Abstract
After drug withdrawal, a key factor triggering relapse is progressively intensified cue-associated drug craving, termed incubation of drug craving. After withdrawal from cocaine self-administration, incubation of cocaine craving develops more reliably in rats compared to mice. This species difference provides an opportunity to determine rat-specific cellular adaptations, which may constitute the critical mechanisms that contribute to incubated cocaine craving in humans. Expression of incubated cocaine seeking is mediated, in part, by cocaine-induced cellular adaptations in medium spiny neurons (MSNs) within the nucleus accumbens (NAc). In rats, decreased membrane excitability in NAc MSNs is a prominent cellular adaptation, which is induced after cocaine self-administration and lasts throughout prolonged drug withdrawal. Here, we show that, similar to rats, mice exhibit decreased membrane excitability of dopamine D1 receptor (D1)-, but not D2 (D2)-, expressing MSNs within the NAc shell (NAcSh) after 1 d withdrawal from cocaine self-administration. However, in contrast to rats, this membrane adaptation does not persist in mice, diminishing after 45-d withdrawal. We also find that restoring the membrane excitability of NAcSh MSNs after cocaine withdrawal decreases cocaine seeking in rats. This suggests that drug-induced membrane adaptations are essential for behavioral expression of incubated cocaine craving. In mice, however, experimentally inducing hypoactivity of D1 NAcSh MSNs after cocaine withdrawal does not alter cocaine seeking, suggesting that MSN hypo-excitability alone is insufficient to increase cocaine seeking. Together, our results demonstrate an overall permissive role of cocaine-induced hypoactivity of NAcSh MSNs in gating increased cocaine seeking after prolonged cocaine withdrawal.
Similar content being viewed by others
Introduction
After withdrawal from drug use, the urge for drug taking becomes progressively intensified, a phenomenon termed incubation of drug craving [1]. Such incubated drug craving contributes to drug relapse, a key barrier in treating substance use disorder [2, 3]. Notably, rodent models of cocaine relapse show that rats, but not mice, exhibit robust incubation of cocaine craving after withdrawal cocaine self-administration [4, 5]. Thus, comparative studies between rats and mice offer an opportunity to explore the key cellular underpinnings of incubated cocaine craving.
Extensive results show that incubated cocaine craving is mediated in part by cocaine-induced cellular adaptations in principal medium spiny neurons (MSNs) within the nucleus accumbens (NAc) [6,7,8]. In rats, decreased membrane excitability of NAc shell (NAcSh) MSNs represents a prominent drug-induced cellular adaptation, which persists throughout prolonged withdrawal periods [9,10,11,12]. This membrane adaptation reduces the responsiveness of NAcSh MSNs to excitatory inputs, and promotes a homeostatic upregulation of Ca2+-permeable AMPA receptors (AMPARs) at excitatory synapses on NAcSh MSNs. Collectively, these changes result in functional alterations of NAcSh MSNs that promote cue-induced cocaine seeking after cocaine withdrawal [9, 13]. In mice, a similar membrane adaptation is induced in NAc MSNs shortly after repeated noncontingent cocaine administration [14,15,16,17,18]. However, it remains unclear whether the NAc MSN membrane adaptation in mice is induced by cocaine self-administration, and if so, whether this adaptation persists after prolonged cocaine withdrawal to contribute to the intensified cocaine-seeking.
Our current results show that, similar to rats, cocaine self-administration in mice decreased the membrane excitability of NAcSh MSNs after short-term withdrawal. However, in contrast to rats, mice exhibited diminished membrane adaptation following prolonged withdrawal, rendering this adaptation much more short-term in mice compared to rats. Furthermore, in mice, such changes in membrane excitability were only induced in dopamine D1 receptor (D1)-, but not D2 (D2)-, expressing NAcSh MSNs. After prolonged withdrawal from cocaine self-administration, partial restoration of membrane excitability in rats decreased cue-induced cocaine seeking. On the other hand, in mice, acutely inducing hypoactivity of NAcSh MSNs did not increase cocaine seeking after prolonged cocaine withdrawal. Together, our results suggest that cocaine-induced hypo-excitability of NAcSh MSNs is required but not sufficient for intensified cocaine seeking after long-term cocaine withdrawal.
Methods and methods
Subjects and reagents
Male wildtype C57BL/6J mice (Jax#000664), D1-tdTomato (B6.Cg-Tg(Drd1a-tdTomato)6Calak/J, Jax# 016204) and Drd1-cre (B6.FVB(Cg)-Tg(Drd1-cre)EY262Gsat/Mmucd, MMRRC# 030989-UCD) mice were acquired from The Jackson Laboratory (Bar Harbor, ME); wildtype male Sprague Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). Although we initiated our experiments in both male and female mice, ultimately, the data presented here is restricted to males. This is because: (1) females’ considerably smaller body size rendered them more likely to have health issues during or after catheter surgery or self-administration training. This resulted in a higher elimination rate of female mice (~85%), compared to male mice (~10%) in our experiments. (2) the cocaine-induced membrane adaptation in NAc MSNs was established and repeatedly replicated in male rats. For effective comparison between the two species, we thus only included males for final data analysis.
Mice and rats were singly housed on a regular 12-h light/dark cycle (lights on/off at 7:00/19:00), with food and water available ad libitum. After acclimation (~10-weeks old), both mice and rats underwent the same surgical and operant training procedures as detailed below. The animals were used in accordance with the NIH Guide for Experimental Animal Use as well as protocols approved by the Institutional Animal Care and Use Committees at the University of Pittsburgh.
AAV-hSyn-DIO-hM4D(Gi)-mCherry (AAV-DIO-hM4D) was purchased from Addgene (Cat # 44362-AAV9, Watertown, MA). All chemicals used for electrophysiological recordings were purchased from Sigma-Aldrich (St. Louis, MO) and Thermo Fisher Scientific (Waltham, MA). Cocaine-HCl was supplied by the Drug Supply Program of the National Institute on Drug Abuse. The water-soluble DREADD agonist 21 (Compound 21) hydrochloride was obtained from Hello Bio Inc. (Princeton, NJ).
Intravenous jugular vein catheterization
Jugular vein catheterization was performed with the animals under combined ketamine/xylazine anesthesia. Briefly, two incisions were made in the neck and back to expose the external jugular vein for intravenous cannula insertion and implantation of a vascular access button (Instech Laboratories, Inc., Plymouth Meeting, PA) that was connected to a silicone cannula. The cannula was placed subcutaneously from the back incision, and the tip of the cannula was gently inserted into the jugular vein. The button was anchored on the back by suturing. A daily flush maintained the patency of the intravenous catheter with heparinized saline (30 USP units/mL). Following catheterization, animals were singly housed in their home cages for 1 week before behavioral training.
Cocaine self-administration
After recovery, catheterized animals were trained for cocaine self-administration in modular operant chambers (Med Associates Inc., St. Albans, VT) under a fixed ratio 1 schedule of reinforcement. For mouse self-administration, the chamber contained two retractable levers on one side of the wall and an LED cue light was located above the active lever. A 3-mL syringe containing 1.5 mg/mL cocaine solution was loaded to an infusion pump (PHM-200; Med Associates Inc.) before each 2-h self-administration session. Each session began by extending the two levers into the chamber and illuminating the house light (continuously on throughout each session). Intravenous injection was triggered by a single active lever press, enabling the mice to receive cocaine solution at a dose of 0.75 mg/kg body weight/infusion and in a volume of 0.5 µL/g body weight/infusion over ~2 sec. The infusion was accompanied by cue light illumination over the course of infusion. To prevent cocaine overdose, there was a 10-sec timeout period between two consecutive infusions, during which pressing the active lever was recorded but did not result in cocaine infusion. Pressing the inactive lever had no programmed consequence. The establishment of self-administration was determined using these criteria: (1) ≥20 active lever presses in each self-administration session; (2) an active/inactive lever press ratio exceeding 2:1.
The self-administration of rats was conducted in operant conditioning chambers (20 × 28 x 20 cm; Med Associates Inc.) as previously described [19, 20]. Briefly, an animal self-administered cocaine at a dose of 0.75 mg/kg per infusion by nose-poking the active hole, accompanied by the switching-on of a light within the hole that served as a conditioned stimulus (CS) and a background light. The CS stayed on for 6 sec, and the background light for 20 sec. To avoid overdose, upper limits of cocaine infusions were set at 100 for the 12-h overnight sessions and 65 for the 2-h daily session.
Cue-induced cocaine seeking
To compare the response of cue-induced cocaine seeking between rats and mice after prolonged cocaine abstinence, the rats and mice previously undergoing 5 days of cocaine self-administration followed by 45 days of home-cage withdrawal were re-exposed to cocaine-associated cues in the same operant chambers. In the 1-h cue-induced cocaine-seeking session, operant responses to the active hole (rats) or active lever (mice) resulted in the presentation of light cues without cocaine infusion. On the other hand, responses to the inactive hole (rats) or inactive lever (mice) triggered no consequences. The number of responses to the active hole/lever was used to assess cue-induced cocaine-seeking.
Electrophysiology
To prepare acute brain slices, animals were decapitated under isoflurane anesthesia. Coronal slices (250 μm) containing the NAc were prepared on a VT1200S vibratome (Leica Biosystems, Nussloch, Germany) in a cutting solution composed as follows (in mM): 135 N-methyl-d-glutamine, 1 KCl, 1.2 KH2PO4, 0.5 CaCl2, 1.5 MgCl2, 20 choline-HCO3, and 11 glucose; the cutting solution was maintained at 4 °C, saturated with 95% O2 / 5% CO2, and pH adjusted to 7.4 with HCl. Osmolality of the solution was adjusted to 300 mOsm. Slices were incubated in the artificial cerebrospinal fluid (aCSF), containing (in mM) 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1 NaH2PO4, 26.2 NaHCO3, and 11 glucose and saturated with 95% O2 / 5% CO2, pH 7.4; aCSF osmolality was adjusted to 290-295 mOsm. The brain slices were incubated at 34 °C for 30 min and allowed to recover for >30 min at 20-22 °C before experimentation. The slices were then transferred into a recording chamber superfused with aCSF on the stage of an upright fluorescent microscope (BX51-WI; Olympus, Tokyo, Japan).
Membrane properties were measured in several discrete regions including in the NAcSh (AP: 1.1-1.6 mm), NAc core (NAcCo), dorsomedial (DMS) and dorsolateral striatum (DLS) (Fig. 1B). Whole-cell current-clamp recordings were obtained using a Multiclamp 700B amplifier, a Digidata 1320 A/D converter and pCLAMP 9.2 software (Axon Instruments, Molecular Devices, San Jose, CA). Glass recording electrodes were filled with a K+-based internal solution (in mM): 130 K-methansulfate, 10 KCl, 10 HEPES, 0.4 EGTA, 2.0 MgCl2, 2.5 MgATP, and 0.25 Na3GT (pH 7.2–7.4, 275–285 mOsm). D1 and D2 MSNs were visually identified depending on the presence or absence of the Drd1a-tdTomato reporter, respectively. Interneurons were distinguished and excluded from data analysis based on their signature electrophysiological properties, i.e., relatively depolarized resting membrane potentials (approximately −72 mV), higher frequency of action potential firing during current injection, or the presence of a large voltage sag at hyperpolarized potentials. If stable resting membrane potentials were not achieved during the ∼5-min stabilization period (oscillating <5 mV) after the current-clamp configuration was formed, the MSNs were excluded from the following recording.
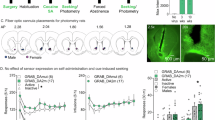
A Schematic showing experimental procedure of self-administration training and electrophysiological recording on withdrawal d1. B Diagram showing the four striatal subregions where MSNs were sampled. Summaries showing the infusion (C) and operant responding (D) in mice used for the withdrawal d1 recording. Examples and summaries of numbers of evoked action potentials in MSNs from the NAcSh (E), NAcCo (F), DMS (G), and DLS (H) in mice 1 d after self-administration training. Insets, example action potentials evoked by 300 pA current steps. I Schematic showing that electrophysiological recording was performed on d 45 after self-administration training. Summaries showing the infusion (J) and operant responding (K) results in mice used for the withdrawal d 45 recording. Examples and summaries of numbers of evoked action potentials in MSNs from the NAcSh (L), NAcCo (M), DMS (N), and DLS (O) in mice on d 45 after self-administration training. All comparisons were performed with animal-based 2-way mixed ANOVA. *p < 0.05.
To measure the intrinsic membrane excitability, the resting membrane potential of each neuron was adjusted to −80 mV by injecting a small positive or negative current (usually <30 pA; recording of the neuron was discontinued if the compensating current was >50 pA). The recorded neuron was then stimulated using step current injection starting at −200pA in progressive 50 pA increments until the strongest depolarization current of +400 pA was reached (interval: 15 sec). The action potential frequency-current curve was obtained by counting the number of action potentials evoked by each current step. The afterhyperpolarization potential (AHP) was defined and measured as previously described [10, 12]. Briefly, the medium component of the AHP was defined as the voltage difference between the action potential threshold and the membrane potential 10 msec after initiation of the action potential. The threshold of action potential was defined as the voltage at which the membrane potential elevation rate (dV/dt) reached 10 during the initiation of the action potential. The fast AHP (fAHP) was defined as the voltage difference between the action potential threshold and the most hyperpolarized membrane potential following the action potential. Both f- and mAHP were measured from the traces evoked by 300-pA current injection.
Chemogenetic inhibition of NAc D1 MSNs activity
D1-Cre mice were anesthetized via i.p. injection of a ketamine (100 mg/kg)–xylazine (10 mg/kg) mixture and held in a stereotaxic frame (Stoelting Co., Wood Dale, IL). Viruses were infused through a 10-μl NanoFil syringe with a 33-gauge needle controlled by UMP3 and Micro4 system (WPI, Sarasota, FL). Specifically, 500 nL of AAV9-hSyn-DIO-hM4Di-mCherry was infused at a rate of 100 nL/min into each side of the NAc in D1-Cre mice (in mm: to bregma AP, 1.54; ML, ±0.6; DV, −4.4). The needle was held in place for 5 min before removal to prevent the backflow of viral solutions. After surgery, the skin was sutured and mice were kept in their home cages for >4 weeks for viral expression before jugular cannulation surgery for cocaine self-administration. The DREADD agonist C21 was used to activate the inhibitory DREADD receptor hM4Di expressed in NAc D1 MSNs in vivo. C21 was freshly dissolved in 0.9% saline on the day of cue-induced cocaine seeking test. Mice were then intraperitoneally injected with either C21 (1 mg/kg) or vehicle solution (0.9% saline) 30 min prior to the placement of mice into the operant chambers where cocaine self-administration was previously conducted.
Data Acquisition and Statistics
All results are shown as mean ± s.e.m. Most experiments were replicated in 5–16 mice. All data collections were randomized and assumed to be normally distributed. Though no statistical methods were used to pre-determine sample sizes, our sample sizes were similar to those reported in previous publications with similar experimental designs [13, 21,22,23]. All data were analyzed offline. For all physiology data sets, sample sizes were presented as the number of recordings (n) and number of animals (m), i.e., n/m. Typically, multiple recordings were made from a single animal, and the multiple data points were averaged to represent the respective parameter for this animal. Statistical significance was assessed using paired or unpaired t-tests, or one-way or two-way mixed ANOVA followed by posttests, as specified in the related text. Two-tailed tests were performed for all analyses, and statistical significance was set at p < 0.05. Statistical analyses were performed in GraphPad Prism version 7 (GraphPad Software, San Diego, CA) or SPSS version 19 (IBM, Armonk, NY).
Results
Cocaine-induced membrane adaptation in mice
We used a cocaine self-administration procedure that included an overnight session followed by a 2-h session over 5 consecutive days (Fig. 1A). While our earlier work showed that this procedure led to robust incubation of cocaine craving in rats [24], we found that this was not the case in mice (see Fig. 5). We trained wildtype mice with this procedure and then examined MSNs in two subregions of the NAc, the NAcCo and NAcSh, as well as two subregions of the dorsal striatum, the DMS and DLS, which are differentially involved in cocaine seeking and cocaine taking [25] (Fig. 1B). After 1 d of withdrawal from self-administration (Fig. 1CD), we assessed the intrinsic membrane excitability of MSNs by recording the evoked action potential firing. NAcSh MSNs in cocaine-trained mice exhibited decreased membrane excitability compared to their control saline counterparts (F1, 8 = 6.4, p = 0.04, cocaine vs saline; Fig. 1E). On the other hand, the membrane excitability of MSNs within either the NAcCo, DMS, or DLS was similar between saline- and cocaine-trained mice (cocaine vs saline: NAcCo, F1, 8 = 0.1, p = 0.73; DMS, F1, 7 = 0.7, p = 0.42; DLS, F1, 8 = 3.2, p = 0.11; Fig. 1F–H). Thus, like rats [12, 13], cocaine self-administration induced a decrease in the membrane excitability of NAcSh MSNs in mice after short-term cocaine withdrawal.
To examine whether the above membrane adaptations are long-lasting, we trained mice with cocaine self-administration and performed slice electrophysiology following 45 d of withdrawal (Fig. 1I-K). At the 45-d withdrawal timepoint, the frequency of evoked action potential firing in NAcSh, NAcCo, DML, and DLS MSNs was similar between saline- and cocaine-trained mice (NAcSh, F1, 10 = 0.0, p = 0.95; NAcCo, F1, 10 = 0.0, p = 0.91; DMS, F1, 10 = 0.0, p = 0.95; DLS, F1, 10 = 0.0, p = 0.90; Fig. 1L–O). Thus, in contrast to rats [10, 12, 13], the decreased membrane excitability of NAcSh MSNs is a short-term adaptation in mice, and diminishes over long-term withdrawal after cocaine self-administration.
Membrane adaptations of D1 versus D2 MSNs
MSNs in the ventral and dorsal striatum can be divided into two largely nonoverlapping subpopulations, one expressing dopamine D1 and the other expressing D2 receptors. D1 and D2 MSNs are embedded differentially in NAc circuits to confer distinct behavioral functions [25, 26]. To explore the potential difference between these two neuronal subpopulations, we employed D1-tdTomato mice, in which D1 MSNs are labeled with tdTomato fluorescence [27]. In brain slices, extensive previous studies from us and others demonstrate that the presence versus absence of tdTomato fluorescence accurately predicts D1 versus D2 MSNs [21, 27,28,29,30].
After training D1-tdTomato mice in either saline or cocaine self-administration, we performed electrophysiology following 1 d of withdrawal (Fig. 2A–C). In the NAcSh, the membrane excitability was selectively decreased in D1 MSNs (tdTomato+), but not in D2 MSNs (tdTomato-) in cocaine-trained compared to saline-trained mice (D1, F1, 19 = 12.8, p = 0.00; D2, F1, 19 = 0.8, p = 0.37; cocaine vs saline; Fig. 2D, E). This cocaine-induced membrane adaptation was not detected in either D1 or D2 MSNs in the NAcCo (D1, F1, 19 = 1.2, p = 0.29; D2, F1, 17 = 0.9, p = 0.37) or DMS (D1, F1, 18 = 0.0, p = 0.98; D2, F1, 18 = 0.1, p = 0.73; Fig. 2F–I). In the DLS of cocaine-trained mice, the membrane excitability was decreased in D1 MSNs but increased in D2 MSNs, compared with saline-trained mice (F1, 30 = 4.4, p = 0.045; D2, F1, 25 = 5.5, p = 0.03; Fig. 2J, K). These differential changes in D1 versus D2 DLS MSNs may be missed in wild-type mice as these opposite changes may cancel one another out when both neuronal types were sampled together (Fig. 1H). Though short-lasting (see Fig. 3), these differential changes in DLS MSN membrane excitability warrant further investigation as they may represent an initial process through which cocaine experience shifts the balance between the direct versus indirect pathways within the dorsal striatum for cocaine-associated habit formation or motor output.
A Schematic showing electrophysiological recording performed on d 1 after self-administration training. Summarized self-administration results showing the infusion (B) and operant responding (C) in mice used for the withdrawal d1 recording. Examples and summaries of numbers of evoked action potentials in D1 or D2 MSNs from the NAcSh (D, E), NAcCo (F, G), DMS (H, I), and DLS (J, K) in mice on d 1 after self-administration training. All comparisons were performed using animal-based 2-way mixed ANOVA. *p < 0.05.
A Schematic showing electrophysiological recording on d 45 after self-administration training. Summaries showing self-administration results, including saline/cocaine infusions (B) and operant responding (C), in mice used for the withdrawal d45 recording. Examples and summaries of numbers of evoked action potentials in D1 or D2 MSNs from the NAcSh (D, E), NAcCo (F, G), DMS (H, I), and DLS (J, K) in mice on d 45 after self-administration training. All comparisons were performed using animal-based 2-way mixed ANOVA.
After 45-d withdrawal from cocaine self-administration (Fig. 3A–C), the membrane excitability of D1 or D2 MSNs across all the ventral and dorsal striatum was comparable between saline- and cocaine-trained mice (NAcSh-D1, F1, 15 = 0.8, p = 0.38; NAcSh-D2, F1, 15 = 1.5, p = 0.24; NAcCo-D1, F1, 14 = 0.9, p = 0.35; NAcCo-D2, F1, 15 = 3.5, p = 0.08; DMS-D1, F1, 15 = 1.3, p = 0.27; DMS-D2, F1, 15 = 0.5, p = 0.49; DLS-D1, F1, 15 = 0.5, p = 0.49; DLS-D2, F1, 14 = 0.0, p = 0.85; Fig. 3D–K). Thus, in contrast to rats, cocaine-induced membrane adaptations of striatal MSNs in mice diminished after prolonged cocaine withdrawal.
Behavioral implications
In our previous studies, the same cocaine self-administration procedure used in the above mouse experiments induced robust incubation of cue-induced cocaine craving in rats [13, 31]. That is, rats exhibited much higher levels of cue-induced cocaine seeking on withdrawal d 45 than withdrawal d 1 [24, 32]. At the cellular level, expression of incubated cocaine seeking in rats is accompanied by persistent decreases in membrane excitability of NAcSh MSNs, which are detected after long-term cocaine withdrawal [12, 13]. The persistence of this cocaine-induced membrane adaptation in rats was further confirmed by our current study in which cocaine-induced membrane adaptations were maintained throughout the 45-d withdrawal following cocaine self-administration (F1,14 = 6.82, p = 0.02, saline vs cocaine, 2-way ANOVA; Fig. 4A–D).
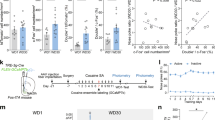
A Schematic showing electrophysiological recording in rats on d 45 after self-administration training. B Summarized infusion results of rats with saline or cocaine self-administration. Examples (C) and summary (D) of numbers of action potentials evoked in NAcSh MSNs from saline- and cocaine-trained rats. E Example AHPs in NAcSh MSNs from a saline- and a cocaine-trained rat after 45 d of withdrawal from self-administration training. F Summaries showing increased amplitudes of mAHP in NAcSh MSNs in cocaine-trained rats compared to saline-trained rats. G Schematic showing intra-NAcSh administration of apamin, followed by cue-induced cocaine seeking test on withdrawal d 45 after self-administration training. H Summaries of self-administration results (infusions) of rats that later received intra-NAcSh injection of aCSF vehicle or apamin. I Schematic illustration showing the postmortem-identified locations of the cannula that delivered apamin within the NAcSh. J Summaries showing that intra-NAcSh injection of apamin on withdrawal d 45 after cocaine self-administration decreased cue-induced cocaine seeking. *p < 0.05; **p < 0.01.
In rats, the decreased membrane excitability of NAcSh MSNs after cocaine withdrawal are attributable to changes in several types of ionic conductance, including the SK2 subtype of Ca2+-activated K+ channels. Specifically, accompanying the decreased membrane excitability after 45-d cocaine withdrawal, NAcSh MSNs exhibited an increase in the amplitude of the SK2-mediated, medium component of afterhyperpolarization potentials (mAHP), which function to dampen recurrent action potential firing [10, 12, 13]. In rat brain slices, we previously showed that application of apamin, an SK2-selective antagonist, effectively reduced the mAHP amplitude and partially reversed cocaine-induced decreased membrane excitability of NAcSh MSNs after 45-d cocaine withdrawal [10]. In line with these findings, we found that the amplitude of mAHP was increased in rats after 45-d withdrawal from cocaine (t1,14 = 2.4, p = 0.03, t-test; Fig. 4E, F). This confirmed that SK2-mediated mAHP is a key cellular substrate contributing to the decreased membrane excitability of NAcSh MSNs in rats after cocaine withdrawal.
To examine whether the decreased membrane excitability of NAcSh MSNs is required for incubated cocaine seeking after long-term cocaine withdrawal, we used apamin to manipulate SK2 in vivo. Specifically, we trained two groups of rats for cocaine self-administration (Fig. 4G, H). On withdrawal d 45, we handled the rats gently for ~10 min until they habituated, and then applied apamin (500 nM in 1 µl aCSF) to the NAcSh to acutely compromise the cocaine-induced membrane adaptation in MSNs. We applied apamin bilaterally through pre-installed cannulae, using rats with intra-NAcSh infusion of vehicle (aCSF) as controls (Fig. 4I). After 10–30 min, we placed the rats into the operant boxes in which nose pokes to the active hole resulted in the presentation of a contingent light cue but without cocaine infusion. Rats with intra-NAcSh apamin infusion exhibited decreased levels of cue-induced cocaine seeking compared to vehicle-treated rats (t 1,10 = 4.4, p = 0.00, t-test; Fig. 4J). These results suggest that the hypo-excitability of NAcSh MSNs is essential for the expression of high levels of cue-induced cocaine seeking after prolonged cocaine withdrawal. It is worth noting that, in addition to MSNs, the NAc contains other neuronal types that also express SK2 and are implicated in cue-conditioned motivated behaviors, including cholinergic neurons [33] and fast-spiking interneurons [34], among others [35, 36]. We could therefore not rule out the possible contribution of non-MSN NAc cells to our observed behavioral changes in response to intra-NAc application of apamin.
In contrast to rats, mice do not develop robust incubation of cocaine seeking after prolonged cocaine withdrawal [4, 37, 38]. NAc D1 and D2 MSNs coordinate their functions to contribute to reward responses, where D1 MSN activity promotes cocaine seeking [25, 39]. Our above results show that cocaine-induced membrane adaptation occurred selectively in NAcSh D1 MSNs but did not persist over long-term cocaine withdrawal (Figs. 2, 3). To determine whether the lack of persistent hypo-excitability of NAcSh D1 MSNs is the key for incubated cocaine craving, we chemogenetically decreased the activity of these MSNs in vivo. We injected AAV9-hSyn-DIO-hM4Di-mCherry into the NAcSh of D1-Cre mice to selectively express inhibitory DREADDs in D1 MSNs. ~4 weeks later, we divided these mice into two groups and trained them with cocaine self-administration. We then tested cue-induced cocaine seeking on both withdrawal d 1 and d 45 (Fig. 5A–C). On withdrawal d 45 (but not on withdrawal d 1), we intraperitoneally injected the mice with DREADD agonist C21 (1 mg/kg, i.p.) or the vehicle (saline) control to induce hypoactivity of D1 NAcSh MSNs; ~30 min later, the mice were placed into operant boxes. No increased cocaine seeking was detected in either vehicle- (t8 = 1.9, p = 0.10, t-test; Fig. 5D, E) or C21-injected (t8 = 2.1, p = 0.07, t-test; Fig. 5F, G) mice on withdrawal d 45 compared to withdrawal d 1. Importantly, on withdrawal d 45, both vehicle and C21 mice exhibited similar levels of cue-induced cocaine-seeking (active, p = 0.36; inactive, p = 0.57, vehicle vs C21, t-test; Fig. 5H). Thus, inducing a hypoactivity of D1 NAcSh MSNs alone is insufficient to increase cocaine seeking after prolonged withdrawal from cocaine self-administration.
A Schematic showing experimental procedures in D1-Cre mice, including intra-NAc expression of hM4D in D1 MSNs, 5-d self-administration training, cue-induced cocaine seeking tests on withdrawal d1 and d45, and C21 administration on withdrawal d45 before operant testing. B Diagram showing intra-NAcSh injection of AAV9-hSyn-DIO-hM4Di-mCherry. C Example image showing viral-mediated expression of hM4Di-mCherry in NAcSh D1 MSNs. D Summaries of cocaine self-administration results in mice used for vehicle injection. E Summaries showing cue-induced cocaine seeking (lever presses) on withdrawal d 1 and d45 in mice that received vehicle injection on withdrawal d 45. F Summaries of cocaine self-administration results in mice used for C21 injection. G Summaries showing cue-induced cocaine seeking on withdrawal d1 and d45 in mice that received C21 injection on withdrawal d45. H Summaries showing that chemogenetic suppression of D1 NAc MSNs in mice on withdrawal d 45 after cocaine self-administration did not affect cue-induced cocaine seeking.
Discussion
Species differences in incubation of cocaine craving
Incubation of cue-induced drug craving serves as a key rodent model to study molecular and cellular mechanisms underlying drug relapse. While robust in rats, incubated cocaine seeking is either weak or absent in mice after withdrawal from a variety of cocaine self-administration procedures [4, 37, 38]. This species difference provides an opportunity to determine incubation-specific cellular underpinnings by examining cocaine-induced changes present in rats but absent in mice. This is exemplified by cocaine-induced membrane adaptation in NAcSh MSNs: while the decrease in membrane excitability persists in rats, this adaptation diminishes in mice following prolonged cocaine withdrawal (Figs. 1–3) [12, 13]. These results prompted us to examine whether this persistent membrane adaptation represents a key factor that differentiates rats versus mice in expressing incubated cocaine seeking.
In rats, partially restoring the cocaine-induced decrease in NAcSh MSN membrane excitability after long-term cocaine withdrawal led to a decrease in cue-induced cocaine seeking. Such a finding indicates an essential role of this membrane adaptation in expressing incubated cocaine seeking (Fig. 4). On the other hand, experimentally inducing hypoactivity of NAcSh D1 MSNs in mice after cocaine withdrawal via inhibitory DREADDs did not increase cocaine seeking (Fig. 5). This suggests that, in addition to membrane adaptation, other cellular alterations within NAcSh MSNs or neurons in other brain regions are also required for the increased cocaine seeking observed after prolonged cocaine withdrawal. Taken together, our results suggest that the hypo-excitability of NAcSh MSNs is permissive, but not sufficient, for the expression of incubated cocaine craving.
Cocaine-induced hypo-excitability
During drug abstinence, the basal activity of ventral striatum in human drug users is lower than in nondrug-using subjects [40]. Similar NAc hypoactivity is also observed in nonhuman primates with a history of cocaine self-administration [41, 42]. Consistent with these results, rats exhibit reduced responsiveness of NAc MSNs to excitatory inputs after abstinence from repeated cocaine administration [43]. This cocaine-induced hypoactivity may partially stem from the persistent decrease in the intrinsic membrane excitability of NAc MSNs.
In rats, the decreased membrane excitability of NAc MSNs is attributable to cocaine-induced changes in several plasma membrane-localized ion channels, including Na+, K+, and Ca2+ channels [10,11,12,13, 15, 44,45,46]. Among these, the SK2 type of Ca2+-activated K+ channels may underlie the difference in cocaine-induced membrane adaptation in rats versus mice described above. In rats, results from earlier work [10, 12, 13] as well as our current study show a persistent increase in mAHP amplitude, a functional readout of SK2 conductance, in NAcSh MSNs throughout prolonged withdrawal from cocaine. After long-term cocaine withdrawal, pharmacological inhibition of SK2 partially restores the decreased membrane excitability of NAcSh MSNs [10]. In mice, however, our current study did not detect similar changes in the mAHP following cocaine withdrawal (p = 0.14 for saline vs cocaine in NAcSh D1 MSNs after 1-d withdrawal, t-test; data analyzed from mice presented in Fig. 2), despite a clear decrease in the membrane excitability of these MSNs at this withdrawal time point. This is consistent with previous mouse studies that similarly did not detect such a change in mAHP [15]. Instead, associated upregulation of voltage-gated K+ channels contributes to the decreased membrane excitability after cocaine in mice [15, 16]. Taken together, although the membrane excitability of NAcSh MSNs is decreased in both rats and mice after short-term withdrawal from cocaine, the underlying ion channels are different, and likely account for the species differences in the persistence of the membrane adaptation following cocaine withdrawal.
Beyond the hypo-excitability
In mice, we show that acutely decreasing the activity of NAcSh D1 MSNs after prolonged cocaine withdrawal does not increase cocaine seeking (Fig. 5). However, this acute manipulation does not fully recapitulate the case in rats, in which the membrane excitability of NAcSh MSNs remains persistently decreased throughout the prolonged cocaine withdrawal. We posit that, in rats, this persistent membrane adaptation triggers cascades of synapse-membrane homeostatic crosstalk (SMHC) in NAcSh MSNs during cocaine withdrawal, and the resulting synaptic changes also serve as essential factors promoting cocaine seeking [13]. In rats, preventing this cocaine-induced membrane adaptation during early withdrawal prevents the subsequent, SMHC-mediated synaptic changes and decreases cue-induced cocaine seeking that are evident after long-term cocaine withdrawal [13]. Consequently, the synaptic and other cellular changes induced by these persistent membrane adaptations after cocaine withdrawal are also part of the cellular drives for the behavioral expression of incubated cocaine seeking. In mice, however, in the absence of persistent membrane adaptations, these secondary synaptic and cellular changes may not be induced, which results in low levels of cocaine-seeking after withdrawal.
Beyond membrane adaptation, a prominent synaptic adaptation in NAc MSNs after long-term cocaine withdrawal is the synaptic accumulation of Ca2+-permeable AMPA receptors (CP-AMPARs). This receptor accumulation is promoted by several early withdrawal adaptations, such as decreased functionality of postsynaptic mGluR1, generation, and maturation of nascent glutamatergic synapses, as well as homeostatic dysregulation induced by decreased membrane excitability [6, 8, 13, 24, 47]. Indeed, such synaptic adaptation is also essential for the expression of incubated cocaine seeking in rats since decreasing the function of mGluR1 or pharmacological inhibition of CP-AMPARs within the NAc after cocaine withdrawal decreases cue-induced cocaine seeking [6, 47]. We expect that the cocaine-trained rats in our experiments exhibit such high levels of NAc CP-AMPARs on withdrawal d 45, similar to rats in previous studies trained with the same or similar cocaine procedures [24, 31, 32, 48,49,50,51,52]. In these rats, increasing the membrane excitability alone without affecting synaptic CP-AMPARs decreased cue-induced cocaine seeking (Fig. 4). In mice, on the other hand, although similar cocaine procedures also induce synaptic accumulation of CP-AMPARs in NAcSh MSNs, these mice do not exhibit incubation of cocaine craving [4]. These results from rats and mice suggest that both membrane adaptation and synaptic CP-AMPAR accumulation are essential but are individually insufficient for the expression of incubated cocaine seeking after long-term cocaine withdrawal.
In rats after cocaine withdrawal, synaptic accumulation of CP-AMPARs is expected to strengthen excitatory transmission to NAc MSNs. The decrease in ambient glutamate is thought to unleash the synaptic inhibition mediated by mGluR II, which promotes glutamatergic synaptic transmission [8, 53, 54]. Moreover, generation and maturation of nascent glutamatergic synapses may also strengthen glutamatergic inputs [9, 20, 24, 32]. Collectively, these synaptic changes may increase the excitatory drive for NAc MSNs. On the other hand, the decreased membrane excitability following cocaine withdrawal may decrease the responsiveness of NAcSh MSNs to excitatory inputs. How are these functionally opposing changes coordinated to promote cocaine seeking? A neuron-based hypothesis is that these changes polarize the synapse-to-soma communication such that only the strong inputs potentially related to cocaine trigger action potentials in NAc MSNs, while the relatively moderate inputs that encode physiological incentives are filtered out [13]. Alternatively, a circuit-based hypothesis suggests that these synapse and membrane alterations are unevenly distributed among individual MSNs, leading to the formation of functionally unique MSN ensembles that promote cocaine seeking [8, 9]. For testing these hypotheses in future studies, our detailed characterization of cocaine-induced membrane adaptations above may provide a solid knowledge foundation.
References
Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 2001;412:141–2.
Dong Y, Taylor JR, Wolf ME, Shaham Y. Circuit and synaptic plasticity mechanisms of drug relapse. J Neurosci. 2017;37:10867–76.
Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–20.
Terrier J, Luscher C, Pascoli V. Cell-type specific insertion of GluA2-lacking AMPARs with cocaine exposure leading to sensitization, Cue-induced seeking, and incubation of craving. Neuropsychopharmacology 2016;41:1779–89.
Steiner N, Rossetti C, Sakurai T, Yanagisawa M, de Lecea L, Magistretti PJ, et al. Hypocretin/orexin deficiency decreases cocaine abuse liability. Neuropharmacology 2018;133:395–403.
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 2008;454:118–21.
Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 2016;17:351–65.
Wright WJ, Dong Y. Psychostimulant-induced adaptations in nucleus accumbens glutamatergic transmission. Cold Spring Harb Perspect Med. 2020;10:a039255.
Wright WJ, Dong Y. Silent synapses in cocaine-associated memory and beyond. J Neurosci. 2021;41:9275–85.
Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM, et al. Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J Neurosci. 2009;29:5820–31.
Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, et al. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–7.
Mu P, Moyer JT, Ishikawa M, Zhang Y, Panksepp J, Sorg BA, et al. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci. 2010;30:3689–99.
Wang J, Ishikawa M, Yang Y, Otaka M, Kim JY, Gardner GR, et al. Cascades of homeostatic dysregulation promote incubation of cocaine craving. J Neurosci. 2018;38:4316–28.
Kourrich S, Calu DJ, Bonci A. Intrinsic plasticity: an emerging player in addiction. Nat Rev Neurosci. 2015;16:173–84.
Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su TP, Bonci A. Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell 2013;152:236–47.
Delint-Ramirez I, Garcia-Oscos F, Segev A, Kourrich S. Cocaine engages a non-canonical, dopamine-independent, mechanism that controls neuronal excitability in the nucleus accumbens. Mol Psychiatry. 2020;25:680–91.
Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–83.
Kim J, Park BH, Lee JH, Park SK, Kim JH. Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biol Psychiatry. 2011;69:1026–34.
Wang J, Li KL, Shukla A, Beroun A, Ishikawa M, Huang X, et al. Cocaine triggers Astrocyte-mediated Synaptogenesis. Biol Psychiatry. 2021;89:386–97.
Neumann PA, Wang Y, Yan Y, Wang Y, Ishikawa M, Cui R, et al. Cocaine-induced synaptic alterations in thalamus to nucleus accumbens projection. Neuropsychopharmacology 2016;41:2399–410.
Xia SH, Yu J, Huang X, Sesack SR, Huang YH, Schluter OM, et al. Cortical and Thalamic interaction with Amygdala-to-Accumbens Synapses. J Neurosci. 2020;40:7119–32.
Ge F, Mu P, Guo R, Cai L, Liu Z, Dong Y, et al. Chronic sleep fragmentation enhances habenula cholinergic neural activity. Mol Psychiatry. 2021;26:941–54.
Wright WJ, Graziane NM, Neumann PA, Hamilton PJ, Cates HM, Fuerst L, et al. Silent synapses dictate cocaine memory destabilization and reconsolidation. Nat Neurosci. 2020;23:32–46.
Lee BR, Ma Y-Y, Huang YH, Wang X, Otaka M, Ishikawa M, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–51.
Zinsmaier AK, Dong Y, Huang YH. Cocaine-induced projection-specific and cell type-specific adaptations in the nucleus accumbens. Mol Psychiatry. 2022;27:669–86.
Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr Opin Neurobiol. 2013;23:546–52.
Shuen JA, Chen M, Gloss B, Calakos N. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J Neurosci. 2008;28:2681–5.
Yu J, Sesack SR, Huang Y, Schluter OM, Grace AA, Dong Y. Contingent Amygdala inputs trigger Heterosynaptic LTP at Hippocampus-to-accumbens synapses. J Neurosci. 2022;42:6581–92.
Yu J, Ishikawa M, Wang J, Schluter OM, Sesack SR, Dong Y. Ventral Tegmental area projection regulates glutamatergic transmission in nucleus accumbens. Sci Rep. 2019;9:18451.
Graziane NM, Sun S, Wright WJ, Jang D, Liu Z, Huang YH, et al. Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci. 2016;19:915–25.
Chen B, Wang Y, Liu X, Liu Z, Dong Y, Huang YH. Sleep regulates incubation of cocaine craving. J Neurosci. 2015;35:13300–10.
Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 2014;83:1453–67.
Collins AL, Aitken TJ, Huang IW, Shieh C, Greenfield VY, Monbouquette HG, et al. Nucleus accumbens cholinergic interneurons oppose cue-motivated behavior. Biol Psychiatry. 2019;86:388–96.
Yu J, Yan Y, Li KL, Wang Y, Huang YH, Urban NN, et al. Nucleus accumbens feedforward inhibition circuit promotes cocaine self-administration. Proc Natl Acad Sci USA. 2017;114:E8750–E59.
Schall TA, Wright WJ, Dong Y. Nucleus accumbens fast-spiking interneurons in motivational and addictive behaviors. Mol Psychiatry. 2021;26:234–46.
Smith ACW, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, et al. Accumbens nNOS interneurons regulate cocaine relapse. J Neurosci. 2017;37:742–56.
Nugent AL, Anderson EM, Larson EB, Self DW. Incubation of cue-induced reinstatement of cocaine, but not sucrose, seeking in C57BL/6J mice. Pharm Biochem Behav. 2017;159:12–17.
Mead AN, Zamanillo D, Becker N, Stephens DN. AMPA-receptor GluR1 subunits are involved in the control over behavior by cocaine-paired cues. Neuropsychopharmacology 2007;32:343–53.
Pardo-Garcia TR, Garcia-Keller C, Penaloza T, Richie CT, Pickel J, Hope BT, et al. Ventral Pallidum is the primary target for accumbens D1 projections driving cocaine seeking. J Neurosci. 2019;39:2041–51.
Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13.
Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, et al. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22:7687–94.
Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci. 2006;23:3109–18.
White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharm Exp Ther. 1995;273:445–54.
Hu XT, Basu S, White FJ. Repeated cocaine administration suppresses HVA-Ca2+ potentials and enhances activity of K+ channels in rat nucleus accumbens neurons. J Neurophysiol. 2004;92:1597–607.
Zhang XF, Cooper DC, White FJ. Repeated cocaine treatment decreases whole-cell calcium current in rat nucleus accumbens neurons. J Pharm Exp Ther. 2002;301:1119–25.
Zhang XF, Hu XT, White FJ. Whole-cell plasticity in cocaine withdrawal: reduced sodium currents in nucleus accumbens neurons. J Neurosci. 1998;18:488–98.
Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, et al. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci. 2014;17:73–80.
Guo R, Wang Y, Yan R, Chen B, Ding W, Gorczyca MT, et al. Rapid eye movement sleep engages melanin-concentrating hormone neurons to reduce cocaine seeking. Biol Psychiatry. 2022;92:880–94.
Wang YQ, Huang YH, Balakrishnan S, Liu L, Wang YT, Nestler EJ, et al. AMPA and NMDA receptor trafficking at cocaine-generated synapses. J Neurosci. 2021;41:1996–2011.
Wang Y, Guo R, Chen B, Rahman T, Cai L, Li Y, et al. Cocaine-induced neural adaptations in the lateral hypothalamic melanin-concentrating hormone neurons and the role in regulating rapid eye movement sleep after withdrawal. Mol Psychiatry. 2021;26:3152–68.
Ge F, Mu P, Guo R, Cai L, Liu Z, Dong Y, et al. Chronic sleep fragmentation enhances habenula cholinergic neural activity. Mol Psychiatry. 2021;26:941–54.
Shukla A, Beroun A, Panopoulou M, Neumann PA, Grant SG, Olive MF, et al. Calcium-permeable AMPA receptors and silent synapses in cocaine-conditioned place preference. EMBO J. 2017;36:458–74.
Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharm Rev. 2016;68:816–71.
Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72.
Acknowledgements
We thank Jaryd Ross and Min Li for excellent technical support. Cocaine-HCl was supplied by the Drug Supply Program of the National Institute on Drug Abuse.
Funding
The authors’ work was partially supported by NIH grants DA023206 (YD), DA040620 (YD), DA047861 (YD), DA051010 (YD), DA052419 (ZF), AA028800 (ZF).
Author information
Authors and Affiliations
Contributions
Design of the work (YH and YD); Acquisition, analysis, or interpretation of data for the work (YH, KL, JW, and YQW); Drafting or revising the work (YH, ZF, and YD).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, Y., Wang, J., Li, Kl. et al. Membrane excitability of nucleus accumbens neurons gates the incubation of cocaine craving. Neuropsychopharmacol. 48, 1318–1327 (2023). https://doi.org/10.1038/s41386-023-01580-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01580-w