Abstract
Trabectedin, approved for the treatment of soft tissue sarcoma (STS), interferes with cell division and genetic transcription processes. Due to its strong anti-tumor activity in only certain histotypes, several studies on trabectedin combinations are currently ongoing to improve its efficacy. In this study, we aimed to investigate novel potential therapeutic strategies to enhance the anti-tumor effect of trabectedin using integrated in silico, in vitro, and in vivo approaches. For in silico analysis, we screened two public datasets, GSEA M5190 and TCGA SARC. Fibrosarcoma, leiomyosarcoma, dedifferentiated, and myxoid liposarcoma cell lines were used for in vitro studies. For in vivo experiments, fibrosarcoma orthotopic murine model was developed. In silico analysis identified Glo1 as the only druggable target upregulated after trabectedin treatment and correlated with poor prognosis. The specific Glo1 inhibitor, S-p-bromobenzylglutathione cyclopentyl diester (BBGC), increased trabectedin cytotoxicity in STS cells, and restored drug sensitivity in myxoid liposarcoma cells resistant to trabectedin. Moreover, the combined treatment with BBGC and trabectedin had a synergistic antitumor effect in vivo without any additional toxicity to mice. Based on these results, we believe that BBGC warrants further investigation to evaluate its potential clinical use in combination with trabectedin.
Similar content being viewed by others
Introduction
Soft tissue sarcoma (STS) is a rare, heterogeneous group of distinct mesenchymal malignancies, accounting for 1–2% of all cancers in adults [1, 2]. Surgical resection is the primary curative treatment for patients with localized disease. However, STS frequently recurs as locally advanced or metastatic disease, which is not amenable to surgical resection, necessitating systemic therapies [3,4,5]. The chemotherapy agents most widely used in sarcomas include anthracyclines (doxorubicin), ifosfamide, gemcitabine, and trabectedin [6,7,8].
Trabectedin was the first marine-derived antineoplastic drug approved in 2007 for the treatment of patients with advanced STS after the failure of doxorubicin [9,10,11]. Trabectedin is a DNA-binding agent with a complex pleiotropic mechanism of action. It selectively targets monocytes and tumor-associated macrophages, downregulating the production of inflammatory mediators in the tumor microenvironment, along with conventional direct cytotoxic effects [12,13,14,15,16]. Specifically, trabectedin interacts with the minor groove of the DNA, interferes with gene transcription and DNA repair machinery, leading to DNA damage accumulation and cell cycle perturbation [12,13,14]. Additionally, trabectedin has a specific mechanism against some translocation-related sarcomas, such as myxoid liposarcoma, where it displaces the oncogenic fusion protein FUS-CHOP from its target promoter [17, 18].
Numerous studies have consistently shown that trabectedin confers long-lasting objective remissions and tumor control in about 20% of various STS types [4, 9, 19,20,21,22]. However, limited information is currently available on the mechanisms leading to secondary resistance. Thus, identifying novel therapeutic strategies to increase trabectedin efficacy is an unmet clinical need.
In this study, starting from an in silico approach, we investigated novel potential therapeutic strategies to enhance the anti-tumor effect of trabectedin using in vitro and in vivo models of STS.
Results
In silico analysis identifies Glyoxalase 1 (Glo1) as “druggable” target
To identify genes modulated by trabectedin exposure that are associated with clinical outcomes in STS patients, we performed a bioinformatic analysis combining two public datasets: GSEA M5190 [23] and TCGA SARC [24]. The GSEA M5190 dataset included cDNA microarray data from nine STS cell lines with varying sensitivity to trabectedin, collected both at baseline and after drug treatment. The TCGA SARC dataset included RNA-Seq data along with survival data from 206 STS patients.
We considered two classes of potential therapeutic targets: genes downregulated after trabectedin exposure (GSEA M5190) and upregulated in patients with good prognosis (TCGA SARC), which could potentially be targeted by agonist compounds (Fig. 1, Gene Set A); and genes overexpressed after trabectedin treatment (GSEA M5190) and upregulated in patients with poor prognosis (TCGA SARC), which could be targeted by specific inhibitors (Fig. 1, Gene Set B). We focused on glyoxalase-1 (GLO1) from Gene Set B, as it was the only gene encoding a “druggable” protein with a commercially available compound suitable for in vivo use.
BBGC enhanced trabectedin anti-tumor activity in STS in vitro models
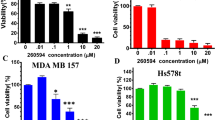
Firstly, we confirmed Glo1 expression in SK-LMS-1 leiomyosarcoma, SW872 liposarcoma, and HT1080 fibrosarcoma cell lines at both gene and protein levels (Supplementary Fig. 1). In contrast to trabectedin, that exhibited dose-dependent cytotoxicity, BBGC treatment at increasing doses (up to the limits of solubility) did not affect cell viability in any of the cell lines, as the half-maximal inhibitory concentration (IC50) was not reached (Fig. 2A–C, left-middle panel). Intriguingly, combination drug analysis revealed a strong synergistic activity between BBGC and trabectedin in all three cell lines (Synergy scores: SK-LMS-1: 38.058; SW872: 35.428; HT1080: 30.395) (Fig. 2A–C, right panel).
Cell viability percentage in SK-LMS-1 (A) SW872 (B), and HT1080 (C) cells treated with trabectedin or BBGC (left and middle panels). HSA synergy score of BBGC and trabectedin combined treatment in SK-LMS-1 (A), SW872 (B), and HT1080 (C) cells (right panel). The square in the plot indicates the highest synergy area.
We then analyzed the potential synergistic effect of BBGC with two other chemotherapy drugs, doxorubicin, and gemcitabine, which are widely used in STS treatment. Similar to trabectedin, both doxorubicin and gemcitabine exerted dose-dependent cytotoxic activity in all three STS cell lines (Supplementary Fig. 3). However, no consistent synergy was observed with BBGC, except for a weak synergy with doxorubicin in HT1080 cells (Synergy score: 14.027) (Fig. 3A–C). These data suggest that BBGC specifically enhances the anti-tumor effect of trabectedin, while it does not enhance the cytotoxicity of doxorubicin and gemcitabine (Fig. 3D).
HSA synergy score of BBGC and doxorubicin or gemcitabine combined treatment in SK-LMS-1 (A) SW872 (B), and HT1080 (C) cells. The square in the plot indicates the highest synergy area. D Histogram summarizes the HSA synergy scores of SK-LMS-1, SW872, and HT1080 cells treated with BBGC in combination with trabectedin or doxorubicin or gemcitabine. The dashed line indicates the score above which the treatment is synergistic. *p < 0.05; **p < 0.01.
BBGC restores trabectedin sensitivity in resistant myxoid liposarcoma cells
To investigate the effectiveness of BBGC in trabectedin-resistant cells, we used the myxoid liposarcoma cell lines 402-91 WT (sensitive to trabectedin) and 402-91 ET (resistant to trabectedin). We observed higher Glo1 gene and protein levels in 402-91 ET cells compared to 402-91 WT cells (Supplementary Fig. 3A). Data confirmed that 402-91 ET cells were less sensitive to trabectedin treatment compared to 402-91 WT cells (Fig. 4A). Similar to other STS cell lines, BBGC treatment did not affect the viability of either myxoid liposarcoma cell lines (Fig. 4B).
Percentage of cell viability of 402-91 ET and 402-91 WT cells treated with trabectedin (A) or BBGC (B). C HSA synergy score of BBGC and trabectedin combined treatment in 402-91 ET and 402-91 WT. The square in the plot indicates the highest synergy area (left panel). Histogram summarizes the HSA synergy scores of 402-91 ET and 402-91 WT and the dashed line indicates the score above which the treatment is synergistic (right panel). D The histogram shows IC50 value of trabectedin, alone or in combination with BBGC in 402-91 ET. The dashed line indicates IC50 trabectedin value in 402-91 WT. *p < 0.05; **p < 0.01.
Drug combination analysis revealed a synergistic effect of BBGC and trabectedin in 402-91 ET cells compared to 402-91 WT cells (Synergy score: 402-91 ET: 37.218; 402-91 WT: 13.662) (Fig. 4C). Notably, BBGC treatment completely restored sensitivity to trabectedin in 402-91 ET cells, reducing the IC50 from 29.1 nM to 5.2 nM, similar to the trabectedin IC50 of 402-91 WT cells (IC50: 7.0 nM) (Fig. 4D).
Next, we investigated whether BBGC increased DNA damage of trabectedin. We identified the optimal trabectedin concentration (2 nM) that did not induce significant cytotoxicity, allowing us to better analyze DNA damage. Data showed that BBGC augmented DNA damage induced by trabectedin in both 402-91 WT and ET cells (Fig. 5A), confirming its ability to enhance trabectedin’s anti-tumor effect. Moreover, apoptosis analysis revealed that BBGC, in combination with trabectedin, significantly increased the percentage of annexin V-positive cells in the 402.91 ET line. In contrast, in the 402.91 WT cells, the combination treatment did not further increase the percentage of apoptotic cells, as trabectedin alone already induced apoptosis in nearly 90% of the cells (Fig. 5B).
Additionally, Glo1 silencing did not make 402-91 ET cells sensitive to trabectedin, as the effect is similar to 402-91 ET and 402-91 ET scramble cells (Fig. 6A), suggesting a compensation mechanism for MG detoxification.
A Cell viability percentage in 402.91 ET, ET lipofectamine and ET siGlo1 cells after trabectedin treatment. B Cell viability percentage in 402.91 ET cells treated with MG (left). HSA synergy score of MG and trabectedin combined treatment (right). The square in the plot indicates the highest synergy area.
To confirm the pivotal role of MG in enhancing trabectedin activity, we treated 402-91 ET cells with MG alone or in combination with trabectedin. Data showed a strong synergistic effect of the two compounds (Synergy score: 25.328), similar to that observed with BBGC plus trabectedin (Fig. 6B).
BBGC enhanced trabectedin anti-tumor activity in mice
To test the potential synergy between BBGC and trabectedin, we used an orthotopic fibrosarcoma mouse model. One week after HT1080 cell implantation, tumor-bearing mice were randomized into four groups: control, BBGC (200 mg/kg), trabectedin (0.100 mg/kg), and BBGC (200 mg/kg) plus trabectedin (0.100 mg/kg). Mice treated with trabectedin showed a significant reduction in tumor volume, while the BBGC-treated group exhibited tumor growth similar to the control group (Fig. 7A). The combination of BBGC and trabectedin significantly enhanced the anti-tumor effect compared to trabectedin alone (p < 0.01, from day 2 to 7; p < 0.01, from day 11 to 16) (Fig. 7A). The combined treatment did not alter the body weight of the mice, suggesting that BBGC does not induce any additional toxic effects (Fig. 7B).
Glo1 expression is associated with trabectedin response in patients affected by myxoid liposarcoma
We analyzed Glo1 expression in nine matched myxoid liposarcoma tumor tissue samples obtained from patients before and after trabectedin treatment. Then, patients were categorized into “high” and “low” Glo1 expression groups based on Glo1 staining in tissue samples collected before trabectedin treatment (score 3: “high”; score 1 or 2: “low”) (Fig. 8A). Immunohistochemical analysis showed greater tumor regression, in terms of radiological response and fibrosis percentage, in patients with low Glo1 expression (p < 0.01) (Fig. 8B), suggesting that Glo1 could be a predictive biomarker of trabectedin response.
Discussion
While trabectedin is approved as a single agent for the treatment of all STS histotypes, it exhibits strong antitumor activity primarily in certain types, such as liposarcoma and leiomyosarcoma [25]. Preclinical and clinical evidence suggests a synergistic effect when trabectedin is combined with other agents, although this is associated with increased toxicity [25,26,27,28,29,30].
The aim of this study was to identify therapeutic strategies to enhance the antitumor effect of trabectedin using integrated in silico, in vitro, and in vivo approaches. Bioinformatics analysis identified Glo1 as a “druggable” target upregulated after trabectedin treatment and associated with poor prognosis in STS patients. Our retrospective analysis in a cohort of patients with myxoid liposarcoma showed that high Glo1 levels at baseline were associated with a lower response to trabectedin. Although based on a small number of cases, these results suggest a potential role of Glo1 in predicting trabectedin response in myxoid liposarcoma patients.
Glo1 is a key enzyme in the glyoxalase system involved in the detoxification of MG preventing its abnormal accumulation, which causes DNA strand breaks, mutagenesis, and cytotoxicity [31, 32]. Increased expression and activity of Glo1 have been found in various cancer types and are closely correlated with tumor aggressiveness and metastasis [33]. Cell-permeable Glo1 inhibitor prodrugs have been developed to induce severe dicarbonyl stress as a potential treatment for cancer, particularly for high Glo1-expressing, multidrug-resistant tumors. The prototype Glo1 inhibitor is BBGC, which has shown antitumor activity particularly in lung cancer and glioblastoma in vitro and in tumor-bearing mice in vivo models [32,33,34,35,36,37].
In our STS cell lines (leiomyosarcoma, fibrosarcoma, myxoid, and dedifferentiated liposarcoma cells), BBGC treatment did not affect cell proliferation when administered alone, but it was effective in increasing trabectedin activity. Conversely, BBGC did not exert any synergistic effect in combination with other chemotherapeutic agents, such as doxorubicin and gemcitabine, in the same cell lines. These data highlighted that the BBGC synergy was restricted to the combination with trabectedin, clearly suggesting a drug-specific enhancing effect in the four histotypes analyzed. Our in vitro results were confirmed in an orthotopic murine fibrosarcoma model, where the combination of BBGC and trabectedin significantly reduced tumor growth compared to trabectedin alone. Moreover, the combination of BBGC with trabectedin did not induce additional adverse effects, as assessed by monitoring body weight evolution, suggesting that the combined treatment could be effective without increasing toxicity.
This strong synergy could be related to the unique mechanism of action of trabectedin [38,39,40]. Specifically, trabectedin has higher cytotoxicity on cells in the G1 phase than other chemotherapy agents that act on proliferative cells [41,42,43,44,45,46,47]. Since MG accumulation causes cell cycle arrest in the G1 phase, we hypothesize that an increase in MG levels after BBGC treatment could induce a cellular quiescent state, making tumor cells more sensitive to trabectedin. In support of our hypothesis, we observed a significant arrest of the cell cycle at the G0-G1 phase following BBGC treatment (Supplementary Fig. 4).
Data available in the literature show that increased expression of Glo1 has a protective effect on tumor cells, contributing to their survival and multidrug resistance, and eventually leading to chemotherapy failure [31, 35]. Therefore, we investigated whether BBGC could enhance the antitumor effect of trabectedin in a well-characterized model of trabectedin resistance. Data showed that resistant cells expressed high levels of Glo1, and BBGC treatment was able to overcome the resistance in these cells. In contrast to BBGC treatment, Glo1 silencing in resistant cells did not resensitize them to trabectedin. The explanation for this differential effect may lie in the activation of compensatory detoxification mechanisms for MG in these cells. Indeed, differently from pharmacological inhibition by BBGC, Glo1 silenced models in several studies did not lead to elevated MG levels, indicating alternative detoxification routes during Glo1 loss [48]. Besides the glyoxalase system, other enzymes are also known to detoxify MG, such as aldo-keto reductases (AKRs), aldehyde dehydrogenases (ALDHs), and the Parkinson-associated protein DJ-1 [49, 50]. Moreover, it was recently discovered that the ketone body acetoacetate can scavenge endogenous MG in a nonenzymatic aldol reaction [51].
Since the intracellular measurement of free MG is known to be scarcely reliable with the currently available methods [52,53,54,55], we employed the strategy of administering MG at known concentrations (within a physiological range) in combination with trabectedin to verify their synergy. Data showed a synergy between MG and trabectedin in resistant cells similar to that observed with BBGC, suggesting that MG detoxification may play a key role in the establishment of resistance to trabectedin.
Some potential limitations should be considered. The study relies heavily on bioinformatic analysis using public datasets that may not fully capture the complexity and variability of real-world patient populations. The use of a single in vitro model of trabectedin resistance, albeit the most characterized, and the lack of in vivo validation represent a limitation of the study. Unfortunately, our trabectedin resistance model had several features that precluded its use in vivo evaluation: the engraftment rate was very low (about 10%), the tumor growth was very heterogeneous, and produced hind back paralysis in some animals. Despite HT1080 cells can not be considered a validated model for trabectedin resistance, they exhibit an IC50 of 30.2 nM, comparable to the 29.1 nM observed in 402.91 ET cells.
Finally, the mechanism by which BBGC potentiates the effect of trabectedin has not been fully elucidated, therefore, further analyses are necessary to deepen our understanding of this specific synergy.
Despite these limitations, the study identifies BBGC as a promising therapeutic approach for enhancing the antitumor effect of trabectedin in STS and overcoming trabectedin resistance.
Methods
Cell lines and drugs
SK-LMS-1 leiomyosarcoma, SW872 liposarcoma, and HT1080 fibrosarcoma cell lines were gifted by PharmaMar. The 402.91 WT and 402.91 ET cells were gifted by the D’Incalci research laboratory [56]. SK-LMS-1, SW872, and HT1080 cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Corning, New York, NY, USA), while the base medium for 402.91 WT and 402.91 ET was RPMI1640 (Corning, New York, NY, USA). All media were supplemented with 10% bovine serum (Sigma Aldrich, St. Louis, Missouri, USA), 2 mM L-glutamine (EuroClone, Milano, Italy), and 1% penicillin/streptomycin (EuroClone, Milano, Italy).
Drugs were used at the following concentrations: trabectedin (PharmaMar, Madrid, Spain) 6 nM, 12 nM, 25 nM, 50 nM; doxorubicin (Selleck Chemicals GmbH, Planegg, Germany) 0.5 μM, 1 μM, 2 μM, 4 μM; gemcitabine (Selleck Chemicals GmbH, Planegg, Germany) 0.1 μM, 0.2 μM, 0.5 μM, 1 μM; S-p-bromobenzyl-glutathione cyclopentyl diester (BBGC) (MedChemExpress, Monmouth Junction, NJ, USA) 2.5 μM, 5 μM, 10 μM, 20 μM; methylglyoxal (MG) (Sigma Aldrich, St. Louis, MO, USA) 0.25 μM, 0.5 μM, 1 μM, 2 μM.
Cell viability
STS cells were treated for 1 h with BBGC or chemotherapy agents (trabectedin, doxorubicin, gemcitabine), alone or in combination with BBGC, and then the drugs were washed out. Moreover, 402.91 ET cells were treated with MG plus trabectedin with the same treatment schedule. After 4 days, cell viability was analyzed using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay (Sigma Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. The synergistic effect of trabectedin/doxorubicin/gemcitabine and BBGC was calculated using SynergyFinder 2.0 software. The combination treatment was considered synergistic with a “synergy score” >10.
Flow cytometric γ-H2AX detection
STS cells were treated for 1 h with BBGC or chemotherapy agents (trabectedin, doxorubicin, gemcitabine), alone or in combination with BBGC, and then the drugs were washed out. After 24 h, DNA damage was analyzed by detecting phospho-histone H2AX (γ-H2AX) expression by flow cytometry. Briefly, cells were fixed and permeabilized with Foxp3/Transcription Factor Staining Buffer Set (eBioscience-Thermo Fisher Scientific, Waltham, MA, USA) for intracellular staining with anti-phospho-histone H2AX (γ-H2AX)-APC (clone 2F3) (Biolegend, San Diego, CA, USA). Moreover, for 402.91 ET cells, we evaluated apoptosis after 1 h of treatment with BBGC and trabectedin, alone or in combination, using the anti-human annexin V antibody (Becton Dickinson, New York, USA).
Samples were analyzed using a CytoFlex instrument (Beckman Coulter, Brea, CA, USA) and with FlowJo Software, v.10.8 (FlowJo, Ashland, OR, USA).
Gene silencing
Glo1 silencing was performed using the following 3 selected validated siRNA (Ambion, Life Technologies):
1) Sequence 5′- 3′: GAGUCAAAUUUGUGAAGAAtt
2) Sequence 5′- 3′: GGCUUAUGAGGAUAAAAAUtt
3) Sequence 5′- 3′: ACUGGAUUUUUAUACUAGAtt
Briefly, 402.91 ET cells were seeded to be 60–80% confluent at the time of transfection. The siRNA-lipidic complexes were prepared and added to the cells according to manufacturer’s instructions. 402.91 ET cells were incubated, for 48 h, with Glo1 siRNAs (siGlo1 402.91 ET cells) and then analyzed for Glo1 gene and protein expression by Real Time PCR and Western Blot, respectively (Supplementary Fig. 3B). siGlo1 ET cells were treated with trabectedin to analyze cell viability.
Immunohistochemical analysis
Representative tumor blocks (4 mm thickness) were obtained from nine patients with advanced localized myxoid liposarcoma enrolled in a phase II clinical trial of neoadjuvant trabectedin [57]. Immunohistochemistry (IHC) was performed by the streptavidin-biotin method using the recombinant rabbit monoclonal antibody against GLO1 protein (JU44-1, Invitrogen). The H-Score system was applied for GLO1 IHC evaluation [58], calculated by a semi-quantitative assessment of the intensity of staining (graded as: 0, non-staining; 1, weak; 2, medium; or 3, strong) and the percentage of positive cells. Immunostaining was assessed by two independent pathologists blinded to clinical characteristics and outcomes.
In vivo experiments
Female athymic nu/nu mice between 4 and 6 weeks of age were purchased from Envigo. Animals were housed in individually ventilated cages (Sealsafe® Plus, Techniplast) on a 12-h light-dark cycle at 21–23 °C and 40–60% humidity. Mice were allowed free access to irradiated standard rodent diet (Tecklad 2914C) and sterilized water. Animals were acclimated for 5 days prior to tumor implantation. To generate the fibrosarcoma model, animals were orthotopically implanted into the gastrocnemius muscle by intramuscular injection using a 26G needle and a 1 cc syringe with 1 × 107 HT1080 cells suspended in medium without serum or antibiotics. Tumor-bearing animals were randomly allocated into experimental groups, and treatment administration was initiated on Day 0. BBGC (200 mg/kg) treatment was intraperitoneally administered on days 0 and 8, and trabectedin (0.100 mg/kg) was intravenously injected on days 0 and 7. Tumor volume and body weight were measured twice a week from day 0 to day 18. Treatment tolerability was assessed by monitoring body weight evolution, clinical signs, and evidence of local damage at the injection site. Control and BBGC-treated mice were humanely sacrificed on day 14, while mice treated with trabectedin, alone or in combination with BBGC, were sacrificed between days 16 and 18. All experimental procedures were reviewed and approved by regional Institutional Animal Care and Use Committees (IACUC) and performed in accordance with national and international laws and policies (EU Directive 2010/63/EU).
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8.2.1 (San Diego, CA, USA). All in vitro experimental procedures consisted of at least three independent biological repeats. Comparisons were performed using a two-sided unpaired Student’s t-test or ANOVA test followed by Tukey’s test.
Data availability
The data generated in this study are available within the article and its supplementary data files.
References
Mastrangelo G, Coindre JM, Ducimetière F, Dei Tos AP, Fadda E, Blay JY, et al. Incidence of soft tissue sarcoma and beyond: a population-based prospective study in 3 European regions. Cancer. 2012;118:5339–48.
Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv51–iv67.
Grünewald TG, Alonso M, Avnet S, Banito A, Burdach S, Cidre-Aranaz F, et al. Sarcoma treatment in the era of molecular medicine. EMBO Mol Med. 2020;12:e11131.
Samuels BL, Chawla S, Patel S, von Mehren M, Hamm J, Kaiser PE, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol. 2013;24:1703–9.
Linch M, Miah AB, Thway K, Judson IR, Benson C. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol. 2014;11:187–202.
Verschoor AJ, Litière S, Marréaud S, Judson I, Toulmonde M, Wardelmann E, et al. Survival of soft tissue sarcoma patients after completing six cycles of first-line anthracycline containing treatment: an EORTC-STBSG database study. Clin Sarcoma Res. 2020;10:18.
Ferraresi V, Ciccarese M, Cercato MC, Nuzzo C, Zeuli M, Di Filippo F, et al. Gemcitabine at fixed dose-rate in patients with advanced soft-tissue sarcomas: a mono-institutional phase II study. Cancer Chemother Pharmacol. 2008;63:149–55.
Gordon EM, Sankhala KK, Chawla N, Chawla SP. Trabectedin for soft tissue sarcoma: current status and future perspectives. Adv Ther. 2016;33:1055–71.
Demetri GD, Chawla SP, von Mehren M, Ritch P, Baker LH, Blay JY, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol. 2009;27:4188–96.
Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34:786–93.
Patel S, von Mehren M, Reed DR, Kaiser P, Charlson J, Ryan CW, et al. Overall survival and histology-specific subgroup analyses from a phase 3, randomized controlled study of trabectedin or dacarbazine in patients with advanced liposarcoma or leiomyosarcoma. Cancer. 2019;125:2610–20.
D’Incalci M, Badri N, Galmarini CM, Allavena P. Trabectedin, a drug acting on both cancer cells and the tumour microenvironment. Br J Cancer. 2014;111:646–50.
D’Incalci M. Trabectedin mechanism of action: what’s new? Future Oncol. 2013;9:5–10.
D’Incalci M, Galmarini CM. A review of trabectedin (ET-743): a unique mechanism of action. Mol Cancer Ther. 2010;9:2157–63.
Vincenzi B, Napolitano A, Frezza AM, Schiavon G, Santini D, Tonini G. Wide-spectrum characterization of trabectedin: biology, clinical activity and future perspectives. Pharmacogenomics. 2010;11:865–78.
Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–62.
Di Giandomenico S, Frapolli R, Bello E, Uboldi S, Licandro SA, Marchini S, et al. Mode of action of trabectedin in myxoid liposarcomas. Oncogene. 2014;33:5201–10.
Forni C, Minuzzo M, Virdis E, Tamborini E, Simone M, Tavecchio M, et al. Trabectedin (ET-743) promotes differentiation in myxoid liposarcoma tumors. Mol Cancer Ther. 2009;8:449–57.
Blay JY, Italiano A, Ray-Coquard I, Le Cesne A, Duffaud F, Rios M, et al. Long-term outcome and effect of maintenance therapy in patients with advanced sarcoma treated with trabectedin: an analysis of 181 patients of the French ATU compassionate use program. BMC Cancer. 2013;13:64.
Le Cesne A, Ray-Coquard I, Duffaud F, Chevreau C, Penel N, Bui Nguyen B, et al. Trabectedin in patients with advanced soft tissue sarcoma: a retrospective national analysis of the French Sarcoma Group. Eur J Cancer. 2015;51:742–50.
Buonadonna A, Benson C, Casanova J, Kasper B, López Pousa A, Mazzeo F, et al. A noninterventional, multicenter, prospective phase IV study of trabectedin in patients with advanced soft tissue sarcoma. Anticancer Drugs. 2017;28:1157–65.
Vincenzi B, Napolitano A, Comandone A, Sanfilippo R, Celant S, Olimpieri PP, et al. Trabectedin use in soft-tissue sarcoma patients in a real-world setting: Data from an Italian national drug-access registry. Int J Cancer. 2023;152:761–8.
Cancer Genome Atlas Research Network.Electronic address: elizabeth.demicco@sinaihealthsystem.ca; Cancer Genome Atlas Research Network Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. 2017;171:950–65.e28.
Anaya J. OncoLnc: linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Computer Science. 2016;2:e67.
Le Cesne A, Martín-Broto J, Grignani G. A review of the efficacy of trabectedin as second-line treatment of advanced soft tissue sarcoma. Future Oncol. 2022;8:5–11.
Takahashi N, Li WW, Banerjee D, Scotto KW, Bertino JR. Sequence-dependent enhancement of cytotoxicity produced by ecteinascidin 743 (ET-743) with doxorubicin or paclitaxel in soft tissue sarcoma cells. Clin Cancer Res. 2001;7:3251–7.
Meco D, Colombo T, Ubezio P, Zucchetti M, Zaffaroni M, Riccardi A, et al. Effective combination of ET-743 and doxorubicin in sarcoma: preclinical studies. Cancer Chemother Pharmacol. 2003;52:131–8.
Blay JY, von Mehren M, Samuels BL, Fanucchi MP, Ray-Coquard I, Buckley B, et al. Phase I combination study of trabectedin and doxorubicin in patients with soft-tissue sarcoma. Clin Cancer Res. 2008;14:6656–62.
Sessa C, Perotti A, Noberasco C, De Braud F, Gallerani E, Cresta S, et al. Phase I clinical and pharmacokinetic study of trabectedin and doxorubicin in advanced soft tissue sarcoma and breast cancer. Eur J Cancer. 2009;45:1153–61.
Sütcüoğlu O, Özet A, Yazıcı O, Özdemir N. Doxorubicin plus trabectedin for metastatic or unresectable leiomyosarcoma. Lancet Oncol. 2022;23:e444.
Alhujaily M, Abbas H, Xue M, de la Fuente A, Rabbani N, Thornalley PJ. Studies of glyoxalase 1-linked multidrug resistance reveal glycolysis-derived reactive metabolite, methylglyoxal, is a common contributor in cancer chemotherapy targeting the spliceosome. Front Oncol. 2021;11:748698.
Sakamoto H, Mashima T, Sato S, Hashimoto Y, Yamori T, Tsuruo T. Selective activation of apoptosis program by S-p-bromobenzylglutathione cyclopentyl diester in glyoxalase I-overexpressing human lung cancer cells. Clin Cancer Res. 2001;7:2513–8.
Kim JY, Jung JH, Lee SJ, Han SS, Hong SH. Glyoxalase 1 as a therapeutic target in cancer and cancer stem cells. Mol Cells. 2022;45:869–76.
Thornalley PJ, Edwards LG, Kang Y, Wyatt C, Davies N, Ladan MJ, et al. Antitumour activity of S-p-bromobenzylglutathione cyclopentyl diester in vitro and in vivo. Inhibition of glyoxalase I and induction of apoptosis. Biochem Pharmacol. 1996;51:1365–72.
Thornalley PJ, Waris S, Fleming T, Santarius T, Larkin SJ, Winklhofer-Roob BM, et al. Imidazopurinones are markers of physiological genomic damage linked to DNA instability and glyoxalase 1-associated tumour multidrug resistance. Nucleic Acids Res. 2010;38:5432–42.
Jandial R, Neman J, Lim PP, Tamae D, Kowolik CM, Wuenschell GE, et al. Inhibition of GLO1 in glioblastoma multiforme increases DNA-AGEs, stimulates RAGE expression, and inhibits brain tumor growth in orthotopic mouse models. Int J Mol Sci. 2018;19:406.
Rabbani N, Thornalley PJ. Emerging glycation-based therapeutics-glyoxalase 1 inducers and glyoxalase 1 inhibitors. Int J Mol Sci. 2022;23:2453.
Erba E, Bergamaschi D, Bassano L, Damia G, Ronzoni S, Faircloth GT, et al. Ecteinascidin-743 (ET-743), a natural marine compound, with a unique mechanism of action. Eur J Cancer. 2001;37:97–105.
Martinez EJ, Corey EJ, Owa T. Antitumor activity- and gene expression-based profiling of ecteinascidin Et 743 and phthalascidin Pt 650. Chem Biol. 2001;8:1151–60.
Takebayashi Y, Goldwasser F, Urasaki Y, Kohlhagen G, Pommier Y. Ecteinascidin 743 induces protein-linked DNA breaks in human colon carcinoma HCT116 cells and is cytotoxic independently of topoisomerase I expression. Clin Cancer Res. 2001;7:185–91.
Rabbani N, Xue M, Weickert MO, Thornalley PJ. Multiple roles of glyoxalase 1-mediated suppression of methylglyoxal glycation in cancer biology—involvement in tumour suppression, tumour growth, multidrug resistance and target for chemotherapy. Semin Cancer Biol. 2018;49:83–93.
Antognelli C, Mezzasoma L, Fettucciari K, Mearini E, Talesa VN. Role of glyoxalase I in the proliferation and apoptosis control of human LNCaP and PC3 prostate cancer cells. Prostate. 2013;73:121–32.
Antognelli C, Mezzasoma L, Fettucciari K, Talesa VN. A novel mechanism of methylglyoxal cytotoxicity in prostate cancer cells. Int J Biochem Cell Biol. 2013;45:836–44.
Sakamoto H, Mashima T, Kizaki A, Dan S, Hashimoto Y, Naito M, et al. Glyoxalase I is involved in resistance of human leukemia cells to antitumor agent-induced apoptosis. Blood. 2000;95:3214–8.
Kang Y, Edwards LG, Thornalley PJ. Effect of methylglyoxal on human leukaemia 60 cell growth: modification of DNA G1 growth arrest and induction of apoptosis. Leuk Res. 1996;20:397–405.
Ghosh S, Pal A, Ray M. Methylglyoxal in combination with 5-Fluorouracil elicits improved chemosensitivity in breast cancer through apoptosis and cell cycle inhibition. Biomed Pharmacother. 2019;114:108855.
He T, Zhou H, Li C, Chen Y, Chen X, Li C, et al. Methylglyoxal suppresses human colon cancer cell lines and tumor growth in a mouse model by impairing glycolytic metabolism of cancer cells associated with down-regulation of c-Myc expression. Cancer Biol Ther. 2016;17:955–65.
Kold-Christensen R, Johannsen M. Methylglyoxal metabolism and aging-related disease: moving from correlation toward causation. Trends Endocrinol Metab. 2020;31:81–92.
Morgenstern J, Fleming T, Schumacher D, Eckstein V, Freichel M, Herzig S, et al. Loss of glyoxalase 1 induces compensatory mechanism to achieve dicarbonyl detoxification in mammalian Schwann cells. J Biol Chem. 2017;292:3224–38.
Lodd E, Wiggenhauser LM, Morgenstern J, Fleming TH, Poschet G, Büttner M, et al. The combination of loss of glyoxalase1 and obesity results in hyperglycemia. JCI Insight. 2019;20:e126154.
Salomón T, Sibbersen C, Hansen J, Britz D, Svart MV, Voss TS, et al. Ketone body acetoacetate buffers methylglyoxal via a non-enzymatic conversion during diabetic and dietary ketosis. Cell Chem Biol. 2017;24:935–43.e7.
Rabbani N, Thornalley PJ. Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nat Protoc. 2014;9:1969–79.
Scheijen JL, Schalkwijk CG. Quantification of glyoxal, methylglyoxal and 3-deoxyglucosone in blood and plasma by ultra performance liquid chromatography tandem mass spectrometry: evaluation of blood specimen. Clin Chem Lab Med. 2014;52:85–91.
Beisswenger PJ, Howell SK, Touchette AD, Lal S, Szwergold BS. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes. 1999;48:198–202.
Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344:109–16.
Uboldi S, Bernasconi S, Romano M, Marchini S, Fuso Nerini I, Damia G, et al. Characterization of a new trabectedin-resistant myxoid liposarcoma cell line that shows collateral sensitivity to methylating agents. Int J Cancer. 2012;131:59–69.
Gronchi A, Bui BN, Bonvalot S, Pilotti S, Ferrari S, Hohenberger P, et al. Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol. 2012;23:771–6.
Jensen K, Krusenstjerna-Hafstrøm R, Lohse J, Petersen KH, Derand H. A novel quantitative immunohistochemistry method for precise protein measurements directly in formalin-fixed, paraffin-embedded specimens: analytical performance measuring HER2. Mod Pathol. 2017;30:180–93.
Acknowledgements
This work was supported by Pharmamar. We thank Prof. D’Incanci for providing us with the 402.91 cell lines.
Author information
Authors and Affiliations
Contributions
FP and BV: Conceptualization; SSi, MI, MJG, CC, PA, LN, GP: Methodology; FP: Formal analysis; SSi, MI, MJG, CC, SC, LN, GS: Investigation; PA, MG, AR, EP, SSt, MB, AG, MS, APDT: Resources; MI, SSi, FP: Writing—Original Draft; FP, SSi, MI, MJG, CC, PA, SC, AN, AC, AM, LN, GS, GP, MG, AR, EP, SSt, MB, AG, SV, MS, APDT, GT, BV: Writing—Review & Editing; SSi, MI, LN, MJG: Visualization; PA, GP, AG, APDT, GT: Supervision; BV: Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
BV and GT provided advisory activity for PharmaMar. MJG, CC, and PA work at Research Department of PharmaMar.
Ethics approval and consent to participate
All experimental procedures were reviewed and approved by an Internal Ethical Committee (reference 06/2015), by the Órgano Evaluador de Proyectos de la Universidad Miguel Hernandez de Elche, an authorized organ by the competent authority (Comunidad Autónoma de Madrid, CAM) to evaluate experimental procedures in animals (reference UMH.VR.MGN.01.16) and, authorized by CAM (regional Institutional Animal Care and Use Committee), reference PROEX 046/16. All experimental procedures were performed in accordance with national and international laws and policies: Spanish directive RD 53/2013 and EU Directive 2010/63/EU.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pantano, F., Simonetti, S., Iuliani, M. et al. S-p-bromobenzyl-glutathione cyclopentyl diester (BBGC) as novel therapeutic strategy to enhance trabectedin anti-tumor effect in soft tissue sarcoma preclinical models. Oncogene (2024). https://doi.org/10.1038/s41388-024-03143-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41388-024-03143-9