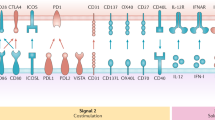
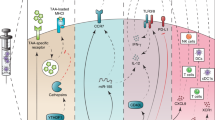
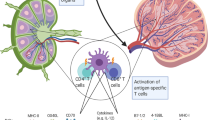
Abstract
Dendritic cells (DCs) play critical roles in recognizing and presenting antigens to T cells. They secrete dendritic cell-derived extracellular vesicles (DC-sEVs), which could mimic the function of DCs. Therefore, we explore the possibility of using DC-sEVs as a potential personalized vaccine in this study. We compared the efficacy of DCs and DC-sEVs on stimulating the immune system to target breast cancer cells and found that DC-sEVs had significantly more MHC molecules on the surface when compared to the parental DCs. In our in vivo and in vitro testing, Dc-sEVs showed significant advantages over DCs, regarding efficacy, safety, storage, and potential delivery advantages. DC-sEVs were able to suppress the growth of immune-cold breast tumors, while DCs failed to do so. These results indicate the strong potential utility of DC-sEVs as a personalized immunotherapy for breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
References
Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7–24.
Mpakali A, Stratikos E. The role of antigen processing and presentation in cancer and the efficacy of immune checkpoint inhibitor immunotherapy. Cancers (Basel). 2021;1:134.
Bandola-Simon J, Roche PA. Dysfunction of antigen processing and presentation by dendritic cells in cancer. Mol Immunol. 2019;113:31–7.
Chen X, Shao Q, Hao S, Zhao Z, Wang Y, Guo X, et al. CTLA-4 positive breast cancer cells suppress dendritic cells maturation and function. Oncotarget. 2017;8:13703–15.
Gervais A, Leveque J, Bouet-Toussaint F, Burtin F, Lesimple T, Sulpice L, et al. Dendritic cells are defective in breast cancer patients: a potential role for polyamine in this immunodeficiency. Breast Cancer Res. 2005;7:R326–35.
Cintolo JA, Datta J, Mathew SJ, Czerniecki BJ. Dendritic cell-based vaccines: barriers and opportunities. Future Oncol. 2012;8:1273–99.
Mastelic-Gavillet B, Balint K, Boudousquie C, Gannon PO, Kandalaft LE. Personalized dendritic cell vaccines-recent breakthroughs and encouraging clinical results. Front Immunol (Review). 2019;10:766.
Tanyi JL, Bobisse S, Ophir E, Tuyaerts S, Roberti A, Genolet R, et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med. 2018;10:eaao5931.
Qian D, Li J, Huang M, Cui Q, Liu X, Sun K. Dendritic cell vaccines in breast cancer: Immune modulation and immunotherapy. Biomed Pharmacother. 2023;162:114685.
Abdi K, Singh NJ, Matzinger P. Lipopolysaccharide-activated dendritic cells: “exhausted” or alert and waiting? J Immunol. 2012;188:5981–9.
Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–7.
van de Loosdrecht AA, van Wetering S, Santegoets SJAM, Singh SK, Eeltink CM, den Hartog Y, et al. A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia. Cancer Immunol Immunother. 2018;67:1505–18.
Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610.
Wu K, Lyu F, Wu S-Y, Sharma S, Deshpande RP, Tyagi A, et al. Engineering an active immunotherapy for personalized cancer treatment and prevention of recurrence. Sci Adv. 2023;9:eade0625.
Huda MN, Nurunnabi M. Potential application of exosomes in vaccine development and delivery. Pharm Res. 2022;39:2635–71.
Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z, et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67:739–48.
Wahlund CJE, Gucluler G, Hiltbrunner S, Veerman RE, Naslund TI, Gabrielsson S. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci Rep. 2017;7:17095.
Andre F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–36.
Admyre C, Johansson SM, Paulie S, Gabrielsson S. Direct exosome stimulation of peripheral humanT cells detected by ELISPOT. Eur J Immunol. 2006;36:1772–81.
Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M, Minami M. MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol Lett. 2003;89:125–31.
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600.
Yang ZZ, Kim HJ, Villasboas JC, Chen YP, Price-Troska T, Jalali S, et al. Expression of LAG-3 defines exhaustion of intratumoral PD-1(+) T cells and correlates with poor outcome in follicular lymphoma. Oncotarget. 2017;8:61425–39.
Wu M, Zheng D, Zhang D, Yu P, Peng L, Chen F, et al. Converting immune cold into hot by biosynthetic functional vesicles to boost systematic antitumor immunity. iScience. 2020;23:101341.
Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux F, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–23.
Deb A, Gupta S, Mazumder PB. Exosomes: a new horizon in modern medicine. Life Sci. 2021;264:118623.
Wang W, Li J, Wu K, Azhati B, Rexiati M. Culture and identification of mouse bone marrow-derived dendritic cells and their capability to induce T lymphocyte proliferation. Med Sci Monit. 2016;22:244–50.
Madaan A, Verma R, Singh AT, Jain SK, Jaggi M. A stepwise procedure for isolation of murine bone marrow and generation of dendritic cells. J Biol Method. 2014;1:e1.
Nair S, Archer GE, Tedder TF. Isolation and generation of human dendritic cells. Curr Protoc Immunol. 2012;Chapter 7:Unit7 32.
Chometon TQ, Siqueira MDS, Sant Anna JC, Almeida MR, Gandini M, Martins de Almeida Nogueira AC, et al. A protocol for rapid monocyte isolation and generation of singular human monocyte-derived dendritic cells. PLoS ONE. 2020;15:e0231132.
Peterson T. Densitometric analysis using NIH image. North American Vascular Biology Organization (NAVBO) eNewsletter. 2010;16:3.
Wu K, Feng J, Lyu F, Xing F, Sharma S, Liu Y, et al. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat Commun. 2021;12:5196.
Wu K, Fukuda K, Xing F, Zhang Y, Sharma S, Liu Y, et al. COX2-MMP1 pathway promotes brain metastasis by tampering with blood-brain barrier and supporting tumor initiating cells in the brain microenvironment. Cancer Res. 2015;75:2250.
Zhao D, Wu K, Sharma S, Xing F, Wu SY, Tyagi A, et al. Exosomal miR-1304-3p promotes breast cancer progression in African Americans by activating cancer-associated adipocytes. Nat Commun. 2022;13:7734.
Acknowledgements
We thank Dr. Ravi Nandan Singh, Ph.D. (Wake Forest University) for kindly providing the assistance in NTA of the sEVs. This work was supported by NIH grants RO1CA173499, R01CA185650, R01CA205067, and W81XWH2110075 from the Department of Defense (to K Watabe), and NIH T32CA247819 (to K Wu). This study used various Core Facilities and Departmental Shared Equipment resources including Cellular Imaging Shared Resources, Tumor Tissue and Pathology Shared Resource Cell and Viral Vector Core Laboratory and FC Shared Resources that are supported by the Comprehensive Cancer Center of Wake Forest University NCI, National Institutes of Health Grant (P30CA012197).
Author information
Authors and Affiliations
Contributions
Conception and design: FL, K Wu, K Watabe. Development of methodology: FL, K Wu, K Watabe. Acquisition of data (provided animals, acquired and managed patient specimens, provided facilities, etc.): FL, K Wu, SYW, RS, RPD, AT, IR, SY, K Watabe. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): FL, K Wu, SYW, K Watabe. Writing, review, and/or revision of the manuscript: FL, K Wu, IR, SY, K Watabe. Administrative, technical, or material support: K Wu, RPD, K Watabe. Study supervision: K Watabe.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lyu, F., Wu, K., Wu, SY. et al. Functional evaluation of dendritic cells and extracellular vesicles as immunotherapy for breast cancer. Oncogene 43, 319–327 (2024). https://doi.org/10.1038/s41388-023-02893-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02893-2