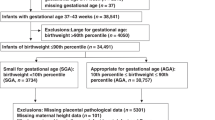
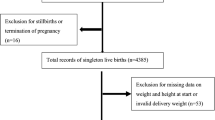
Abstract
Background
Maternal physical condition (reflected by maternal body mass index (BMI) at delivery) and pregnancy complications influence neonatal health outcomes. High BMI during pregnancy increases various health problems’ risks, but studies about the synthesized effect of these factors on fetal growth, are scarce.
Methods
The retrospective cohort study was conducted in Zhejiang Province, China from 1 January 2019 to 31 December 2021. The associations between complications and small-for-gestational-age (SGA) and large-for-gestational-age (LGA) were measured by the Fine-Gray model and subgroup analysis. Effect modification and interaction analyses were conducted to explore BMI’s modification effect and complications’ interaction.
Results
Several complications increased the risk for SGA and LGA, some significance varied in different subgroups. There was a positive effect modification of gestational diabetes mellitus (GDM) across BMI strata on LGA (relative excess risk due to interaction (RERI) [95% CI] = 0.57 [0.09,1.04]). Several pairwise complications’ interactions were synergistic (e.g., pregestational diabetes and intraamniotic infection for SGA (ratio of ORs [95% CI] = 8.50 [1.74,41.37]), pregestational diabetes and assisted reproductive technology (ART) for LGA (ratio of ORs [95% CI] = 2.71 [1.11,6.62])), one was antagonistic (placental problems and ART for LGA (ratio of ORs [95% CI] = 0.58 [0.35,0.96])).
Conclusions
High-BMI positively modified the risk of GDM on LGA. Many interactions existed when two specific pregnancy complications occurred simultaneously.
Impact
-
This is the largest retrospective study covering more than 10 pregnancy complications to date in this aspect.
-
High-BMI (BMI > 28 kg/m2) positively modifies the risk of GDM on LGA. Many pregnancy complications influence the risk of SGA and LGA, with several interactions that may create a “syndrome” effect.
-
Pregnant women with different BMIs should consider the additional risks caused by pregnancy complications for their heterogeneous effects on abnormal fetal growth.
-
Measures should be taken to prevent the occurrence of other exposure factors in the “syndrome”. This study may aid in developing a new strategy for improving neonatal outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data available on request due to restrictions, e.g., privacy or ethical restrictions. The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to participant privacy.
References
Opondo, C. et al. Variations in neonatal mortality, infant mortality, preterm birth and birth weight in England and Wales according to ethnicity and maternal country or region of birth: an analysis of Linked National Data from 2006 to 2012. J. Epidemiol. Community Health 74, 336–345 (2020).
Za’im Sahul Hameed, M., Sutan, R., Mahdy, Z. A., Tamil, A. M. & Sulong, S. Maternal variables as determinant of fetal growth: study protocol on customized fetal growth charts in Malaysia (Grow-My). Front. Med. (Lausanne) 8, 592462 (2021).
Chung, J. H., Boscardin, W. J., Garite, T. J., Lagrew, D. C. & Porto, M. Ethnic differences in birth weight by gestational age: at least a partial explanation for the hispanic epidemiologic paradox? Am. J. Obstet. Gynecol. 189, 1058–1062 (2003).
Capital Institute of Pediatrics, T. C. S. G. O. N. C. O. t. P. G. & Development of, C. [a National Survey on Physical Growth and Development of Children under Seven Years of Age in Nine Cities of China in 2015]. Zhonghua Er Ke Za Zhi 56, 192–199 (2018).
Mikolajczyk, R. T. et al. A global reference for fetal-weight and birthweight percentiles. Lancet 377, 1855–1861 (2011).
Cunningham F. G. et al. Williams Obstetrics. 23 edn. (Mcgraw–Hill, 2010).
Lee, A. C. et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob. Health 1, e26–e36 (2013).
Harvey, L., van Elburg, R. & van der Beek, E. M. Macrosomia and large for gestational age in Asia: one size does not fit all. J. Obstet. Gynaecol. Res. 47, 1929–1945 (2021).
Pilliod, R. A., Cheng, Y. W., Snowden, J. M., Doss, A. E. & Caughey, A. B. The risk of intrauterine fetal death in the small-for-gestational-age fetus. Am. J. Obstet. Gynecol. 207, 318.e311–316 (2012).
Williams, R. L. et al. Fetal growth and perinatal viability in California. Obstet. Gynecol. 59, 624–632 (1982).
Ray, J. G., Park, A. L. & Fell, D. B. Mortality in infants affected by preterm birth and severe small-for-gestational age birth weight. Pediatrics 140, e20171881 (2017).
Esakoff, T. F., Cheng, Y. W., Sparks, T. N. & Caughey, A. B. The association between birthweight 4000 G or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am. J. Obstet. Gynecol. 200, 672.e671–674 (2009).
Baer, R. J. et al. Population-based risks of mortality and preterm morbidity by gestational age and birth weight. J. Perinatol. 36, 1008–1013 (2016).
Bizerea-Moga, T. O. et al. Extreme birth weight and metabolic syndrome in children. Nutrients 14, 204 (2022).
Evagelidou, E. N. et al. Prothrombotic state, cardiovascular, and metabolic syndrome risk factors in prepubertal children born large for gestational age. Diabetes Care 33, 2468–2470 (2010).
Sacchi, C. et al. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: a systematic review and meta-analysis. JAMA Pediatr. 174, 772–781 (2020).
Li, F. et al. Adverse pregnancy outcomes among mothers with hypertensive disorders in pregnancy: a meta-analysis of cohort studies. Pregnancy Hypertens. 24, 107–117 (2021).
Martineau, M. G. et al. The metabolic profile of intrahepatic cholestasis of pregnancy is associated with impaired glucose tolerance, dyslipidemia, and increased fetal growth. Diabetes Care 38, 243–248 (2015).
Gullo, G. et al. Neonatal outcomes and long-term follow-up of children born from frozen embryo, a narrative review of latest research findings. Medicina (Kaunas.) 58, 1218 (2022).
Di Tommaso, M. et al. Influence of assisted reproductive technologies on maternal and neonatal outcomes in early preterm deliveries. J. Gynecol. Obstet. Hum. Reprod. 48, 845–848 (2019).
Goldstein, R. F. et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA 317, 2207–2225 (2017).
Pileggi, V. N. et al. Maternal BMI at the time of birth and selected risk factors associated with severe neonatal outcomes: a secondary analysis of the who better outcomes in labour difficulty (Bold) project. Br. J. Nutr. 124, 1086–1092 (2020).
Fakhraei, R. et al. Predictors of adverse pregnancy outcomes in pregnant women living with obesity: a systematic review. Int. J. Environ. Res. Public Health 19, 2063 (2022).
Gao, M. et al. The cut-off points of body mass index and waist circumference for predicting metabolic risk factors in Chinese adults. Zhonghua Liu Xing Bing Xue Za Zhi 40, 1533–1540 (2019).
Group, H. S. C. R. Hyperglycaemia and adverse pregnancy outcome (hapo) study: associations with maternal body mass index. BJOG 117, 575–584 (2010).
Atalah, E., Castillo, C., Castro, R. & Aldea, A. [Proposal of a new standard for the nutritional assessment of pregnant women]. Rev. Med Chil. 125, 1429–1436 (1997).
Noor, F. et al. Body mass index and serum thyroid stimulating hormone in third trimester of pregnancy. Mymensingh Med. J. 30, 69–72 (2021).
Ortega-Senovilla, H., van Poppel, M. N. M., Desoye, G. & Herrera, E. Angiopoietin-like protein 4 (Angptl4) is related to gestational weight gain in pregnant women with obesity. Sci. Rep. 8, 12428 (2018).
Zhu, L. et al. Chinese neonatal birth weight curve for different gestational age. Zhonghua Er Ke Za Zhi 53, 97–103 (2015).
Higgins, R. D. et al. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet. Gynecol. 127, 426–436 (2016).
Nolan, E. K. & Chen, H. Y. A comparison of the Cox model to the fine-gray model for survival analyses of Re-fracture rates. Arch. Osteoporos. 15, 86 (2020).
Knol, M. J. & VanderWeele, T. J. Recommendations for presenting analyses of effect modification and interaction. Int. J. Epidemiol. 41, 514–520 (2012).
Nie, Z. Q. et al. Application of SAS macro to evaluated multiplicative and additive interaction in logistic and cox regression in clinical practices. Zhonghua Liu Xing Bing. Xue Za Zhi 37, 737–740 (2016).
Ahlbom, A. Modern epidemiology, 4th edition. Tl Lash, Tj Vanderweele, S Haneuse, Kj Rothman. Wolters Kluwer, 2021. Eur. J. Epidemiol. 36, 767–768 (2021).
VanderWeele, T. J. Principles of confounder selection. Eur. J. Epidemiol. 34, 211–219 (2019).
Schulz, L. C., Schlitt, J. M., Caesar, G. & Pennington, K. A. Leptin and the placental response to maternal food restriction during early pregnancy in mice. Biol. Reprod. 87, 120 (2012).
Sacks, D. A. Determinants of fetal growth. Curr. Diab. Rep. 4, 281–287 (2004).
Zhang, J. et al. Early prediction of preeclampsia and small-for-gestational-age via multi-marker model in Chinese pregnancies: a prospective screening study. BMC Pregnancy Childbirth 19, 304 (2019).
Bernardes, T. P. et al. Early and late onset pre-eclampsia and small for gestational age risk in subsequent pregnancies. PLoS One 15, e0230483 (2020).
Cohen, J. M. et al. Maternal antioxidant levels in pregnancy and risk of preeclampsia and small for gestational age birth: a systematic review and meta-analysis. PLoS One 10, e0135192 (2015).
Gotsch, F. et al. Preeclampsia and small-for-gestational age are associated with decreased concentrations of a factor involved in angiogenesis: soluble tie-2. J. Matern. Fetal Neonatal Med. 21, 389–402 (2008).
Longo, S., Borghesi, A., Tzialla, C. & Stronati, M. Iugr and infections. Early Hum. Dev. 90, S42–S44 (2014).
Vrachnis, N., Botsis, D. & Iliodromiti, Z. The fetus that is small for gestational age. Ann. N. Y. Acad. Sci. 1092, 304–309 (2006).
Wang, M., Wang, X., Chen, Z. & Zhang, F. Gestational hypertensive disease and small for gestational age infants in twin pregnancy: a systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 48, 2677–2685 (2022).
Tsujimoto, Y. et al. Association of low birthweight and premature birth with hypertensive disorders in pregnancy: a systematic review and meta-analysis. J. Hypertens. 40, 205–212 (2022).
Siargkas, A. et al. The impact of lateral placenta on preeclampsia and small for gestational age neonates: a systematic review and meta-analysis. J. Perinat. Med. 51, 468–476 (2022).
Räisänen, S., Kancherla, V., Kramer, M. R., Gissler, M. & Heinonen, S. Placenta previa and the risk of delivering a small-for-gestational-age newborn. Obstet. Gynecol. 124, 285–291 (2014).
Weiner, E. et al. The effect of placenta previa on fetal growth and pregnancy outcome, in correlation with placental pathology. J. Perinatol. 36, 1073–1078 (2016).
Hu, H. et al. The mediating role of gestational diabetes mellitus in the associations of maternal prepregnancy body mass index with neonatal birth weight. J. Diabetes 14, 26–33 (2022).
Yue, S. et al. Clinical consequences of gestational diabetes mellitus and maternal obesity as defined by Asian BMI thresholds in Viet Nam: a prospective, hospital-based, cohort study. BMC Pregnancy Childbirth 22, 195 (2022).
Yarde, F. et al. Prenatal famine, birthweight, reproductive performance and age at menopause: The Dutch Hunger Winter Families Study. Hum. Reprod. 28, 3328–3336 (2013).
Takaya, J. Calcium-deficiency during pregnancy affects insulin resistance in offspring. Int. J. Mol. Sci. 22, 7008 (2021).
Wei, Y. et al. Preconception diabetes mellitus and adverse pregnancy outcomes in over 6.4 million women: a population-based cohort study in China. PLoS Med. 16, e1002926 (2019).
Bettencourt-Silva, R. et al. Small for Gestational Age and Gestational Diabetes—Should We Be More Permissive?. <https://www.endocrine-abstracts.org/ea/0049/ea0049ep564> (2017 May).
Mills, H. L. et al. The effect of a lifestyle intervention in obese pregnant women on gestational metabolic profiles: findings from the UK pregnancies better eating and activity trial (Upbeat) randomised controlled trial. BMC Med. 17, 15 (2019).
Jacob, S. et al. Targeted metabolomics demonstrates distinct and overlapping maternal metabolites associated with BMI, glucose, and insulin sensitivity during pregnancy across four ancestry groups. Diabetes Care 40, 911–919 (2017).
Kelly, R. S. et al. Integration of metabolomic and transcriptomic networks in pregnant women reveals biological pathways and predictive signatures associated with preeclampsia. Metabolomics 13, 7 (2017).
Taylor, K. et al. Differences in pregnancy metabolic profiles and their determinants between White European and South Asian women: findings from the born in Bradford Cohort. Metabolites 9, 190 (2019).
Catalano, P. M. et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 35, 780–786 (2012).
Alfadhli, E. M. Maternal obesity influences birth weight more than gestational diabetes author. BMC Pregnancy Childbirth 21, 111 (2021).
Zymperdikas, C. F., Zymperdikas, V. F., Mastorakos, G., Grimbizis, G. & Goulis, D. G. Assisted reproduction technology outcomes in women with infertility and preexisting diabetes mellitus: a systematic review. Hormones (Athens) 21, 23–31 (2022).
Lascar, N. et al. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 6, 69–80 (2018).
Cai, S. et al. Nutritional status impacts epigenetic regulation in early embryo development: a scoping review. Adv. Nutr. 12, 1877–1892 (2021).
Pirtea, P., Ziegler, D. & Ayoubi, J. M. Children born from frozen embryo transfers: is there a difference? Fertil. Steril. 114, 502–503 (2020).
Sheiner, E. et al. Nuchal cord is not associated with adverse perinatal outcome. Arch. Gynecol. Obstet. 274, 81–83 (2006).
Osak, R., Webster, K. M., Bocking, A. D., Campbell, M. K. & Richardson, B. S. Nuchal cord evident at birth impacts on fetal size relative to that of the placenta. Early Hum. Dev. 49, 193–202 (1997).
Hoh, J. K., Sung, Y. M. & Park, M. I. Fetal heart rate parameters and perinatal outcomes in fetuses with nuchal cords. J. Obstet. Gynaecol. Res. 38, 358–363 (2012).
Wu, G., Bazer, F. W., Cudd, T. A., Meininger, C. J. & Spencer, T. E. Maternal nutrition and fetal development. J. Nutr. 134, 2169–2172 (2004).
Denison, F. C. et al. Care of women with obesity in pregnancy: green-top guideline no. 72. BJOG 126, e62–e106 (2019).
Acknowledgements
We thank all participants, coaches, research midwives/nurses, and health professionals who collaborated in the recruitment and procedures. In particular, we would like to thank The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University for their valuable data and sufficient support. We would like to express our gratitude to Springer Nature (https://www.springernature.com) for the expert linguistic services provided.
Funding
This research was partially supported by the Medical Science and Technology Project of Zhejiang Province, China (No. 2023KY149, 2020KY185), and the Public Welfare Science and Technology Plan Project of Wenzhou, China (No. Y20200087). The funders had no role in any aspect of the study beyond funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, F.W., Z.Y., and P.W.; Data curation, W.L.; Formal analysis, P.W. and Y.H.; Funding acquisition, F.W.; Investigation, P.W., Z.Y., W.L.; Methodology, F.W., Z.Y., and P.W.; Project administration, F.W.; Resources, F.W.; Software, Z.Y., P.W., and Y.H.; Supervision, F.W.; Validation, F.W., Z.Y., P.W., W.L.; Visualization, Z.Y., P.W., Y.H., F.D., X.L.; Writing—original draft, F.W., Z.Y., P.W., W.L.; Writing—review & editing, F.W., Z.Y., P.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (2021-K-318-02). This study was granted exemption for ethics approval by the Ethics Committee of The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (2021-K-318-02). This study did not involve human intervention.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, P., Yu, Z., Hu, Y. et al. BMI modifies the effect of pregnancy complications on risk of small- or large-for-gestational-age newborns. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03298-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03298-x