Abstract
Study design
Longitudinal community survey.
Objectives
To describe the treatment for secondary health conditions as reported by individuals living with spinal cord injury (SCI) and to identify potential predictors of treatment.
Setting
Community (people with SCI living in Switzerland).
Methods
Data on the frequency, severity, and treatment of 14 common health conditions (HCs) in the past three months were collected in two surveys by the Swiss Spinal Cord Injury (SwiSCI) cohort study, in 2012 and 2017. Variation in treatment was analyzed using descriptive statistics, by survey period and severity of HC. Conditional multilevel random-effects logistic regression was used to describe differences in self-reported treatment with respect to sociodemographic and socioeconomic factors in addition to SCI characteristics and severity and number of HCs.
Results
Severe or chronic autonomic dysreflexia and sleep problems showed in the self-report as the HCs with the lowest occurrence/frequency of treatment. Across all HCs, higher age, shorter time since injury, the total number of HCs, and level of severity were associated with a higher propensity for reporting treatment. Individuals with severe financial difficulties additionally had 1.40 greater odds of receiving treatment (95% CI 1.09–1.80).
Conclusions
This study identified systematic differences in the report of HCs and their treatment within the Swiss SCI community. This study thus provides a basis to guide future research on identifying targets of intervention for long-term clinical management of SCI.
Similar content being viewed by others
Introduction
Among individuals with spinal cord injury (SCI), secondary health conditions (HCs), when not properly treated, have the potential to increase the rate of hospitalization, and ultimately reduce overall functioning, quality of life (QoL), and survival [1,2,3,4,5,6]. HCs are therefore one of the main targets for intervention across the continuum of care post-SCI. Equitable and adequate access to specialized care—irrespective of lesion characteristics, demographics, or socioeconomic status—is required to ensure the best possible maintenance of health and social participation, and to avoid premature death [7]. Understanding the sources of systematic variation in this usage of health services is thus necessary to ensure health equity and to prevent undertreatment of HCs, as usage can be influenced by availability, accessibility, and affordability [8]. Therefore, it is imperative to identify vulnerable groups of patients with untreated HCs to determine potential policy targets for health system design and functioning interventions [3, 9, 10].
Community surveys that capture information on HCs and their treatment may provide indicators of health inequity and can aid in identifying influential determinants of apparent disparities [11, 12]. In particular, self-report data may capture between-person variation in health—such as disease burden, clinically undiagnosed illness, or the synergistic effect of untreated conditions—that are not captured in more objective measures of health [13, 14]. Furthermore, self-report health information of people living with SCI can be a valuable indicator of their health state and provide a tool to analyze the effectiveness of the health care system for this population [13]. This can be a particularly valuable precursor to the identification of targets for intervention and ensuring that adequate treatment is provided for the SCI community. Currently, evidence regarding self-report health care in community-dwelling individuals with SCI is limited and often derived from convenience samples that may not be representative of the local SCI population.
This study used data from two community surveys that were implemented in 2012 and 2017 as part of the population-based Swiss Spinal Cord Injury (SwiSCI) cohort study [15]. Both surveys recruited a substantial proportion of the eligible population, with marginal non-response bias with respect to demographic and lesion characteristics [16, 17]. Furthermore, self-report information on demographics and lesion characteristics in the 2012 survey showed adequate agreement with available medical record data [16, 17]. Previous analysis from the SwiSCI study population revealed a high prevalence of self-reported HCs, with many HCs identified as untreated [2]. Therefore, the overall objective of this study is to describe the percentage of treatment for HCs reported by individuals living with SCI and to identify potential predictors of treatment.
Methods
Study description and population
This study utilizes data collected in two SwiSCI surveys. The first SwiSCI community survey was conducted between September 2011 and March 2013 (Survey 2012) [16] and the second between March 2017 and March 2018 (Survey 2017) [17]. The questionnaires are available online [18]. Three types of participants are distinguished in this study: individuals who participated in the Survey 2012 only, individuals who participated in Survey 2012 and Survey 2017, and individuals who only participated in Survey 2017. Individuals with a Swiss residency, a traumatic or non-traumatic SCI, and aged 16 years and older were eligible for the study. Individuals with congenital conditions leading to SCI, patients receiving palliative care, those with neurodegenerative diseases (e.g., Guillain-Barré syndrome) were excluded [15].
Measures
Health conditions and treatment
A list of 14 secondary HCs that are commonly diagnosed in people living with SCI were included in the present study. Methods for the collection of data on HCs and treatment have been outlined extensively in a previous SwiSCI study [2]. In brief, information on the presence and impact of HCs that are known to impact health and physical functioning were collected using the Spinal Cord Injury Secondary Conditions Scale (SCI-SCS) [19]. Self-report of impact is over the past three months and on a 4-point Likert scale, with ordinal levels 0 = “not existing or insignificant”; 1 = “mild or infrequent”, 2 = “moderate or occasional”, and 3 = “severe or chronic”. The SCI-SCS includes 16 HCs, of which 13 were included in the present study: chronic pain, spasticity, circulatory problems, bladder dysfunction, bowel dysfunction, contractures, urinary tract infections, autonomic dysreflexia, postural hypotension, injury caused by loss of sensation, respiratory problems, pressure injuries, and heterotopic ossification. Sexual dysfunction was excluded from the present study, which is concerned with a global analysis of determinants of treatment (see section “Statistical analysis” for details). Unlike treatment for other HCs, morphological and physiological differences inherently explain sex differences for treatment of sexual dysfunction and require stratified analyses for males and females. Diabetes and joint and muscle pain were also excluded, because the SwiSCI survey used a different response scale to capture severity of these HCs. Finally, an item on sleep problems, which was included based on the Brief ICF Core Set for SCI [20], completed the set of 14 HCs for this study. A binary response scale (response options “yes”/“no”) was used to capture whether participants had received treatment for a reported health condition.
Sociodemographic characteristics
The variables sex, age at questionnaire completion (in years), and nationality (Swiss vs. non-Swiss) were included as person factors. Participants received a questionnaire in one of three main languages spoken in Switzerland, i.e., German, French, or Italian, corresponding to their preference. Based on the International Spinal Cord Society (ISCoS) recommendations [21], age was categorized in the following groups: 16–30, 31–45, 46–60, 61–75, and 76 years or older.
Socioeconomic factors
Socioeconomic factors were level of education and perceived financial hardship. Education was classified according to the International Standard Classification of Education as years of formal education, combining school and vocational training. For bivariate and multivariate analyses, education was recoded re-grouped into four categories: “compulsory education ≤9 yrs.”, “vocational training 10–12 yrs.”, “secondary education 13–16 yrs.”, and “university ≥17 yrs.”. Financial hardship was operationalized with the question “Did you experience financial difficulties that restricted your everyday life over the past four weeks?” Answer categories were “not applicable”, “had no impact”, “has complicated my life somewhat”, and “has complicated my life massively”. The first two categories indicating “no financial issues” were combined to create a three-level categorical variable for use in data analysis.
SCI characteristics
Information on SCI characteristics was derived through the self-report. SCI etiology was classified as traumatic or non-traumatic. Lesion severity was grouped according to completeness and lesion level: “paraplegia, incomplete”, “paraplegia, complete”, “tetraplegia, incomplete”, “tetraplegia, complete”. Due to low cell counts, it was not possible to group time since injury according to ISCoS recommendations [21], therefore the following groups were used in analyses (in years): <6, 6–15, 16–25, and 26 years or more.
Statistical analysis
All analyses were implemented in Stata, version 14.2 for Windows (College Station, TX).
Descriptive statistics included raw numbers and percentages for differences in HCs and treatment across both survey years.
The analysis of treatment of a HC was restricted to HCs with a severity level of one to three on the 4-point Likert scale, implying that cases with severity “0” (“not existent or insignificant”) were excluded. Descriptive statistics on those receiving treatment are presented across the three levels of severity for a given HC (i.e., categories 1–3 on the Likert scale).
The variation in reporting treatment (binary response “yes”/“no”) across health conditions, persons and surveys, by predictor variables was analyzed using a multilevel mixed-effects logistic regression model with crossed random effects [22, 23]. The use of crossed random effects accounts for sources of variance at the response level (i.e., reporting treatment; n = 15,016 records in multivariable analysis) that are not hierarchically nested, including HC (n = 14), person ID (n = 1819) and survey (2012 and 2017). The following mixed-effects logistic model was implemented:
The log-odds of receiving treatment for a health condition j of person i in survey t was thus estimated, including an overall intercept α, a vector X with a set of coefficients β representing the predictor variables (fixed effects; first column in Table 2), with three crossed error terms (or random effects) θ, η, and μ (for health condition j in person i in survey t), and an overall error term ε. Laplacian approximation, equivalent to adaptive Gaussian quadrature with one integration point for each level in the model, was used to calculate log-likelihoods. This approximation provided a computationally fast and valid alternative to adaptive quadrature with multiple integration, because the main interest of the study was the fixed effects rather than the variance components, which have been reported to be more prone to bias [24]. The analysis was on cases with complete data, because mixed-effects regression modeling has been shown to be robust to the issue of missing data [25].
Results
Participant characteristics
Table 1 summarizes the characteristics of the study participants. The number of participants was 1295 in 2012 and 1117 participants in 2017. The total number of unique participants was 1819, with 593 individuals participating in both surveys. The sample is composed predominantly of Swiss males with an average age of 51 (2012) or 56 (2017) years across surveys, with the largest age group between 46–60 years. Traumatic SCI was the most commonly reported etiology (85 and 81%), and mostly incomplete paraplegia was reported (36 and 40%). Of note, more than 96% of participants had long-standing SCI (diagnosed with SCI for at least 2 years). While the majority of respondents reported that they had no financial hardship (70 and 75%), 8% in each survey indicated severe financial hardship.
In the Survey 2012 (initial N = 1549) and Survey 2017 (initial N = 1530) the number of participants thus excluded was 9.2% (n = 143) and 10.1% (n = 155), respectively. From the resulting pool of 2115 unique participants (2012: 1406; 2017: 1375) with at least one report of treatment for any given secondary health condition, 296 (14%) of participants (2012: 111 (8%); 2017: 258 (19%)) were excluded due to missing data. Missing data categories (n, %) included etiology (27, 1%), lesion characteristics (177, 6%), time since SCI (120, 4%), Nationality (47, 2%), education (90, 3%), financial hardship (87, 3%), and severity of health condition (51, 2%) (Supplementary Table S1).
Treatment for health conditions
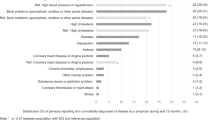
Figure 1 displays the percentage of self-reported HCs with treatment, across survey (2012 and 2017) and by the severity of each health condition (for numerical details see Supplementary Table S2). For example, in 2017, 57.0% of individuals who reported moderate chronic pain (95% CI 51.1–62.8) indicated receiving treatment, as compared to 80.1% of individuals with severe chronic pain (95% CI 75.7–84.0). The percentage of HCs for which treatment was received varied by HC and severity. For example, urinary tract infections were identified as the most reported health condition, with over 90% of individuals with severe or chronic cases receiving treatment (in both 2012 and 2017). In comparison, severe or chronic autonomic dysreflexia (50.0% in 2012, 41.3% in 2017) and sleep problems (50.3% in 2012, 49.1% in 2017) had the lowest percentage of treatment. Variation in treatment reporting between survey years was minimal for most health conditions.
Percentage of treatment as reported in Survey 2012 and Survey 2017 across health conditions of increasing levels of severity (legend bars, greyscale/color version: white/green = "mild or infrequent"; light grey/yellow = "moderate or occasional"; dark grey/red = "severe or chronic"). Health conditions are positioned in a hierarchically descending order in the percentage of treatment rate within the "severe or chronic" category. Numbers in column label "% HC" indicate the proportional distribution of reported levels of severity within HCs. For numerical detail see supplementary Table S2.
Percentage of receiving treatment
Estimates from the multilevel analysis of receiving treatment for HCs are presented in Table 2. The multivariable analysis indicated that the likelihood of treatment increased with age, e.g., individuals over the age of 76 were more than two times as likely to receive treatment than those in the youngest age group (adjusted OR 2.31, 95% CI 1.54–4.85). Swiss nationals were 22% less likely to report treatment (OR 0.78, 95% CI 0.64–0.95). Furthermore, individuals more than 5 years since injury had 17–28% reduced odds of receiving treatment compared to those <5 years since SCI diagnosis, with minimally attenuated effect sizes following full-model adjustment. The odds of receiving treatment were nearly 1.5 times higher for individuals with severe financial difficulties (OR 1.45, 95% CI 1.13–1.85). Furthermore, individuals suffering a greater number of health conditions were more likely to indicate receiving treatment. For example, individuals who reported 11–14 HCs were 32% more likely to receive treatment (OR 1.32, 95% CI 0.99–1.77) than individuals reporting 1–3 HCs. Finally, treatment was strongly associated with the severity of the reported HC, with health issues in the “severe or chronic” category showing nearly 13 times higher odds of treatment than those in the “mild or infrequent” category.
The remaining parameters in the multivariable model, including gender, etiology, lesion characteristics, and education were not clearly associated with treatment. Compared to the univariable model, unadjusted ORs for these parameters were generally attenuated or showed wider 95% confidence intervals (Table 2), thus indicating the relevance of control for confounding.
Discussion
This study found that the percentage receiving treatment for 14 common SCI-common secondary HCs varied substantially with the type and severity of the HC. Among HCs of severe or chronic nature, the reporting of treatment ranged from at least 90% for urinary tract infections to at most 50% for sleep problems or autonomic dysreflexia. The main predictors of treatment were age, years since injury, number of HCs reported, severity of a HC, and financial hardship. The impact of secondary HCs on survival and functioning in SCI is well-documented [4, 9], but the scope of literature that explores which groups are least likely to receive treatment remains limited. With the constant improvement of diagnostic methods and increase in treatment availability, at least in countries with universal health care coverage such as Switzerland, it is imperative to identify groups that are unlikely to seek or try to receive treatment.
Predictors of receiving treatment
Age was an important predictor for receiving treatment for HCs, with the report of treatment showing a steady increase from the youngest to the oldest age group. This association is most likely explained by the common finding that as individuals age, many report poorer health and are more likely to develop health conditions and complications, and therefore require more physical assistance and help with activities of daily living [26]. The association between vulnerability to loss of function, physiological changes, and duration of injury in this population, suggests the need for early preventative measures and routine assessments to detect changes. Thus, aging individuals may have a growing need for frequent access to health care and supportive services—to alleviate or minimize the effect of these changes [26].
Independent of age, and also time since injury, having a greater number of health conditions was an additional predictor for receiving treatment. The positive relationship between the number of health conditions and odds of receiving treatment may be indicative of the comprehensive clinical screening and management for those with multimorbidity [7]. Furthermore, the synergistic effects of multiple health conditions could potentially require an increasing need for treatment. In a similar vein, increasing severity of health conditions was predictive of receiving treatment, possibly suggesting that health problems with a larger burden are more likely to prompt treatment.
Financial hardship emerged in the present study as an additional potential determinant of receiving treatment. A previous SwiSCI study similarly found a higher tendency to use more health services among individuals who reported financial hardship [27]. The positive relationship may support the conclusion that access to health care is not perceived as restrained by financial resources of persons living with SCI in Switzerland. This encouraging finding may be partly explained by the Swiss health system, which involves an obligatory health insurance with a minimal out-of-pocket deductible for health care expenses. In the annually renewed policy, this out-of-pocket deductible can be increased, to receive a reduction in fixed monthly costs, thus facilitating policy-holders to attune anticipated expenses for health care to their financial situation. Yet, some financial costs that are associated with seeking treatment, such as traveling costs, are commonly not covered by the health or other insurances. Therefore, the possibility of reverse causation cannot be excluded, as out-of-pocket costs associated with seeking treatment may have affected financial hardship. It is unfortunately unfeasible to obtain representative information on the individual financial situation via self-report, as relevant questions in the SwiSCI surveys received the highest levels of non-response (up to 24%). To gain insight on financial issues, administrative data on the socioeconomic situation of persons living with SCI would need to be collected, which is also challenging in view of the Swiss ethics and privacy regulations.
Strengths and limitations
It is important to discuss some of the major strengths and limitations of the self-report of health conditions and treatment. The questionnaire survey is, compared to other methods, a practical and relatively cheap way to obtain health information from a large group of individuals. We thus characterize the ability of this study to capture data regarding treatment of secondary HCs from a representative cohort living with SCI in Switzerland as a notable strength [17]. Such health information assists in identifying relevant areas for improvement of physical health and health care, to increase participation, QoL, and life expectancy in this population.
Yet, the self-report of health information also comes with major limitations that may curtail the utility of the evidence in guiding policy interventions to improve health care in SCI. One limitation is the inherent risk of various forms of measurement and reporting bias [28]. The reliability of the reports in the present study may have suffered from recall bias. While the SCI-SCS appears a reliable and valid scale for the reporting of health conditions over the past 3 months [19], we currently lack formal support for the validity of the revised scale as well as for the treatment scale. This study is also potentially subject to sampling bias, as participation in the survey as well as selective non-response regarding questions on HCs and treatment may have been affected by individual health and health care provisioning. Information bias is a further potential limitation, as reports of health conditions and their treatment may systematically deviate from the clinical care data, for instance in relation to health literacy. Also, health problems of a potentially insidious nature, such as urinary tract infections and sleep problems (e.g., sleep-disordered breathing), may not be apparent to the participant prior to a clinical diagnosis and associated therapy. Finally, predictor variables may also be subject to reporting bias which can lead to spurious associations with self-reports of treatment in regression analysis. Financial hardship represents such a predictor variable in the present study, as the positive association with treatment may reflect, albeit to an unknown degree, a shared effect of psychological personal factors. Following, between-person differences in psychological personal factors may misguide statistical inference and has moreover been related to the so-called generalizability crisis in the human sciences [29].
In light of these strengths and limitations, the main value of the self-report health evidence provided by the present study, is to indicate areas of concern that necessitate further research. A critical next step in advancing policy-relevant research is the validation of the self-report using clinical data. Without such validation it remains speculative whether HCs with poor report of treatment reflect areas for improvement of clinical management or rather areas that require improvement of health literacy regarding HCs and treatments. The self-report data are of great value in the focusing of these efforts. For instance, the low percentage of treatment reported in face of highest severity level for autonomic dysreflexia, sleep, and injury caused by loss of sensation needs further investigation.
Conclusion
This community survey of persons living with SCI suggests that several HCs may frequently remain untreated. While the percentage of treatment was consistently highest for the highest severity level across HCs, some HCs (e.g., autonomic dysreflexia and sleep problems) showed worryingly low treatment frequency across all levels of severity. Furthermore, a large number of HCs with a mild or moderate level of severity are not being treated, which may enable their evolution to more severe conditions or complications that ultimately demand greater treatment efforts. To define the best strategy for improvement, an evaluation of clinical records could provide crucial information and insight and thus inform future interventions.
Data archiving
Owing to our commitment to SwiSCI study participants and their privacy, datasets generated during the current study are not made publicly available but can be provided by the SwiSCI Study Center based on reasonable request (contact@swisci.ch).
References
Dryden DM, Saunders LD, Rowe BH, May LA, Yiannakoulias N, et al. Utilization of health services following spinal cord injury: a 6-year follow-up study. Spinal Cord. 2004;42:513–25.
Brinkhof MW, Al-Khodairy A, Eriks-Hoogland I, Fekete C, Hinrichs T, et al. Health conditions in people with spinal cord injury: Contemporary evidence from a population-based community survey in Switzerland. J Rehabil Med. 2016;48:197–209.
van der Meer P, Post MW, van Leeuwen CM, van Kuppevelt HJ, Smit CA, et al. Impact of health problems secondary to SCI one and five years after first inpatient rehabilitation. Spinal Cord. 2017;55:98–104.
Krause JS, Saunders LL. Health, secondary conditions, and life expectancy after spinal cord injury. Arch Phys Med Rehabil. 2011;92:1770–5.
Chamberlain JD, Gmunder HP, Hug K, Jordan X, Moser A, et al. Differential survival after traumatic spinal cord injury: evidence from a multi-center longitudinal cohort study in Switzerland. Spinal Cord. 2018;56:920–30.
Buzzell A, Chamberlain JD, Gmünder HP, Hug K, Jordan X, et al. Survival after non-traumatic spinal cord injury: evidence from a population-based rehabilitation cohort in Switzerland. Spinal Cord. 2018;57:267–75.
Noonan VK, Fallah N, Park SE, Dumont FS, Leblond J, et al. Health care utilization in persons with traumatic spinal cord injury: the importance of multimorbidity and the impact on patient outcomes. Top Spinal Cord Inj Rehabil. 2014;20:289–301.
Penchansky R, Thomas JW. The concept of access: definition and relationship to consumer satisfaction. Med Care. 1981;19:127–40.
Krause JS, Carter RE. Risk of mortality after spinal cord injury: relationship with social support, education, and income. Spinal Cord. 2009;47:592–6.
Krueger H, Noonan VK, Trenaman LM, Joshi P, Rivers CS. The economic burden of traumatic spinal cord injury in Canada. Chronic Dis Inj Can. 2013;33:113–22.
Lahelma E, Martikainen P, Laaksonen M, Aittomaki A. Pathways between socioeconomic determinants of health. J Epidemiol Community Health. 2004;58:327–32.
Muller R, Landmann G, Bechir M, Hinrichs T, Arnet U, et al. Chronic pain, depression and quality of life in individuals with spinal cord injury: Mediating role of participation. J Rehabil Med. 2017;49:489–96.
Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–63.
Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84.
Post MW, Brinkhof MW, von Elm E, Boldt C, Brach M, et al. Design of the Swiss spinal cord injury cohort study. Am J Phys Med Rehabil. 2011;90:5–16.
Brinkhof MW, Fekete C, Chamberlain JD, Post MW, Gemperli A. Swiss national community survey on functioning after spinal cord injury: protocol, characteristics of participants and determinants of non-response. J Rehabil Med. 2016;48:120–30.
Gross-Hemmi MH, Gemperli A, Fekete C, Brach M, Schwegler U, et al. Methodology and study population of the second Swiss national community survey of functioning after spinal cord injury. Spinal Cord. 2020; https://doi.org/10.1038/s41393-020-00584-3.
Swiss Spinal Cord Injury Cohort Study (SwiSCI). Survey 2017 [Internet]. Nottwil: Schweizer Paraplegiker-Forschung; 2018; https://www.swisci.ch/en/research-projects-home/study-design/community-survey.
Kalpakjian CZ, Scelza WM, Forchheimer MB, Toussaint LL. Preliminary reliability and validity of a Spinal Cord Injury Secondary Conditions Scale. J Spinal Cord Med. 2007;30:131–9.
Cieza A, Kirchberger I, Biering-Sorensen F, Baumberger M, Charlifue S, et al. ICF core sets for individuals with spinal cord injury in the long-term context. Spinal Cord. 2010;48:305–12.
DeVivo MJ, Biering-Sorensen F, New P, Chen Y. Standardization of data analysis and reporting of results from the international spinal cord injury core data set. Spinal Cord. 2011;49:596–9.
Baayen RH. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2007;59:390–412.
Yu H-T. Applying linear mixed effects models with crossed random effects to psycholinguistic data: multilevel specification and model selection. Quant Meth Psych. 2015;11:78–88.
Pinheiro JC, Chao EC. Efficient laplacian and adaptive gaussian quadrature algorithms for multilevel generalized linear mixed models. J Comput Graph Stat. 2006;15:58–81.
Twisk J. Missing data in logitudinal studies. In: applied longitudinal data analysis for epidemiology: a practical guide. Cambridge: Cambridge University Press; 2013. pp 212–36.
Thompson L. Functional changes in persons aging with spinal cord injury. Assist Technol. 1999;11:123–9.
Ronca E, Scheel-Sailer A, Koch HG, Essig S, Brach M, et al. Satisfaction with access and quality of healthcare services for people with spinal cord injury living in the community. J Spinal Cord Med. 2018;43:111–21.
Wetzel E, Böhnke JR, Brown A. Response biases. In: Long FTL, Bartram D, Cheung FM, Geisinger KF, Iliescu D, editors. The ITC International Handbook of Testing and Assessment. UK: Oxford University Press Oxford; 2016. pp 349–63.
Yarkoni T. The generalizability crisis. 2019. https://doi.org/10.31234/osf.io/jqw35.
Acknowledgements
We thank the SwiSCI Steering Committee with its members Xavier Jordan, Fabienne Reynard (Clinique Romande de Réadaptation, Sion); Michael Baumberger, Hans Peter Gmünder (Swiss Paraplegic Center, Nottwil); Armin Curt, Martin Schubert (University Clinic Balgrist, Zürich); Margret Hund-Georgiadis, Kerstin Hug (REHAB Basel, Basel); Laurent Prince (Swiss Paraplegic Association, Nottwil); Heidi Hanselmann (Swiss Paraplegic Foundation, Nottwil); Daniel Joggi (Representative of persons with SCI); Nadja Münzel (Parahelp, Nottwil); Mirjam Brach, Gerold Stucki (Swiss Paraplegic Research, Nottwil); Armin Gemperli (SwiSCI Coordination Group at Swiss Paraplegic Research, Nottwil).
Funding
SwiSCI is hosted and funded by Swiss Paraplegic Research.
Author information
Authors and Affiliations
Contributions
KC and MWGB were responsible for designing and planning the conceptual framework of the present study. AB, KC, and MWGB were responsible for data analysis, interpretation, and development of the present manuscript. IEH, KH, XJ, and MS provided clinical support, feedback on the manuscript, and support in data collection at their respective clinics. JC contributed critical feedback on the manuscript. All authors participated in a critical revision of this manuscript for important intellectual content and ultimately approved of the final version based on this submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval for Survey 2012 was granted by the principal ethics committee on research involving humans of the Canton of Lucerne (KEK Luzern, internal application 11042, approved 28.06.2011) and subsequently endorsed by the additional involved cantonal ethics committees of Cantons Basel-Stadt (EK Basel, internal application 306/11, approved 06.09.2011) and Valais (CCVEM Sion, internal application CCVEM042/11, approved 06.12.2011). Ethical approval for Survey 2017 was granted by the leading ethical institution Ethikkommision Nordwest-und Zentralschweiz (EKNZ, Project-ID: 11042 PB_2016-02608, approved Dec 2016). We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Buzzell, A., Camargos, K.C., Chamberlain, J.D. et al. Self-reports of treatment for secondary health conditions: results from a longitudinal community survey in spinal cord injury. Spinal Cord 59, 389–397 (2021). https://doi.org/10.1038/s41393-020-00596-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-020-00596-z