complete MSNT SCI-rats and 7-fold in partial MSNT SCI-rats. Bladder morphology was similar in complete MSNT SCI and non-SCI rats, while partial MSNT SCI rats exhibited detrusor hypertrophy characterised by increased detrusor thickness and decreased connective tissue to smooth muscle ratio. The authors also reported that nerve density was significantly increased in complete MSNT SCI rats while it was significantly decreased in partial MSNT SCI rats. The proportion of NF200-positive afferent nerves was significantly decreased in complete MSNT SCI rats compared to partial MSNT SCI and non-SCI rats, while NPY-positive fibres density was significantly decreased in partial MSNT SCI rats.
Discussion
To our knowledge, the present systematic review is the first to synthesise the scientific literature focusing on early interventions to prevent the emergence of NLUTD after acute supra-sacral SCI. Since NLUTD remains the leading cause of hospitalisation and the fifth largest cause of mortality after SCI [7]—despite multiple advances in NLUTD management over the last three decades—its prevention should be considered a priority by the scientific community.
Even if we have regularly specified throughout the manuscript the role of neurotransmitters and their receptors involved in the micturitional reflex pathway [43, 44], the present review should not be considered an attempt to precisely describe the complex mechanisms underlying the genesis, consolidation and prevention of LUTD after SCI. It should rather be seen as a guide for clinical studies to prevent NLUTD in this specific population.
We can regret the lack of studies focusing on the prevention of LUTD after SCI in humans. Animal models, mostly rodents, have been widely used in the last two decades in the literature focusing on LUTD after SCI. The validity of these models was reinforced a decade ago by Andersson et al. [45] who participated in the standardisation of the practice of urodynamic in rodent models, and by Dietz and al. [46] who more recently emphasised the importance of translational basic research in rodent SCI models.
The high number of studies (29/30) reporting significant improvement in urodynamic parameters clearly supports the concept of a preventive approach, aiming to interfere in the uncontrolled spinal pathways reorganisation occurring below the level of SCI—at least in some animal models. Early post-SCI interventions were mostly reported to inhibit C-fibres hyperexcitability and promote neuroplasticity, including the promotion of sprouting, regeneration or sparing of descending projections to the lumbosacral cord as well as the inhibition of sprouting of primary afferents in the lumbosacral cord. However, methodological heterogeneity of the included studies in terms of rationals, design, in-vivo model studied, outcomes considered, as well as their frequent unclear or even high RoB—prevent us from drawing any definitive conclusion on the best strategy to promote. Although all the proposed approaches deserve to be explored in humans, some of them will be difficult to implement in the early phase of SCI, given the time-consuming, complex and invasive care that will be needed. For these reasons, we feel that some orally administered drugs (antimuscarinic, α-blocker and phosphodiesterase type 5 inhibitor) and electrical nerve modulation seem the most mature candidates for medium-term application in humans. With 9 studies reported here, including 1 in humans, electrical nerve modulation is the therapeutic approach with the longest history and greatest visibility, with SNM and TNS—two minimally invasive therapies—already considered as second-line therapies to treat NLUTD [8, 9]. This finding is supported by recent findings suggesting that manipulation of neuronal activity can drive plasticity-related growth mechanisms and increase collateral sprouting, thereby enhancing the functional effect of axonal remodelling [47]. Furthermore, while electrical stimulation has long been known to enhance regeneration of peripheral axons [48], it has been reported recently that electrical modulation can also enhance CNS plasticity in rodent models, and modulate and strengthen spared circuitry in individuals with SCI [49]. However, given the limited data available in humans, it is impossible to propose electrical nerve modulation therapies as a preventive approach for SCI-related NLUTD in clinical practice. Clinical trials, such as the TASCI research protocol (transcutaneous tibial nerve stimulation in patients with acute spinal cord injury to prevent neurogenic detrusor overactivity) proposed by Birkhäuser [50] et al., are mandatory to confirm the value of such a preventive strategy in SCI humans, in both the short and long term.
Conclusion
The present systematic review supports the concept of early interventions to prevent NLUTD after SCI, allowing for the emergence of a potential preventive approach. Electrical nerve modulation should be considered as the most mature strategy for medium-term application in humans.
Data availability
All references cited in the manuscript are available on PubMed.
References
World Health Organization, International Spinal Cord Society, editors. International perspectives on spinal cord injury. Geneva, Switzerland: World Health Organization; 2013.
Srivastava E, Singh A, Kumar A. Spinal cord regeneration: A brief overview of the present scenario and a sneak peek into the future. Biotechnol J. 2021;e2100167. https://doi.org/10.1002/biot.202100167
Beattie MS, Farooqui AA, Bresnahan JC. Review of Current Evidence for Apoptosis After Spinal Cord Injury. Journal of Neurotrauma. 2000;17:915–25.
Chen M, Zheng B. Axon plasticity in the mammalian central nervous system after injury. Trends Neurosci. 2014;37:583–93.
Schurch B, Tawadros C, Carda S. Dysfunction of lower urinary tract in patients with spinal cord injury. Handbook of Clinical Neurology [Internet]. Elsevier; 2015 [cited 2021 Aug 2]. p. 247–67. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780444632470000146
Kakizaki H, Koyanagi T. Current view and status of the treatment of lower urinary tract symptoms and neurogenic lower urinary tract dysfunction. BJU Int. 2000;85:25–30.
Savic G, DeVivo MJ, Frankel HL, Jamous MA, Soni BM, Charlifue S. Causes of death after traumatic spinal cord injury—a 70-year British study. Spinal Cord. 2017;55:891–7.
Groen J, Pannek J, Castro Diaz D, Del Popolo G, Gross T, Hamid R, et al. Summary of European Association of Urology (EAU) Guidelines on Neuro-Urology. Eur Urol. 2016;69:324–33.
Welk B, Schneider MP, Thavaseelan J, Traini LR, Curt A, Kessler TM. Early urological care of patients with spinal cord injury. World J Urol. 2018;36:1537–44.
Moher D, Liberati A, Tetzlaff J, Altman DG. for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535–b2535.
Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Biardeau X, Przydacz M, Aharony S, Loutochin G, Campeau L, Kyheng M, et al. Early Fesoterodine Fumarate Administration Prevents Neurogenic Detrusor Overactivity in a Spinal Cord Transected Rat Model. Rabchevsky A, editor. PLoS ONE. 2017;12:e0169694.
Temeltas G, Tikiz C, Dagci T, Tuglu İB, Yavasoglu A. The Effects Of Botulinum-A Toxin On Bladder Function And Histology In Spinal Cord Injured Rats: Is There Any Difference Between Early And Late Application? J Urol. 2005;174:2393–6.
Lee KK, Lee MY, Han DY, Jung HJ, Joo MC. Effects of Bladder Function by Early Tamsulosin Treatment in a Spinal Cord Injury Rat Model. Ann Rehabil Med. 2014;38:433.
Kadekawa K, Majima T, Kawamorita N, Okada H, Yoshizawa T, Mori K, et al. Effects of an alpha1A/D-adrenoceptor antagonist, naftopidil, and a phosphodiesterase type 5 inhibitor, tadalafil, on urinary bladder remodeling in rats with spinal cord injury. Neurourol Urodyn. 2017;36:1488–95.
Munoz A, Yazdi IK, Tang X, Rivera C, Taghipour N, Grossman RG, et al. Localized inhibition of P2X7R at the spinal cord injury site improves neurogenic bladder dysfunction by decreasing urothelial P2X3R expression in rats. Life Sciences. 2017;171:60–7.
Wang Z, Liao L. Effect of urethral wall injection of replication-defective herpes simplex virus-mediated gene transfer of kynurenine aminotransferase on urethral pressure in spinal cord-injured rats: Gene Therapy Reduced Urethral Pressure in Rats. Neurourol Urodynam. 2017;36:1046–51.
Miyazato M, Sugaya K, Saito S, Chancellor MB, Goins WF, Goss JR, et al. Suppression of Detrusor-Sphincter Dyssynergia by Herpes Simplex Virus Vector Mediated Gene Delivery of Glutamic Acid Decarboxylase in Spinal Cord Injured Rats. Journal of Urology. 2010;184:1204–10.
Oliveira R, Coelho A, Franquinho F, Sousa MM, Cruz F, Cruz D. C. Effects of early intravesical administration of resiniferatoxin to spinal cord‐injured rats in neurogenic detrusor overactivity. Neurourol Urodyn. 2019;38:1540–50.
Thomas C, Kim JH, Torimoto K, Kwon DD, Kim YT, Tyagi P, et al. Early capsaicin intervention for neurogenic bladder in a rat model of spinal cord injury. Biomed Res. 2007;28:255–9.
Shunmugavel A, Khan M, Hughes FM, Purves JT, Singh A, Singh I. S-Nitrosoglutathione protects the spinal bladder: Novel therapeutic approach to post-spinal cord injury bladder remodeling: GSNO Protects Spinal Bladder. Neurourol Urodyn. 2015;34:519–26.
David BT, Sampath S, Dong W, Heiman A, Rella CE, Elkabes S, et al. A Toll-Like Receptor 9 Antagonist Improves Bladder Function and White Matter Sparing in Spinal Cord Injury. J Neurotrauma. 2014;31:1800–6.
Mothe AJ, Coelho M, Huang L, Monnier PP, Cui Y-F, Mueller BK, et al. Delayed administration of the human anti-RGMa monoclonal antibody elezanumab promotes functional recovery including spontaneous voiding after spinal cord injury in rats. Neurobiol Dis. 2020;143:104995.
Schneider MP, Sartori M, Schwab E, Kessler M. Anti-Nogo-A antibodies: Promising treatment for neurogenic lower urinary tract dysfunction after spinal cord injury. Eur. Urol. Supplements. 2019;18:e2.
Wada N, Yoshimura N, Kurobe M, Saito T, Tyagi P, Kakizaki H. The early, long‐term inhibition of brain‐derived neurotrophic factor improves voiding, and storage dysfunctions in mice with spinal cord injury. Neurourol. Urodyn. 2020;39:1345–54.
Wada N, Shimizu T, Shimizu N, de Groat WC, Kanai AJ, Tyagi P, et al. The effect of neutralization of nerve growth factor (NGF) on bladder and urethral dysfunction in mice with spinal cord injury. Neurourol. Urodyn. 2018;37:1889–96.
Mitsui T, Fischer I, Shumsky JS, Murray M. Transplants of fibroblasts expressing BDNF and NT-3 promote recovery of bladder and hindlimb function following spinal contusion injury in rats. Exp Neurol. 2005;194:410–31.
Mure P-Y, Galdo M, Compagnone N. Bladder function after incomplete spinal cord injury in mice: quantifiable outcomes associated with bladder function and efficiency of dehydroepiandrosterone as a therapeutic adjunct. J Neurosurg.: Spine. 2004;100:56–61.
Chung YG, Seth A, Doyle C, Franck D, Kim D, Cristofaro V, et al. Inosine Improves Neurogenic Detrusor Overactivity following Spinal Cord Injury. Di Giovanni S, editor. PLoS ONE. 2015;10:e0141492.
Mitsui T, Neuhuber B, Fischer I. Acute administration of AMPA/Kainate blocker combined with delayed transplantation of neural precursors improves lower urinary tract function in spinal injured rats. Brain Res. 2011;1418:23–31.
Mitsui T, Kakizaki H, Tanaka H, Shibata T, Matsuoka I, Koyanagi T. Immortalized Neural Stem Cells Transplanted Into the Injured Spinal Cord Promote Recovery of Voiding Function in the Rat. J Urol. 2003;170:1421–5.
Mitsui T. Transplantation of Neuronal and Glial Restricted Precursors into Contused Spinal Cord Improves Bladder and Motor Functions, Decreases Thermal Hypersensitivity, and Modifies Intraspinal Circuitry. J Neurosci. 2005;25:9624–36.
Keller EE, Patras I, Hutu I, Roider K, Sievert K, Aigner L, et al. Early sacral neuromodulation ameliorates urinary bladder function and structure in complete spinal cord injury minipigs. Neurourol. Urodyn. 2020;39:586–93.
Li P, Liao L, Chen G, Zhang F, Tian Y. Early low-frequency stimulation of the pudendal nerve can inhibit detrusor overactivity and delay progress of bladder fibrosis in dogs with spinal cord injuries. Spinal Cord. 2013;51:668–72.
Chen G, Liao L, Dong Q, Ju Y. The Inhibitory Effects of Pudendal Nerve Stimulation on Bladder Overactivity in Spinal Cord Injury Dogs: Is Early Stimulation Necessary?: PUDENDAL NERVE STIMULATION TO INHIBIT BLADDER OVERACTIVITY. Neuromodulation: Technology at the Neural. Interface. 2012;15:232–7.
Lee YJ, Yoon CY, Lee MS, Song BD, Lee SW, Jeong SJ. Effect of Early Sacral Neuromodulation on Bladder Function in a Rat Model of Incomplete Spinal Cord Injury Due to Focal Contusion. Neuromodulation: Technology at the Neural. Interface. 2019;22:697–702.
Shi P, Fang Y, Yu H. Bladder response to acute sacral neuromodulation while treating rats in different phases of complete spinal cord injury: a preliminary study. Int Braz J Urol. 2015;41:1194–201.
Hassouna M, Li JS, Sawan M, Duval F, Latt R, Elhilali MM. Effect of early bladder stimulation on spinal shock: Experimental approach. Urology. 1992;40:563–73.
Li J, Hassouna M, Sawan M, Duval F, Latt R, Carter K, et al. Role of Electric Stimulation in Bladder Evacuation Following Spinal Cord Transection. J Urol. 1992;147:1429–34.
Sievert K-D, Amend B, Gakis G, Toomey P, Badke A, Kaps HP, et al. Early sacral neuromodulation prevents urinary incontinence after complete spinal cord injury. Ann Neurol. 2010;67:74–84.
Horst M, Heutschi J, van den Brand R, Andersson K-E, Gobet R, Sulser T, et al. Multisystem Neuroprosthetic Training Improves Bladder Function After Severe Spinal Cord Injury. J Urol. 2013;189:747–53.
de Groat WC, Yoshimura N. Chapter 5 - Anatomy and physiology of the lower urinary tract. In: Vodušek DB, Boller F, editors. Handbook of Clinical Neurology [Internet]. Elsevier; 2015 [cited 2022 Feb 9]. p. 61–108. Available from: https://www.sciencedirect.com/science/article/pii/B9780444632470000055
de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp. Neurol. 2012;235:123–32.
Andersson K-E, Soler R, Füllhase C. Rodent models for urodynamic investigation. Neurourol Urodyn. 2011;30:636–46.
Dietz V, Schwab ME. From the Rodent Spinal Cord Injury Model to Human Application: Promises and Challenges. J Neurotrauma. 2017;34:1826–30.
Carmel JB, Martin JH. Motor cortex electrical stimulation augments sprouting of the corticospinal tract and promotes recovery of motor function. Front Integr Neurosci. [Internet]. 2014 [cited 2021 Aug 2];8. Available from: http://journal.frontiersin.org/article/10.3389/fnint.2014.00051/abstract
Hoffman H, Binet F. Acceleration and retardation of the process of axon-sprouting in partially denervated muscles. Aust J Exp Biol Med. 1952;30:541–66.
Jara JS, Agger S, Hollis ER. Functional Electrical Stimulation and the Modulation of the Axon Regeneration Program. Front Cell Dev Biol. 2020;8:736.
Birkhäuser V, Liechti MD, Anderson CE, Bachmann LM, Baumann S, Baumberger M, et al. TASCI—transcutaneous tibial nerve stimulation in patients with acute spinal cord injury to prevent neurogenic detrusor overactivity: protocol for a nationwide, randomised, sham-controlled, double-blind clinical trial. BMJ Open. 2020;10:e039164.
Author information
Authors and Affiliations
Contributions
NV was responsible for designing the review protocol, writing the protocol and report, conducting the search, screening potentially eligible studies, extracting and analysing data, interpreting results, updating reference lists, creating ’Summary of findings’ tables and writing the report. XB contributed to designing the review protocol, writing the protocol and report, conducting the search, screening potentially eligible studies, extracting and analysing data, interpreting results, updating reference lists, creating 'Summary of findings’ tables and writing the report. PLD and DS contributed to conducting the search and screening potentially eligible studies. PV and SDW contributed to designing the review protocol and provided feedback on the report.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare. XB has received compensation as a member of the scientific advisory board of Medtronic.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Vamour, N., Dequirez, PL., Seguier, D. et al. Early interventions to prevent lower urinary tract dysfunction after spinal cord injury: a systematic review. Spinal Cord 60, 382–394 (2022). https://doi.org/10.1038/s41393-022-00784-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-022-00784-z