Abstract
Background
Necrotizing enterocolitis (NEC) is a severe gastrointestinal inflammatory disease in neonates. Fucosyltransferase 2 (Fut2) regulates intestinal epithelial cell fucosylation. In this study, we aimed to investigate butyrate-mediated upregulation of Fut2 expression and the underlying mechanisms.
Methods
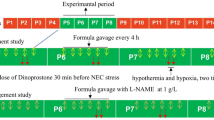
In vivo and in vitro models were established. SP600125 was used to inhibit the MEK4-JNK pathway, and anisomycin was used to activate the MEK4-JNK pathway. Fut2, occludin, and ZO-1 expressions were assessed. Furthermore, intestinal permeability was analyzed by FITC-Dextran. The expression of proteins in the MEK-4-JNK pathway was examined by western blotting.
Results
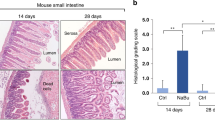
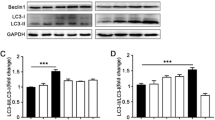
In vivo, the addition of exogenous butyrate notably upregulated Fut2, occludin, and ZO-1 expressions and reduced intestinal permeability in mice with NEC. Butyrate may increase the phosphorylation of MEK4, JNK, and c-jun, which are key components of the MEK4-JNK pathway. Additionally, SP600125 inhibited their phosphorylation, which was reversed by anisomycin treatment. In vitro, butyrate substantially increased occludin and ZO-1 expressions. Butyrate considerably increased Fut2 expression and markedly upregulated p-MEK4, p-JNK, and p-c-jun expressions. SP600125 administration decreased their expressions, while anisomycin administration increased their expressions.
Conclusion
Butyrate upregulated Fut2 expression via activation of the MEK4-JNK pathway, improved intestinal barrier integrity, and protected neonatal mice from NEC.
Impact
-
We found that exogenous butyrate could improve intestinal barrier integrity and protect against NEC in neonatal mice.
-
Our data showed that exogenous butyrate supplementation upregulated Fut2 expression by activating the MEK4-JNK pathway.
-
Our study provides novel insights into the pathogenesis of NEC, thereby laying an experimental foundation for future clinical research on the use of butyrate in NEC treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated for this study are available from the corresponding author upon request.
References
Nolan, L. S., Wynn, J. L. & Good, M. Exploring Clinically-relevant Experimental Models Of Neonatal Shock And Necrotizing Enterocolitis. Shock 53, 596–604 (2020).
Meister, A. L., Doheny, K. K. & Travagli, R. A. Necrotizing Enterocolitis: It’s not all in the gut. Exp. Biol. Med. 245, 85–95 (2020).
Duess, J. W. et al. Necrotizing Enterocolitis, GUT MICROBES, AND Sepsis. Gut Microbes 15, 2221470 (2023).
Kovler, M. L. et al. Toll-like Receptor 4-Mediated Enteric Glia loss is critical for the development of necrotizing Enterocolitis. Sci. Transl. Med. 13, eabg3459 (2021).
Hold, G. L., Schwiertz, A., Aminov, R. I., Blaut, M. & Flint, H. J. Oligonucleotide probes that detect quantitatively significant groups of butyrate-producing bacteria in human feces. Appl Environ. Microbiol. 69, 4320–4324 (2003).
Sun, M., Wu, W., Liu, Z. & Cong, Y. Microbiota metabolite short chain fatty acids, Gpcr, and inflammatory bowel diseases. J. Gastroenterol. 52, 1–8 (2017).
Liu, X. C. et al. Gut microbiota and short-chain fatty acids may be new biomarkers for predicting neonatal necrotizing Enterocolitis: A pilot study. Front Microbiol 13, 969656 (2022).
Sun, Q. et al. Sodium Butyrate alleviates intestinal inflammation in mice with necrotizing Enterocolitis. Mediators Inflamm. 2021, 6259381 (2021).
Li, Y. et al. Exogenous L-Fucose protects the intestinal mucosal barrier depending on upregulation of Fut2-mediated fucosylation of intestinal epithelial cells. FASEB J. 35, e21699 (2021).
Pickard, J. M. et al. Rapid Fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature 514, 638–641 (2014).
Pickard, J. M. & Chervonsky, A. V. Intestinal Fucose as a mediator of host-microbe symbiosis. J. Immunol. 194, 5588–5593 (2015).
Goto, Y. et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345, 1254009 (2014).
She, X. et al. The decrease of fucosylation in intestinal epithelium is related to the development of Necrotizing Enterocolitis. Mol. Immunol. 131, 23–32 (2021).
Bourgonje, A. R. et al. The Effect of phenotype and genotype on the plasma proteome in patients with inflammatory bowel disease. J. Crohns Colitis 16, 414–429 (2022).
Park, D. et al. Enterocyte glycosylation is responsive to changes in extracellular conditions: implications for membrane functions. Glycobiology 27, 847–860 (2017).
Dang, G., Wu, W., Zhang, H. & Everaert, N. A new paradigm for a new simple chemical: butyrate & immune regulation. Food Funct. 12, 12181–12193 (2021).
Gasaly, N., Hermoso, M. A. & Gotteland, M. Butyrate and the fine-tuning of colonic homeostasis: implication for inflammatory bowel diseases. Int J. Mol. Sci. 22, 3061 (2021).
Feng, X., Du, C. & Wang, C. Structural characterization of polysaccharide from yellow sweet potato and ameliorates Dss-induced mice colitis by active Gpr41/Mek/Erk 1/2 Signaling Pathway. Int J. Biol. Macromol. 192, 278–288 (2021).
Meng, D. et al. Bacterial symbionts induce a Fut2-dependent fucosylated niche on colonic epithelium via Erk and Jnk signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G780–G787 (2007).
Ji, Y. C. et al. Exogenous Autoinducer-2 rescues intestinal dysbiosis and intestinal inflammation in a neonatal mouse necrotizing Enterocolitis model. Front Cell Infect. Microbiol 11, 694395 (2021).
Su, Y. et al. Astragaloside Iv ameliorates sepsis-induced myocardial dysfunction by regulating Nox4/Jnk/Bax Pathway. Life Sci. 310, 121123 (2022).
Tang, Z. et al. In vivo toxicological evaluation of Anisomycin. Toxicol. Lett. 208, 1–11 (2012).
Yu, X., Radulescu, A., Zorko, N. & Besner, G. E. Heparin-binding Egf-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology 137, 221–230 (2009).
Zhang, X. et al. Β-Glucan protects against necrotizing Enterocolitis in mice by inhibiting intestinal inflammation, improving the gut barrier, and modulating gut microbiota. J. Transl. Med. 21, 14 (2023).
Shi, J., Zhao, X. H., Fu, Y. & Lametsch, R. Transglutaminase-mediated caseinate oligochitosan glycation enhances the effect of caseinate hydrolysate to ameliorate the Lps-induced damage on the intestinal barrier function in Iec-6 Cells. J. Agric Food Chem. 69, 8787–8796 (2021).
Zhao, X. et al. Probiotics mixture reinforces barrier function to ameliorate necrotizing enterocolitis by regulating Pxr-Jnk pathway. Cell Biosci. 11, 20 (2021).
van Gorp, C. et al. Antenatal Ureaplasma infection induces ovine small intestinal goblet cell defects: a strong link with Nec pathology. Tissue Barriers 11, 2158016 (2023).
Koh, A., De Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016).
Flint, H. J., Scott, K. P., Louis, P. & Duncan, S. H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 9, 577–589 (2012).
Wong, J. M., de Souza, R., Kendall, C. W., Emam, A. & Jenkins, D. J. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 40, 235–243 (2006).
Machiels, K. et al. A decrease of the butyrate-producing species Roseburia Hominis and Faecalibacterium Prausnitzii defines dysbiosis in patients with ulcerative Colitis. Gut 63, 1275–1283 (2014).
Offermanns, S. Hydroxy-carboxylic acid receptor actions in metabolism. Trends Endocrinol. Metab. 28, 227–236 (2017).
Huang, S. et al. Anti-inflammatory actions of Acetate, Propionate, and Butyrate in fetal mouse Jejunum cultures ex vivo and immature small intestinal cells in vitro. Food Sci. Nutr. 10, 564–576 (2022).
Liu, J. et al. Beneficial effects of butyrate in intestinal injury. J. Pediatr. Surg. 55, 1088–1093 (2020).
Goto, Y., Uematsu, S. & Kiyono, H. Epithelial glycosylation in gut homeostasis and inflammation. Nat. Immunol. 17, 1244–1251 (2016).
Wang, Z. et al. Fut2-dependent Fucosylation of Hyou1 protects intestinal stem cells against inflammatory injury by regulating unfolded protein response. Redox Biol. 60, 102618 (2023).
Tang, X. et al. Gut microbiota-mediated Lysophosphatidylcholine generation promotes colitis in intestinal epithelium-specific Fut2 deficiency. J. Biomed. Sci. 28, 20 (2021).
Wang, Y. et al. Fucosylation deficiency in mice leads to colitis and adenocarcinoma. Gastroenterology 152, 193–205.e110 (2017).
Pham, T. A. et al. Epithelial Il-22ra1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe 16, 504–516 (2014).
Lei, C. et al. Enteric Vip-producing neurons maintain gut microbiota homeostasis through regulating Epithelium Fucosylation. Cell Host Microbe 30, 1417–1434.e1418 (2022).
Kononova, S., Litvinova, E., Vakhitov, T., Skalinskaya, M. & Sitkin, S. Acceptive Immunity: The role of fucosylated glycans in human host-microbiome interactions. Int J. Mol. Sci. 22, 3854 (2021).
Yang, T. et al. Protective roles of Sodium Butyrate in Lipopolysaccharide-induced bovine ruminal epithelial cells by activating G protein-coupled receptors 41. Front Nutr. 9, 842634 (2022).
Xu, H. et al. Maternal antibiotic exposure Enhances Ilc2 activation in neonates via downregulation of Ifn1 signaling. Nat. Commun. 14, 8332 (2023).
Katzengruber, L., Sander, P. & Laufer, S. Mkk4 inhibitors-recent development status and therapeutic potential. Int J. Mol. Sci. 24, 7495 (2023).
Bavaria, M. N., Jin, S., Ray, R. M. & Johnson, L. R. The mechanism by which Mek/Erk regulates Jnk and P38 activity in polyamine depleted Iec-6 cells during apostosis. Apoptosis 19, 467–479 (2014).
Yuan, J., Che, S., Zhang, L. & Ruan, Z. Reparative effects of ethanol-induced intestinal barrier injury by Flavonoid Luteolin Via Mapk/Nf-Κb/Mlck and Nrf2 signaling pathways. J. Agric Food Chem. 69, 4101–4110 (2021).
Shi, L. et al. Oral administration of Lentinus Edodes Β-Glucans Ameliorates Dss-Induced ulcerative colitis in mice via Mapk-Elk-1 and Mapk-Pparγ pathways. Food Funct. 7, 4614–4627 (2016).
Zhang, M. et al. Lncrna Cbr3-As1 regulates of breast cancer drug sensitivity as a competing endogenous Rna through the Jnk1/Mek4-Mediated Mapk signal Pathway. J. Exp. Clin. Cancer Res 40, 41 (2021).
Funding
This work was supported by the Natural Science Foundation of Chongqing municipality (cstc2021jcyj-msxmX0063), the Joint Medical Research Project of Chongqing Science and Technology Commission (2021MSXM206,2022MSXM039), and the Project of Nestle Health Science (KY210030, China).
Author information
Authors and Affiliations
Contributions
All the authors made substantial contributions to the study. D.-D.Z. and Z.-X.H. led the experiments. D.-D.Z., Z.-X.H., X.-C.L. and X.-P.D. conceived and conducted the experiments and analyzed the data. L.L., Q.A., Y.H., L.B. and L.-Q.L. provided resources and equipment. D.-D.Z. wrote the original draft. X.-C.L. and X.-P.D. reviewed and edited the article. L.B. and L.-Q.L. contributed to the critical revision and final approval of the manuscript. All authors contributed to the article and approved the submitted version. D.-D.Z. and Z.-X. Huang contributed equally to this work and share first authorship.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, DD., Huang, ZX., Liu, XC. et al. Butyrate protects the intestinal barrier by upregulating Fut2 expression via MEK4-JNK signaling pathway activation. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03419-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03419-6
This article is cited by
-
Guardian of the gut: butyrate-regulated FUT2 protects against experimental NEC
Pediatric Research (2024)