Abstract
Design
Descriptive.
Setting
Community, Bangladesh.
Objectives
To determine the costs associated with providing a community-based model of care delivered as part of the CIVIC trial to people discharged from hospital with recent spinal cord injury (SCI), and to determine the economic burden to households.
Methods
Records were kept of the costs of providing a community-based model of care to participants of the CIVIC trial. Data were also collected at discharge and 2 years post discharge to capture out-of-pocket healthcare costs over the preceding 2 years, and the number of participants suffering catastrophic health expenditure and illness-induced poverty.
Results
The mean cost of providing the community-based model of care to participants assigned to the intervention group (n = 204) was US$237 per participant. The mean out-of-pocket healthcare cost over the first 2 years post discharge was US$472 per participant (n = 410), and US$448 per control participant (n = 206). Median (IQR) equivalent annual household incomes prior to SCI and at 2 years post discharge were US$721 (US$452–1129) and US$464 (US$214–799), respectively. Of the 378 participants alive at 2 years, 324 (86%) had catastrophic health expenditure, and 161 of 212 participants who were not in poverty prior to injury (76%) were pushed into illness-induced poverty within 2 years of injury.
Conclusion
The cost of providing community-based support to people with SCI for 2 years post discharge in Bangladesh is relatively inexpensive but an overwhelming majority of households rapidly experience financial catastrophe, and most fall into poverty.
Similar content being viewed by others
Introduction
Very little is known about the financial implications of living with a spinal cord injury (SCI) in a low- and middle-income country (LMIC). Even less is known about the cost of supporting people from these countries living with a SCI in their communities and the burden this poses to their households. Yet SCI are far more common in LMICs than high-income countries with an estimated incidence of between 10 and 83 per million per year [1, 2]. Given that 70% of the world’s population lives in LMIC, it is appropriate that attention be directed at the plight of people with SCI in LMICs.
Bangladesh is a LMIC in which healthcare is largely funded by out-of-pocket healthcare payments. In 2017, around 2.2% of Bangladesh’s gross domestic product was spent on healthcare; of which 73% was attributed to the out-of-pocket healthcare costs of individuals. It is therefore not surprising that each year an estimated 5 million Bangladeshi (3.5% of the population) fall into poverty as a result of ill health or serious injury such as SCI [3, 4]. Against this background, the Government of Bangladesh has been implementing a 20-year plan to achieve universal health coverage through a combination of taxation-based financing and social insurance programs [5]. In spite of such initiatives and a general commitment to the United Nations’ Sustainable Development Goals [6], progress toward universal health coverage has been slow [7]. To achieve universal health coverage for people living with SCI in Bangladesh (and other LMICs), there are two key tasks for policy makers: first, they need to ensure that all people have access to appropriate support and health services, and second, they need to provide financial support to individuals to cover the costs of the support and health services. In order to address these tasks, data are required to highlight the cost of providing ongoing support services, and the financial implications of living with serious disabilities such as SCI [2, 8,9,10]. We provide data to tackle both these issues in this study.
Our team recently completed a large randomised controlled trial (called the CIVIC trial). The trial looked at the effectiveness of a model of care for providing ongoing community-based support to people with SCI recently discharged from hospital in Bangladesh. The CIVIC model of care was posited as a low resource and potentially sustainable way of providing long-term support. It involved the assignment of a case manager to each participant who was then followed up regularly by telephone and a few home visits over the first 2 years following discharge from hospital (details of the intervention are provided in this reference [11]). Whilst our model of care was not found to be effective for reducing mortality at 2 years post discharge (primary outcome) [12], the observed costs and resource implications of our model of care may inform the planning and testing of future comprehensive services for people with SCI in Bangladesh and in other LMIC where community-based care and follow-up support for people with SCI is often ad hoc, limited or non-existent. With increasing awareness on the need to improve long-term well-being and inclusion for people with SCI and with widespread mobile phone coverage/usage, it is likely that similar programs based on telephone support and a limited number of home visits will be taken up, tested and potentially adopted in future service models in Bangladesh and other resource constrained settings. An initial aim of this study was therefore to determine the cost incurred by the trial of providing our community-based model of care designed to support people with SCI in the community in Bangladesh.
The second aim of this study was to provide the first detailed analysis of the burden of out-of-pocket healthcare costs and income loss of SCI in Bangladesh over the first 2 years post discharge. This entailed determining the number of participants who suffered catastrophic health expenditure and illness-induced poverty following their SCI. There are a lot of data on the costs associated with SCI from high-income countries [13,14,15] but very little from LMICs. We and others have presented data on the financial implications of the loss of the income of a person with SCI on their household incomes in Bangladesh and other low LMICs [16, 17], but no one has yet provided a detailed costing of how and where money is spent due to SCI, or quantified the number of people who suffer catastrophic health expenditure and illness-induced poverty due to their SCI. A better understanding of all these issues will help highlight the financial plight of these people and their families, and contribute data towards the Bangladeshi goal of universal health coverage.
Methods
An economic analysis was undertaken alongside a pragmatic randomised controlled trial (the CIVIC trial) conducted between July 2015 and March 2020 [12]. The aim of the trial was to determine the effectiveness of a community-based model of care designed to reduce mortality at 2 years post discharge. The economic analysis assessed the costs incurred by the trial of delivering the model of care, and the out-of-pocket healthcare costs incurred by individuals and their families.
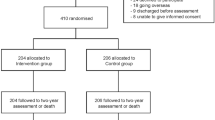
The model of care consisted of providing participants with a case manager for 2 years. The case managers were healthcare professionals who were instructed as part of the trial protocol to ring participants 38 times over the first 2 years post discharge, visit them in their homes three times and provide them with up to AU$80 (US$59) of financial support for essential items of care (as required). At each point of contact, participants were screened for early signs of complications, provided with ongoing advice and support, and encouraged to participate in family and social life. The CIVIC trial participants were 410 people with SCI (sustained within the preceding 2 years) who were wheelchair dependent and who were recently discharged from the Centre for the Rehabilitation of the Paralysed in Bangladesh. The participants were reflective of those admitted to CRP but not necessarily reflective of all people with SCI in Bangladesh. CRP is a not-for-profit hospital that admits all people irrespective of their capacity to pay. Those with high incomes or health insurance are more likely to be admitted to private hospitals elsewhere or to travel to other countries for treatment and rehabilitation. The participants were randomised to either usual care (control group: n = 206) or our model of community-based care (intervention group; n = 204). The trial was prospectively registered (ACTRN 12615000630516, Universal Trial Number U1111-1171-1876) and received ethics permission from the Centre for the Rehabilitation of the Paralysed (CRP-R&E-0401-126) and the University of Sydney Ethics Committees. The protocol for the trial [18] along with the trial results [12] and findings from a process evaluation [11] has been previously published. We have also previously published the demographic and economic data collected at baseline in the cohort from this trial to highlight the vulnerability of this group to poverty and disadvantage [16]. This paper specifically examines the trial delivery costs and out-of-pocket healthcare costs incurred by trial participants over the 2 years following discharge from hospital.
Cost of providing the community-based model of care
A bottom-up costing of the CIVIC model of care was conducted. All costs incurred by the trial associated with providing the community-based model of care to the 204 intervention participants were prospectively collected in Bangladeshi Taka (BDT) and later converted to American dollars (US$).
Cost of employing staff
Detailed records were kept of the time trial staff spent training and providing the intervention. This included the time associated with:
-
Attending training and self-training.
-
Co-ordinating the telephone calls and home visits, and keeping records.
-
Providing the telephone calls.
-
Conducting the home visits.
-
Travelling to and from home visits.
Staff time was costed at standard hourly rates of pay and apportioned according to time administering the intervention (excluding research-related costs).
Cost of consumables
Detailed records were kept to capture the cost of the following consumables:
-
Cost of telephone call charges: the start and end times of all telephone calls with participants were recorded to attain a total telephone call time per participant. The cost of telephone call charges was based on an estimated cost of US$0.024 per minute.
-
Transport, food and accommodation for home visits: detailed records (and receipts) were kept of all costs associated with transport, food and accommodation for the home visits.
-
Information booklets for participants: all intervention participants were provided with a booklet that contained information about self-management. The cost of printing the booklets was recorded.
-
Discretionary money for participants: detailed records (and receipts) were kept of all financial support provided to participants. Participants were not provided with cash-in-hand but rather provided with equipment and medical accessories such as dressings for pressure ulcers, medication and nutritional supplements. The total cost per participant was calculated.
Out-of-pocket healthcare costs
Data were collected at 2 years post discharge to estimate the out-of-pocket healthcare costs incurred by the 410 participants and their families. Participants were interviewed in their homes using a structured questionnaire with both open-ended and close-ended questions to capture all costs (see Supplementary File 1 of the Case Report Forms used for this purpose). Thirty-two participants were deceased; data for these participants were collected by interviewing family members over the telephone (31 participants had died by 2 years post discharge/randomisation and another one participant died by the time the 2-year assessment was conducted. The difference was due to the inclusion of a 1-month window for the completion of the 2-year assessments). The items of expense that were billed for, and determined separately, were medical and treatment costs (hospitalisations, visits to a doctor, visits to other healthcare professionals and visits to traditional healers) and equipment costs (equipment or supplies for bladder and bowel management, orthoses or aids, special equipment, bed mattresses, cushions for wheelchairs and miscellaneous items).
Number of participants suffering catastrophic health expenditure and illness-induced poverty
Data were collected at discharge and 2 years post discharge to determine the number of participants who experienced catastrophic health expenditure and illness-induced poverty. Participants were asked about their and their families’ incomes and the number of adults and children in their households at both times (see Supplementary File 1 of the Case Report Forms used for this purpose). Participants were asked at discharge to report on their situation prior to injury. Two-year data were only collected in those alive at the time (n = 378). Equivalent annual household incomes prior to injury and at 2 years post injury were determined by adjusting household incomes by the number and composition of families at the time using the reported equivalence scale for Bangladesh [19]. This provides an income per individual that takes into account the household income and the number of family members. Households were classified as having incurred catastrophic health expenditure if their annual out-of-pocket healthcare costs were more than 30% of their equivalent annual household income prior to injury [20, 21]. Participants were classified as having suffered illness-induced poverty if their equivalent annual household income prior to injury was more than the prevailing poverty level in Bangladesh of US$693.50 and this was reduced to <US$693.50 after deducting out-of-pocket healthcare costs and household income loss [22] (the prevailing poverty level is equivalent to US$1.90 per day; the standard poverty line used by the World Bank and other international organisations) [23].
Analysis
Data were initially captured in paper format and then transferred across to a Redcap database. STATA-16 was used for all analyses. BDT was converted to US$ applying the exchange rate (US$1 = 84.9 BDT). Mean (SD) costs were calculated despite the highly skewed nature of the data in keeping with economic theory.
Results
The demographic characteristics of the intervention participants (n = 204) are provided in Table 1. The family situation, employment status and incomes of all participants and their households prior to injury (n = 410) and at 2 years post discharge (n = 378) are provided in Table 2.
Cost of providing the community-based model of care
The total cost of providing the community-based model of care to the 204 intervention participants was US$48,327 (on average, US$237 per participant; see Table 3 for details). The most expensive part of the intervention was the transport, food and accommodation required by staff to make the home visits (mean: US$97 per participant, or US$19,840 in total; see Table 3 for details). Staff spent on average 13 hours in travel time per home visit and often needed to stay overnight in local villages because participants lived in all corners of Bangladesh, and travel was time consuming with poor roads and public transport infrastructure. The other major cost was associated with providing participants with discretionary money (mean: US$51 per participant, or US$10,318 in total; see Table 3 for details).
Out-of-pocket healthcare costs
The mean out-of-pocket healthcare cost for all participants (n = 410) over the 2 years post discharge was US$472 per participant, US$375 per participant for medical and treatment costs and US$97 per participant for equipment costs (see Supplementary File 2 and Table 1). Some of the more expensive items, such as nights in hospital and visits to healthcare professionals, were only borne by a small number of participants. Nearly all participants (n = 395) incurred costs associated with medication (mean: US$170 per participant). The costs for the control participants were less than for the intervention participants: US$505 per intervention participant (n = 204) and US$448 per control participant (n = 206; see Table 4).
Number of participants suffering catastrophic health expenditure and illness-induced poverty
The median (IQR) equivalent annual household incomes prior to injury and at 2 years post discharge were US$721 (US$452–1129) and US$464 (US$214–799), respectively (see Table 2). Three hundred and twenty-four participants (86%) incurred catastrophic health expenditure, and 161 out of 212 participants not previously in poverty (76%) were pushed into illness-induced poverty within 2 years of injury.
Discussion
This study provides the first detailed analysis of the costs of delivering a community-based model of care for 2 years post discharge from a hospital in Bangladesh. The cost of our community-based model of care is modest at US$237 per participant over 2 years. Future studies could look at the effectiveness of a more sophisticated and costly intervention if looking to achieve the same minimum effect size posed in the original protocol of 10% absolute risk reduction in mortality at 2 years from 17% to 7%. This assumes the intervention could be priced at up to US$360 per person to fall within a cost effectiveness threshold of US$3600 per life saved. (This is based on a GDP per capita in 2019 of around US$1800 [24], and an assumed cost effectiveness threshold of two times per capita GDP.) Given that the cost of the current intervention is around US$237 and an effective intervention will most likely also deliver offsetting reductions in healthcare use, there appears to be some, albeit limited, scope for intensifying the resources used in future versions of this intervention in an effort to cost-effectively achieve the proposed intervention effect.
A notable finding was that the cost of providing telephone-based support is low but the cost of visiting people in their homes is high. Nonetheless the present costs could be substantially reduced if the travel costs associated with the three home visits could be minimised and the home visits could be better coordinated to ensure all home visits to one region were conducted at the same time (this was not always possible because the home visits needed to be conducted within a specified time frame in keeping with the trial protocol). Most of the costs here were related to the transport, food and accommodation costs for staff. These were necessary because staff were travelling from one central location (CRP) to all corners of Bangladesh. Bangladesh is a relatively small country so distances are not large. However, the roads and infrastructure are poor and many people live in rural areas. Consequently, staff spent on average 13 hours in travel per home visit and often needed to stay overnight in local towns. This cost could clearly be reduced if a community-based model of care was organised and coordinated at a divisional/provincial level rather than from CRP’s central location close to the capital of Dhaka. When the study started there was not infrastructure to enable the use of a decentralised system to provide the home visits. However, in recent years, CRP has expanded and established a network of rehabilitation centres in all eight divisions of the country. There is now scope to coordinate and roll out a community-based model of care from these divisional centres as an integral component of CRP’s decentralised model of service provision. Access to appropriate divisional level of SCI services closer to people’s home may also help reduce out-of-pocket expenditure. These divisional centres may not have the capacity and expertise to deal with all the various complications people with SCI experience and some people with SCI may not be able or willing to visit the divisional centres for routine follow-up or as complications occur. In both scenarios telemedicine could be considered (1) to connect the divisional centres to CRP’s central facility for expert SCI consultations and (2) to connect the person with SCI in his/her home with CRP divisional and central SCI services (if a future trial could prove that such an approach in this type of setting was effective).
This study also indicates the substantial economic burden to households associated with supporting people with SCI in Bangladesh is in most part due to the costs of medication. The out-of-pocket healthcare costs incurred by the control participants over the first 2 years following discharge (US$448) provide the best reflection of the real costs outside the context of participation in a trial. The costs were substantial when one considers that the median (IQR) annual household income was only US$1131 (US$565–2121). In households that were already struggling, many not only lost the income of the injured person but also now needed to direct approximately 20% of annual household incomes to the costs associated with the person’s SCI. It is not known how many of these families took out loans or sold possessions to meet these costs, or sourced money and support from elsewhere such as from families, communities or charities. It is also not known how many people with SCI missed essential medication and items of care because of financial distress. However, it is clear that many of the families of participants were struggling financially before the injuries and were thrown into further extreme poverty after their injuries. This is highlighted by the number of participants that had catastrophic health expenditure (86%) and suffered illness-induced poverty (76%). Despite these grim statistics, they probably underestimate the severity of the problem because 198 participants (48%) had an equivalent annual household income prior to injury of <US$693.50. These participants were not classified as having illness-induced poverty because they were living in poverty prior to, and irrespective of, their injuries. Our figures may also underestimate the financial implications of participants’ SCI because out-of-pocket healthcare costs did not include indirect (non-healthcare) costs related to home modifications, setting up businesses or loss of income by carers.
The out-of-pocket healthcare costs associated with living with a SCI were probably less than what might be the case for other people in similar situations in Bangladesh and other LMICs because participants received support and services prior to discharge from CRP. For example, all participants received wheelchairs, cushions for wheelchairs and bed mattresses from CRP prior to discharge (mostly free of charge—a small number who could pay were required to self-fund). In addition, the participants allocated to the intervention group received an additional US$51 worth of goods and services which they may otherwise have needed to fund. Interestingly, they still had greater out-of-pockets costs (US$505) than the control participants who were not provided with this support (US$448; see Table 4). The difference was primarily due to the intervention participants spending more on health-related costs (US$408 per participant) than the control participants (US$350 per participant). This was attributable to the intervention participants visiting doctors and purchasing more medication than the control participants. This may indicate that the case managers were either directly or indirectly prompting intervention participants to visit doctors who were then in turn prescribing medication. The increased costs associated with visiting doctors and purchasing medication for the intervention participants were somewhat offset by the reduced costs associated with purchasing dressings for pressure ulcers (that were often provided to intervention participants as part of the allocated (AU$80/US$59) allowance) and visiting traditional healers (which was discouraged by the case managers).
A strength of this study is that the participants were reflective of those discharged from a large centre for SCI in Bangladesh over two and a half years (a consecutive series of people discharged from CRP were included in the trial). Importantly, all participants were wheelchair dependent at discharge so the costings are only reflective of this subgroup of people with SCI. Also 41 potentially eligible participants declined to be involved in the trial (24 declined, 9 discharged before assessments and 8 were unable to provide consent). Their exclusion may have introduced a small selection bias. A weakness of this study is that the out-of-pocket healthcare costs were collected through self-report at 2 years. We considered collecting these costs through diaries or regular phone calls, but neither option was viable because many participants were illiterate and because regular contact with the control participants could have introduced contamination to the trial.
In all, the results of our trial are relevant for the management of people with SCI in LMICs, particularly when one considers that 70% of the world’s population lives in LMICs, and SCI are far more common in these countries than high-income countries [2]. It is therefore right that attention be directed at the plight of those with SCI living in these countries. Our results will help guide the roll out of community-based models of care that progress upon the current work directed at reducing mortality and complications post discharge in this under-served and under-researched population. Our results also serve to again highlight the financial burden in LMICs of individuals living with SCI and their families, and by extension the potential for the transmission of such disadvantage over generations. With such data, organisations such as the World Health Organisation or the International Spinal Cord Society can more effectively lobby governments around the world for enhanced financial protection measures for this population by linking such support to longstanding commitments to universal health coverage and the United Nations’ Sustainable Development Goals [2, 6, 8,9,10].
Data availability
The authors will consider any reasonable requests for deidentified individual participant data.
References
Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44:523–9.
World Health Organization. International perspectives on spinal cord injury. Geneva: World Health Organisation; 2013.
World Health Organisation Global Health Expenditure Database. Out-of-pocket expenditure (% of current health expenditure)—Bangladesh. https://data.worldbank.org/indicator/SH.XPD.OOPC.CH.ZS?locations=BD. Accessed 3 Sept 2020.
Khan JAM, Ahmed S, Evans TG. Catastrophic healthcare expenditure and poverty related to out-of-pocket payments for healthcare in Bangladesh-an estimation of financial risk protection of universal health coverage. Health Policy Plan. 2017;32:1102–10.
Health Economics Unit, Ministry of Health and Family Welfare Government of the People’s Republic of Bangladesh. Expanding Social Protection for Health: Towards Universal Coverage. Health care financing strategy 2012-32. Health Economics Unit, Ministry of Health and Family Welfare Government of the People’s Republic of Bangladesh, Dhaka, Bangladesh; 2012.
United Nations Development Progamme (UNDP). Sustainable development goals: Bangladesh. https://www.bd.undp.org/content/bangladesh/en/home/sustainable-development-goals.html#:~:text=The%20Sustainable%20Development%20Goals%20(SDGs,peace%20and%20prosperity%20by%202030. Accessed 15 Sept 2020.
Rahman MM, Karan A, Rahman MS, Parsons A, Abe SK, Bilano V, et al. Progress towards Universal Health Coverage: A Comparative Analysis in 5 South Asian Countries. JAMA Intern Med. 2017;177:1297–305.
World Health Organization and The World Bank. World report on disability. Geneva: World Health Organization; 2011.
The World Bank. Poverty assessment for Bangladesh: creating opportunities and bridging the East-West Divide. In: Bangladesh development series. The World Bank Office, Dhaka, Bangladesh; 2008.
Islam D, Sayeed J, Hossain N. On determinants of poverty and inequality in Bangladesh. J Poverty. 2016;21:352–71.
Liu H, Hossain MS, Islam MS, Rahman MA, Costa PD, Herbert RD, et al. Understanding how a community-based intervention for people with spinal cord injury in Bangladesh was delivered as part of a randomised controlled trial: a process evaluation. Spinal Cord. 2020;58:1166–75.
Hossain MS, Harvey LA, Islam MS, Rahman MA, Muldoon S, Biering-Sorensen F, et al. A community-based intervention to prevent serious complications and death two years after discharge in people with spinal cord injury in Bangladesh (CIVIC): a randomised trial. Spinal Cord. 2020. https://doi.org/10.1038/s41393-020-00546-9.
Access Economics for the Victorian Neurotrauma Initiative. The economic cost of spinal injury and traumatic brain injury in Australia. Access Economics for the Victorian Neurotrauma Initiative, Victoria, Australia; 2009.
Krueger H, Noonan VK, Trenaman LM, Joshi P, Rivers CS. The economic burden of traumatic spinal cord injury in Canada. Chronic Dis Inj Can. 2013;33:113–22.
DiPiro ND, Murday D, Corley EH, Krause JS. Prevalence of chronic health conditions and hospital utilization in adults with spinal cord injury: an analysis of self-report and South Carolina administrative billing data. Spinal Cord. 2019;57:33–40.
Hossain MS, Harvey LA, Islam MS, Rahman MA, Liu H, Herbert RD, et al. Loss of work-related income impoverishes people with SCI and their families in Bangladesh. Spinal Cord. 2020;58:423–9.
Kawu AA, Olawepo A, Salami AO, Kuranga SA, Abdulhameed S, Esenwah VC. A cost analysis of conservative management of spinal cord-injured patients in Nigeria. Spinal Cord. 2011;49:1134–7.
Hossain MS, Harvey LA, Rahman MA, Muldoon S, Bowden JL, Islam MS, et al. Community-based InterVentions to prevent serIous Complications (CIVIC) following spinal cord injury in Bangladesh: protocol of a randomised controlled trial. BMJ Open. 2016;6:e010350.
Hasan SA. Engel curves and equivalence scales for Bangladesh. J Asia Pac Econ. 2016;21:301–15.
Jan S, Lee SW, Sawhney JP, Ong TK, Chin CT, Kim HS, et al. Catastrophic health expenditure on acute coronary events in Asia: a prospective study. Bull World Health Organ. 2016;94:193–200.
Group AS, Jan S, Kimman M, Peters SA, Woodward M. Financial catastrophe, treatment discontinuation and death associated with surgically operable cancer in South-East Asia: results from the ACTION study. Surgery. 2015;157:971–82.
Jan S, Laba T-L, Essue BM, Gheorghe A, Muhunthan J, Engelgau M, et al. Action to address the household economic burden of non-communicable diseases. Lancet. 2018;391:2047–58.
World Bank. Poverty and shared prosperity 2018: piecing together the poverty puzzle. Washington: World Bank; 2018.
The World Bank. GDP per capita (current US$)—Bangladesh. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=BD. Accessed 15 Sept 2020.
Acknowledgements
MSH is a recipient of an International Postgraduate Research Scholarship (IPRS) from the University of Sydney. We acknowledge the assistance of the following people: Murali Dhakshinamurthy, Mohammad Muddasser, Md. Naushad Alam, Sarath Gudivada, Jitendra Rathore, Ambika Yoganathan, Faruq Ahmed, Md. Shahoriar Ahmed, SM Iftekhar Alam, Md. Jubair Hassan, Masud Ur Rahman, Pangkaz Kanti Dash, Habibur Rahman and Md. Gourab Hasan.
Funding
This trial was funded by the Australian National Health and Medical Research Council (project grant APP1080259). The funder was not involved in any aspect of the study. MSH and LAH have full access to all the data.
Author information
Authors and Affiliations
Contributions
LAH, MSH, SM and VT conceived the study. LAH, MSH, SM, VT, RIL, FB-S, IDC and SJ secured funding. LAH, MSH, HL, SM, LB, RIL, FB-S, IDC and SJ wrote or reviewed the study protocol. LAH and MSH coordinated the trial. MSI, MSH, MAR, PDC and VT managed or contributed to the management of the site. MSI, MAR and PDC provided the intervention. LAH conducted the statistical analyses. MSI, LAH, MSH, MAR, PDC, HL, SM, VT, LB, RIL. FB-S, IDC and SJ interpreted the results. MSI, LAH, MSH, MAR, PDC, HL, SM, VT, LB, RIL, FB-S, IDC and SJ wrote or reviewed the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was obtained from the ethics committees of the Centre for the Rehabilitation of the Paralysed (CRP), Savar, Bangladesh and the University of Sydney, Australia. Institutional and governmental regulations concerning the ethical use of human volunteers were followed.
Informed consent
Written consent was obtained from all participants involved in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Islam, M.S., Harvey, L.A., Hossain, M.S. et al. The cost of providing a community-based model of care to people with spinal cord injury, and the healthcare costs and economic burden to households of spinal cord injury in Bangladesh. Spinal Cord 59, 833–841 (2021). https://doi.org/10.1038/s41393-020-00600-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-020-00600-6