Abstract
Study design
Cross sectional.
Objectives
To identify variables associated with severe bowel symptoms in spinal cord injured people.
Setting
National cohort.
Methods
Adult spinal cord injured (SCI) people were recruited for an online registry and 1373 were included for analysis. Univariate analysis and logistic regression was used to evaluate associations between severity of bowel symptoms and variables. Bowel symptoms were assessed by the Neurogenic Bowel Dysfunction (NBD) score and patients scoring ≥14 were categorized as having severe bowel symptoms. Autonomic dysreflexia (AD) severity was measured using a six item questionnaire and reported as total AD score (0–24). Bladder management was categorized as: voiding, clean intermittent catheterization (CIC), surgery (augmentation/diversion) or indwelling catheter.
Results
Severe bowel symptoms were reported in 570 (42%) On multivariable logistic regression, every point increase of AD total score was associated with 5% increased odds of having more severe bowel symptoms [OR 1.05 95% CI 1.03–1.10]. Type of bladder management was also associated with more severe symptoms (p = 0.0001). SCI people with indwelling catheters (OR = 2.16, 95% CI 1.40–3.32) or reconstructive surgery (OR = 1.79, 95% CI 1.08–3.32) were almost twice as likely to report more severe bowel symptoms than those performing CIC.
Similar content being viewed by others
Introduction
Over 2.5 million people worldwide live with a spinal cord injury (SCI) [1] and the incidence of SCI has remained high in the United States over the past decade at 54 cases per 1 million people [2]. Common long-term complications from SCI include respiratory compromise, bladder dysfunction, chronic pain, cardiovascular changes, and bowel dysfunction. Untreated sequela of SCI can result in high emergency care utilization [3] and overall health care costs [4]. Consequently, it is important to understand factors that can either potentially increase or decrease severity of long-term complications in this population.
Bowel symptoms are particularly notable for negatively impacting quality of life after a SCI. Between 40–75% of people with a SCI suffer from constipation, fecal incontinence, or both [5, 6]. Risk factors for severe bowel symptoms in SCI patients are multifocal, but the injury location predicts the resulting enteric physiology. In general, people with injuries above L1–L2 have symptoms of increased bowel motility and a poorly relaxing sphincter. People with injuries below L2 are more likely to have reduced bowel motility and low sphincter tone. Complete spinal cord injuries also tend to have slower colonic transit time, likely from loss of coordinated peristalsis, and more severe symptoms compared to incomplete injuries[7]. In addition to changes in bowel motility, people with injuries above T6 are more likely to have autonomic dysreflexia (AD) and experience symptoms of hypertension, diaphoresis, and headache which can be triggered by changes in bowel or bladder function.
It has been previously demonstrated that SCI people with more severe bowel symptoms are more likely to have more severe bladder symptoms [8]. A central question is to better understand differences between association and causation relationships between bladder and bowel systems in SCI people. It is understood that a SCI changes the neurologic afferent and efferent communication between both bladder and bowels. Consequently, changes in both systems are associated with the underlying injury. However, it is not well understood if modifying one system will cause changes in the other system. Recently, the Neurogenic Bladder Research Group has published results from its registry, a prospectively studied national cohort of 1479 people with SCI, which showed that SCI people with indwelling urinary catheters or who had previous urinary reconstructive surgery reported significantly fewer bothersome urinary symptoms compared to people performing intermittent catheterization [9]. There is little information on whether a higher satisfaction with the urinary symptoms due to chosen management strategy is also associated with higher satisfaction in bowel function when other associative variables are controlled.
Our aim was to identify variables associated with severe bowel symptoms among spinal cord injured people. Based on our previous work, we hypothesized that SCI people with different bladder management will have higher odds of more symptoms, depending of type of management used, independent of variables that commonly impact both bladder and bowel function.
Methods
The Neurogenic Bladder Research Group (www.nbrg.org), a multi-institutional collaborative study group, prospectively enrolled adult SCI people between January 1, 2016 to June 30, 2017 in a SCI registry. Adult patients with acquired SCI who were able to communicate in English, provide consent, and answer web-based questionnaires were included in the study. Patients with congenital SCI (e.g., spina bifida) were excluded. The details on trial protocol and methods have been previously published [10] and trial registration numbers are NCT0261608, www.clinicaltrials.gov and HSRP20153564. U.S. National Library of Medicine, wwwcf.nlm.nih.gov. Grant funding was obtained through Patient Centered Outcomes Research Institute.
The analysis includes baseline data acquired during the initial enrollment of patients. Patient self-reported demographic, clinical, and injury information were collected. The Neurogenic Bowel Dysfunction (NBD) [11], Neurogenic Bladder Symptom Score (NBSS) [12], and modified SCI SF-12 [13] questionnaires concomitantly administered to measure bowel, bladder, and generalized QOL.
The primary outcome measure in this study was the NBD Score, a validated ten item questionnaire that measures impact of fecal incontinence, constipation, and obstructed defecation among people with neurogenic bowel. Scores were dichotomized by severity, defined as none/mild/moderate symptoms (0–13) or severe (14 or higher). The questionnaire has established validity for these categorizations. Bladder management strategy was characterized as either non-instrumented voiding, any intermittent catheterization, indwelling urinary catheter (both suprapubic or urethral), or reconstructive urinary surgery such as urinary diversion/bladder augment/continent stoma. SCI level was self-reported by level, complete versus incomplete, and ASIA classification. The severity of AD was assessed through AD questions taken from the validated ADFSCI instrument [14] and included domains of exercise, sexual activity, bladder function, bowel function, provoked activities, and unprovoked activities. Total AD score ranged from 0–24. Chronic pain was examined as a dichotomous variable for this study (yes/no). Bowel management was classified as medication (laxative, stool softener) and stimulation (digital, enema, suppository). SF-12, NBSS scores were also included in the analysis.
Statistical software used for analysis was SAS (Version 9.2, SAS Institute Inc, Cary, NC). Continuous variables were reported as means and standard deviation or medians and ranges, discrete variables were reported as percent and proportions. ANOVA and Wilcoxon tests were used for continuous variables, and the Chi-square test was used for comparison of binary/categorical variables with statistical significance defined as p ≤ 0.05. Mixed binomial logistic regression was used to evaluate predictors of severe bowel dysfunction. The following variables were chosen a priori to include in the model: age, complete vs incomplete injury, cervical or thoracic injury, ASIA, pain, AD score, SF12 scores, bladder management system, bowel management, and years since injury.
Results
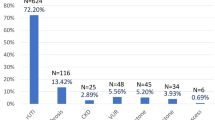
Of 2284 SCI people screened, 1479 people met the PCORI cohort inclusion criteria and were enrolled in the study. One hundred and six patients were excluded (69 with colostomy) due to missing NBD score which left 1373 patients in the cohort for analysis. CIC was the most common bladder management method (52%), followed by indwelling catheter (18%), voiding (18%), and surgical reconstruction (13%). Mean NBD score (SD) for the cohort was 12 [6]. AD was reported by 605 people (40%) and mean AD total score (SD) was 7 [4]. Mean time from the initial injury was 14 (SD 11) years for the cohort (Table 1).
When stratifying by NBD, 570 (42%) reported severe and 803 (58%) reported mild/moderate bowel symptoms. On univariate analysis, severe bowel symptoms were more common among people with cervical/thoracic injuries, complete spinal cord injuries, and education less than a bachelor’s degree. Severe bowel symptoms were also more common among people reporting chronic pain, and with higher total AD, SF-12 scores. Comparing NBD scores across bladder management type, severe bowel symptoms were more prevalent with indwelling catheter and bladder reconstructive surgery groups. Table 2 provides the specific differences between these groups.
A mixed binomial logistic regression analysis showed each point increase on AD questionnaire was associated with an ~5% increased odds of having severe bowel symptoms (OR 1.05, [1.03–10], p < 0.01). Type of bladder management was strongly associated with severe bowel symptoms (global p < 0.001). When compared to intermittent catheterization, SCI patients with indwelling catheters (OR = 2.16, [1.40–3.32]) and reconstructive surgery (OR = 1.79 [1.08–3.32]) were approximately twice more likely to have severe bowel symptoms. Also, taking medication (OR 1.97 [1.11–3.51]) or using bowel stimulation (OR 1.92 [1.32–2.80]) was associated with severe bowel symptoms (Table 3).
Discussion
Neurogenic bowel can greatly impact quality of life among spinal cord injured patients. Our data showed that 42% of SCI in this cohort reported severe bowel symptoms, 73% were using a medication and 32% were using stimulation for a bowel regimen. When controlling for other variables known to impact both bladder and bowel function, the study showed that rising AD scores increase the odds of having more severe bowel symptoms. The study also showed that SCI people with an indwelling urinary catheter and previous urinary reconstructive surgery had a higher odd’s (OR 2.2, 1.8, respectively) of reporting severe bowel symptoms compared to those performing intermittent catheterization.
The prevalence of bowel symptoms reported in this cohort mirrors symptom severity reported in other studies. The mean NBD score for the entire cohort of 1373 patients was 12. Cameron et al. retrospectively examined 175 spinal cord patients from an institutional neurogenic bladder database and reported a mean NBD of 11 [8]. Liu et al surveyed 128 SCI patients with a neurogenic bowel QOL instrument and found ~50% reported moderate-to-severe bowel symptoms [15]. Other studies also note similar prevalence of moderate to severe symptoms[5, 6, 16] among spinal cord injured people. Overall, our data confirm the high prevalence of severe bowel symptoms reported in other, smaller series.
It was striking that patients with an indwelling catheter (OR 2.16) had the highest odds of severe bladder symptoms among the bladder management types. Although we can not verify causality because of the cross-sectional analysis study design, it is possible to speculate that chronic inflammation in the related to bladder management strategy could result in changes in bowel function. An SCI rat model has also shown that chronic inflammation after SCI was associated with extensive neuromuscular enteric remodeling which impacted bowel functioning [17]. Indwelling catheters can cause chronic inflammation as evidenced through loss of bladder compliance and UTI’s. Weld et al. reviewed urodynamic data from 316 SCI patients and showed that people who voided or performed intermittent catheterization were 9x more likely to have normal bladder compliance compared to indwelling catheters [18]. Therefore, it is possible that inflammatory changes in the bladder due to changes such as loss of compliance could conceivably worsen bowel function.
An alternative speculative explanation for observing a favorable bladder but severe bowel symptoms among indwelling catheter patients could be that people with severe bowel symptoms chose an indwelling catheter because it is an easier to maintain compared to intermittent catheterization and has less self-care demands. Myers and the Neurogenic Bladder Research Group published data from this patient cohort showing a clear relationship in this cohort between the SCI people with indwelling catheters and a higher perceived urinary quality of life, compared to those performing intermittent catheterization [9]. Long-term outcomes on bowel management programs are lacking per a recent systemic review of the literature which may make SCI patients reluctant to change bowel programs [19] and rely on bladder management changes to improve quality of life. Further research should be undertaken to test this conjecture and to better understand how severity of bowel symptoms could potentially influence the choice of bladder management prospectively.
Similarly, we noted more severe bowel symptoms were also associated with previous urologic reconstructive surgery (augmentation cystoplasty, urinary diversion) (OR 1.8). Of the 192 people who had reconstructive surgery, 15% reported severe symptoms on the NBD. There are few other prospective or retrospective studies that have used validated bowel-specific questionnaires after urinary reconstruction in the SCI population. Hoen et al. performed a systematic review for augmentation cystoplasty (20 studies, 551 patients) and noted that a similar 15% of patients reported long-term bowel dysfunction [20]. There are no comparable studies examining long-term bowel dysfunction after urinary diversion. Guillot-Tantay reviewed 102 SCI people who underwent urinary diversion [21]. After a median follow-up of 60 months, the authors reported 35% overall late complication rate with no procedures needed to improve bowel function but residual bowel symptom severity was not quantified [22]. Given the cross-sectional nature of this analysis, we can not state causality between reconstructive urology surgery and more severe bowel symptoms. However, we explain the findings by speculating that harvesting bowel segments for use in reconstructive surgery may affect neurologic continuity of the enteric tract and thus increase bowel symptoms.
AD is a concerning condition among SCI people with lesions above T7 and there is a strong association with bowel irritation. Krassiouskov et al. performed a systematic review of AD in SCI people and noted that pain and irritation in the perirectal area is the second most common cause of AD in this population [21]. The severity of AD has also been shown to reduce overall quality of life in other studies [23]. Our data confirmed a high prevalence of AD in the patient population with ~40% reporting having some AD. Given the negative impact between AD and QOL, it is not surprising that univariate analysis demonstrated that SCI people with more severe bowel symptoms had more severe AD symptoms (AD score 8.0 vs 6.8) and higher AD scores were associated with more severe bowel symptoms in the logistic regression model. However, similar to the bladder management findings, we can not state causality between AD and severe bowel symptoms due to the cross-sectional nature of the study.
This study has limitations. Females were overrepresented in the cohort compared to other national cohorts and it was not possible to confirm level, completeness of injury, ASIA classification, or bowel transit information since investigators did not have access to medical records for participants. We were also not able to separate bladder management into subgroups based on location of indwelling catheter (urethral vs suprapubic) or define the specifics regarding urinary reconstruction such as length or location of bowel segments used. There also could be a component of recall bias in how patients filled out the questionnaire, which is a limitation of all cross-sectional questionnaire-based studies. Most importantly, although the cross-sectional nature of this study allows us attempting to control for common variables affecting bladder and bowel, we recognize that this does not allow us to establish true causality between associations.
Despite these limitations, this study has aspects that allow for generalization of findings. First, the study used a national cohort that was not tied to a specific region or care center. This reduces some of the potential risk of institutional bias that can occur in single or multi-center cohort studies and it is likely that our cohort resembles a national SCI population. Second, recruitment was not based through a urology-specific clinic. Patients who participated in the study may or may not have had regular urologic care. This also minimized bias toward more severe urologic complications in the cohort. Third, the quality of life questionnaires had been validated for the SCI population which also allows for accurate assessment and comparisons. Finally, the findings in this study can be used to generate communication between providers and spinal cord injured patients to facilitate a team approach when developing plans for bladder and bowel symptom management. Future research should focus on investigating causality between bladder interventions and bowel symptoms.
Conclusions
Forty percent of SCI people in this cohort reported severe bowel symptoms on the NBD questionnaire. More severe AD was associated with more severe bowel symptoms. People with indwelling urethral catheters or previous urinary reconstructive surgery were more likely to have severe bowel symptoms compared to people performing intermittent catheterization or voiding.
Data availability
Data from this study are archived at the University of Utah and the University of Michigan.
References
Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–31.
Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O’Connor KC, et al. Traumatic spinal cord injury in the United States, 1993-2012. JAMA. 2015;313:2236–43.
Crescenze IM, Lenherr SM, Myers JB, Elliott SP, Welk B, O’Dell D, et al. Self-reported urologic hospitalizations or emergency room visits in a contemporary spinal cord injury cohort. J Urol. 2020. https://doi.org/10.1097/JU.0000000000001386.
Chan BC, Cadarette SM, Wodchis WP, Krahn MD, Mittmann N. The lifetime cost of spinal cord injury in Ontario, Canada: a population-based study from the perspective of the public health care payer. J Spinal Cord Med. 2019;42:184–93.
Ng C, Prott G, Rutkowski S, Li Y, Hansen R, Kellow J, et al. Gastrointestinal symptoms in spinal cord injury: relationships with level of injury and psychologic factors. Dis Colon Rectum. 2005;48:1562–8.
Tate DG, Forchheimer M, Rodriguez G, Chiodo A, Cameron AP, Meade M, et al. Risk factors associated with neurogenic bowel complications and dysfunction in spinal cord injury. Arch Phys Med Rehabil. 2016;97:1679–86.
Stoffel JT, Van der Aa F, Wittmann D, Yande S, Elliott S. Neurogenic bowel management for the adult spinal cord injury patient. World J Urol. 2018;36:1587–92.
Cameron AP, Rodriguez GM, Gursky A, He C, Clemens JQ, Stoffel JT. The severity of bowel dysfunction in patients with neurogenic bladder. J Urol. 2015;194:1336–41.
Myers JB, Lenherr SM, Stoffel JT, Elliott SP, Presson AP, Zhang C, et al. Patient reported bladder related symptoms and quality of life after spinal cord injury with different bladder management strategies. J Urol. 2019;202:574–84.
Patel DP, Lenherr SM, Stoffel JT, Elliott SP, Welk B, Presson AP, et al. Study protocol: patient reported outcomes for bladder management strategies in spinal cord injury. BMC Urol. 2017;17:95.
Krogh K, Christensen P, Sabroe S, Laurberg S. Neurogenic bowel dysfunction score. Spinal Cord. 2006;44:625–31.
Welk B, Morrow S, Madarasz W, Baverstock R, Macnab J, Sequeira K. The validity and reliability of the neurogenic bladder symptom score. J Urol. 2014;192:452–7.
Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33.
Hubli M, Gee CM, Krassioukov AV. Refined assessment of blood pressure instability after spinal cord injury. Am J Hypertens. 2015;28:173–81.
Liu CW, Huang CC, Yang YH, Chen SC, Weng MC, Huang MH. Relationship between neurogenic bowel dysfunction and health-related quality of life in persons with spinal cord injury. J Rehabil Med. 2009;41:35–40.
Elmelund M, Klarskov N, Biering-Sorensen F. Fecal incontinence and neurogenic bowel dysfunction in women with traumatic and nontraumatic spinal cord injury. Dis Colon Rectum. 2019;62:1095–104.
White AR, Holmes GM. Anatomical and functional changes to the colonic neuromuscular compartment after experimental spinal cord injury. J Neurotrauma. 2018;35:1079–90.
Weld KJ, Dmochowski RR. Association of level of injury and bladder behavior in patients with post-traumatic spinal cord injury. Urology. 2000;55:490–4.
Musco S, Bazzocchi G, Martellucci J, Amato MP, Manassero A, Putignano D, et al. Treatments in neurogenic bowel dysfunctions: evidence reviews and clinical recommendations in adults. Eur J Phys Rehabil Med. 2020;56:741–55.
Hoen L, Ecclestone H, Blok BFM, Karsenty G, Phe V, Bossier R, et al. Long-term effectiveness and complication rates of bladder augmentation in patients with neurogenic bladder dysfunction: a systematic review. Neurourol Urodyn. 2017;36:1685–702.
Krassioukov A, Warburton DE, Teasell R, Eng JJ.Spinal Cord Injury Rehabilitation Evidence Research Team. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil. 2009;90:682–95.
Guillot-Tantay C, Chartier-Kastler E, Perrouin-Verbe MA, Denys P, Leon P, Phe V. Complications of non-continent cutaneous urinary diversion in adults with spinal cord injury: a retrospective study. Spinal Cord. 2018;56:856–62.
Inskip JA, Lucci VM, McGrath MS, Willms R, Claydon VE. A community perspective on bowel management and quality of life after spinal cord injury: the influence of autonomic dysreflexia. J Neurotrauma. 2018;35:1091–105.
Author contributions
PBR—data analysis, manuscript writing. SML and JBM—study design, data analysis, manuscript edit. SPE, BW, and JTS—study design, data analysis. DO—data analysis.
Funding
Grant funding was obtained through Patient Centered Outcomes Research Institute (PCORI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
PBR, SML, JBM, SPE and DO—no conflict. JTS—Editorial Board—J Urology, Leadership—Neurogenic Bladder Research Group.
Ethics approval
This study was approved and overseen by the University of Utah Internal Review Board. At each Co Investigator’s participating site (University of Michigan, University of Minnesota), institutional approval was obtained for the study IRB.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stoffel, J.T., Barboglio-Romo, P., Lenherr, S.M. et al. Factors impacting bowel symptoms in a contemporary spinal cord injury cohort: results from the Neurogenic Bladder Research Group Registry. Spinal Cord 59, 997–1002 (2021). https://doi.org/10.1038/s41393-021-00667-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00667-9
This article is cited by
-
Editorial special edition neuro-urology
Spinal Cord (2021)