Abstract
Our skin contributes critically to health via its role as a barrier tissue, carefully regulating passage of key substrates while also providing defense against exogenous threats. Immunological processes are integral to almost every skin function and paramount to our ability to live symbiotically with skin commensal microbes and other environmental stimuli. While many parallels can be drawn to immunobiology at other mucosal sites, skin immunity demonstrates unique features that relate to its distinct topography, chemical composition and microbial ecology. Here we provide an overview of skin as an immune organ, with reference to the broader context of mucosal immunology. We review paradigms of innate as well as adaptive immune function and highlight how skin-specific structures such as hair follicles and sebaceous glands interact and contribute to these processes. Finally, we highlight for the mucosal immunology community a few emerging areas of interest for the skin immunity field moving forward.
Similar content being viewed by others
Introduction
Our skin is an immune-rich tissue through which we mediate continual interactions with our external surroundings. While not a ‘mucosal’ surface in the strictest sense, cutaneous biology parallels in many ways that of other barrier tissues, such as the intestine, oropharynx or genital mucosa. As our body’s outermost surface, skin also displays notably distinct structural characteristics and endures specific functional challenges and environmental exposures. The skin’s immune system has evolved to integrate and respond to these various signals and accordingly encompasses unique tissue-specific features. We highlight these here for the readership of Mucosal Immunology in honor of the journal’s decision to expand its scope to also include research focused on cutaneous immunity.
Tissue topography sets the stage for biology
The dry, cool, hair-bearing surface of skin makes it immediately distinguishable from the warm, moist and smooth niches typical of other barrier tissues. These differences extend to the biochemical level1, with skin being comparatively more acidic, nutrient-deficient and high in saline than the neutral pH, carbon- and nitrogen-rich intestinal environment. The skin epithelium is also rich in a variety of lipid species2,3. Free fatty acids, ceramides, and cholesterol are produced by keratinocytes in the upper layers of the epidermis4,5, and sebum secreted onto the skin’s surface contains triglycerides, wax esters, squalene, and free fatty acids6. These fatty acids, along with poly-carboxylic acids generated through deamination of epithelial amino acids, contribute to skin acidification – a process which in turn supports skin barrier integrity7.
Oxygen density is another key distinguishing feature of skin as compared to some mucosal sites. In contrast to the highly anaerobic lumen of the colon and the steep oxygen gradient across the intestinal epithelium8, the skin surface is largely aerobic with the exception of microaerophilic invaginations created by hair follicles and other adnexal structures9. Alteration of these microenvironments can impact skin immunobiology. For example, a shift towards more anaerobic conditions in hair follicles can lead to outgrowth of Cutibacterium acnes—a commensal bacterial species native to this niche—and augment its production of short chain fatty acids (SCFAs)10. These SCFAs, in turn, enhance proinflammatory cytokines through epigenetic changes in epithelial keratinocytes11,12. Thus, the cutaneous environment provides a distinct, tissue-specific topography for microbial commensals and a unique metabolic milieu for immune cell13,14. How the latter shapes cutaneous immunity, as distinguished from other mucosal sites, however, remains to be fully elucidated.
The skin epidermis – at the immunological forefront
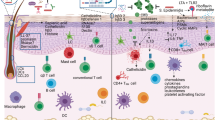
The epidermis constitutes the primary physical barrier between us and our external environment, providing a first line of defense against various physical, chemical and infectious threats (Fig. 1). Unlike the single layer of columnar enterocytes found in the intestinal epithelium, the skin’s epidermis is a stratified, squamous epithelium comprised of keratinocyte cells that proliferate in the basal layer then migrate upward and undergo a process of terminal differentiation into corneocytes. This transition is denoted by transglutaminase-mediated protein cross-linking, secretion of lamellar bodies, and ultimately nuclear loss15,16. In the upper outer layers, lipids synthesized by keratinocytes are released to fill the intercellular spaces between these corneocytes, providing a tight and effective barrier that limits both transepidermal water loss and penetration of exogenous compounds17. Similar to other mucosal barrier sites, tight junction proteins such as occludins, claudins, and zona occludens, are crucial to maintain skin barrier integrity and restrict harmful substances, while also allowing transport of essential molecules18,19.
Top panel: The epidermis is a stratified, squamous epithelium and comparatively lipid rich, nutrient poor, and acidic. Beneath the epidermis are a diverse set of immune cells that augment the physical barrier. Similar to mucus, the skin surface is coated with lipids and waxes produced by the sebaceous gland and other exocrine glands. Bottom panel: The gastrointestinal tract is a single layer of columnar enterocytes. In contrast to the skin, the healthy gastrointestinal tract contains tertiary lymphoid organs known as Peyer’s Patches. NMF natural moisturizing factor, AMP antimicrobial protein, NK Natural Killer, DC Dendritic cell, ILC Innate lymphoid cells.
This robust physical barrier is complemented by a plethora of innate and adaptive immune defenses, as discussed in more depth below. The skin’s epidermis is replete with antimicrobial molecules that both limit microbial entry and serve additional immunological functions20. Antigen presenting cells, namely Langerhans cells, also serve as immune sentries of the epidermis, extending their dendrites through tight junctions to sample antigens on the skin surface21. T cells, in particular CD103+ resident memory T cells in human skin22, and a specific subset of Vδ1 γδ T cells in murine skin23, also display significant epidermotrophism.
Skin Adnexal Structures & Immunologic Sub-Niches
Skin contains several exocrine adnexal structures, including sebaceous glands, and apocrine and eccrine sweat glands. Analogous to saliva, pulmonary surfactant, and intestinal mucus, skin secretions are controlled by a combination of circulating and local hormones24. Sebaceous glands release their lipid-rich products, known as sebum, by holocrine secretion25. These glands are typically found in association with hair follicles, forming so-called pilosebaceous units. At specific body sites, sebaceous glands are also found independent of hair follicles, most notably near the eye, lips and genitalia. Depending on their location, sebaceous glands fulfill a number of functions, including protection of the skin and hair, thermoregulation, formation of the tear lipid film, and pheromone-based communication26. With regard to skin immune function, both sebaceous and sweat glands contribute to the pool of cutaneous antimicrobial peptides27,28. Eccrine sweat glands and sebocytes are also capable of producing cytokines and chemokines29,30. Reciprocally, innate lymphoid cells and T cells31,32, as well as the cytokines TSLP, IL-4 and IL-1331,33 have been shown to influence sebaceous activity in human and mouse models.
Hair follicles are the most structurally distinct adnexal structures in skin. While concentrated on the scalp, axilla and genitalia, hair follicles are present everywhere on skin except for acral surfaces. The bulge region of hair follicles, analogous to the base of intestinal crypts, represents a key epithelial stem cell niche34. These stem cells support re-epithelization of the interfollicular epidermis after injury and facilitate the process of hair follicle cycling. Skin regulatory T cells (Tregs), γδ T cells35,36, and inflammatory cytokines, such as interleukin (IL)-1, IL-17 and TNFα37, have all been shown to influence epidermal stem cell differentiation and thus serve an immune mediators of hair follicle cycling.
Hair follicle keratinocytes, in turn, produce various chemokines that direct homing of myeloid and lymphoid cells. For example, during inflammation keratinocytes in the upper hair follicle isthmus and infundibulum produce CCL2 and CCL20 and facilitate epidermal accumulation of monocyte-derived dendritic cells38. Likewise, hair follicle derived IL-7 and IL-15 support epidermal and hair follicle tropism of skin memory T cells39. Regulatory T cells (Tregs), in particular, have been demonstrated to preferentially localize to the perifollicular dermis40, and their tissue density is correlated with that of hair follicles both in fetal and adult skin41,42. In murine studies, CCL20 from infundibular keratinocytes participates in early recruitment of Tregs to this tissue niche43. How Tregs are recruited and retained within the hair follicles of human skin remains unclear. These observations, in tandem with the fact that hair follicles represent a dense niche for skin microbial symbionts, underscore the fascinating role these structures play in cutaneous immunity44.
A discussion of skin immunology is also not complete without mention of the subcutaneous layer, comprised primarily of adipocytes, fibroblasts and other stromal cells. Subcutaneous adipocytes help limit skin infections by producing the antimicrobial peptide, cathelicidin45, a capacity that is diminished in the skin of elderly or obese subjects46,47. Fibroblasts on the other hand express cytokines and receptors that facilitate their interaction with adjacent immune cells. One subset of skin fibroblasts was recently shown to express type 2 cytokine receptors and, in the right pathological context, to reciprocally support expansion of subcutaneous type 2 helper (Th2) CD4+ T cells48. Other dermal fibroblast populations can influence recruitment, activation and differentiation of immune cells, both during homeostasis or in inflammatory contexts such as wound healing49.
Spatial organization of immune cells in barrier tissues is intrinsically related to their function. This is shaped in part by interactions with tissue structures and parenchymal cells, as discussed above. Separately, mucosal tissues can contain tertiary lymphoid structures, so called mucosa-associated lymphoid tissue (MALT), which contribute to tissue immune function, in particular antibody production in the intestines and nasopharynx50,51. The concept and nomenclature of skin-associated lymphoid tissue (SALT) was proposed as early as 197852. However, like the human lung where tertiary structures tend to be found mostly in association with disease53, healthy skin generally lacks substantial tertiary lymphoid structures54. Inducible SALT (iSALT), seen in tandem with skin inflammation55, consists of dendritic cells, perivascular macrophages and T cells clustered near post-capillary venules. In murine models, IL-1R1 signaling and CXCL2 are required for the formation of iSALT and the ability to activate T cells through antigen presentation56. The contributions of iSALT to inflammation and disease in human skin remain to be fully elucidated, but analogous structures have been described in the context of allergic, autoimmune and infectious skin disease56,57.
Skin microbiota as a natural cutaneous adjuvant
While the intestines house the body’s largest biomass of microbial symbionts, the skin is a close second supporting up to a million live bacterial cells per square centimeter58. As has been shown for other mucosal sites, skin commensals provide continual signals to keratinocytes and skin immune cells, which help shape the tissue’s homeostatic immune function59. Metagenomic sequencing has revealed that skin bacteria are accompanied, albeit at lower levels, by cutaneous fungi and viruses60. While many skin microbes reside in the upper layers of the stratum corneum, hair follicles and other adnexal structures provide protected invaginations and unique microenvironments for bacteria to thrive. While live bacteria are not thought to readily penetrate past the epidermis in healthy skin, studies have suggested that low amounts of viable bacteria can be found in the dermis61, thus presenting other opportunities for direct immune interaction.
Commensal bacteria contribute to cutaneous innate immune defense, both by producing their own antimicrobial peptides (AMPs) and inducing expression of host AMPs in epithelial cells47,62,63. Studies in gnotobiotic mice suggest that commensal microbes also elicit homeostatic levels of cytokine production by keratinocytes and sebocytes29, augment skin expression of complement pathways64, expand the pool of skin CD4+ and CD8+ T cells, and stimulate skin T cell cytokine production9,65,66,67,68. The broader effect is a more robustly activated skin immune system that is primed for defense against pathogenic microbes69.
Given the distinct cutaneous architecture and nutrient availability, the composition of skin microbiota differs substantially from that of the intestines and other mucosal sites, and demonstrates compositional diversity across different skin body sites, as dictated by. Just as bacterial composition differs along the length of the intestine as dictated by changes in luminal composition and transit speed70, each microenvironment of the skin favors its own array of microbial communities70,71,72. Lipophilic microbes, including Cutibacterium species dominate sebaceous sites whereas Staphylococcus and Corynebacterium species thrive in humid environments such as the moist areas of the groin and feet73,74. Strain-level skin bacterial diversity is often also spatially organized within a given individual75, for example, genetically similar species of Staphylococcus epidermidis are found more consistently on the feet versus other body sites76,77.
These regional differences in composition of bacterial communities across the skin’s surface are notable from an immunological perspective because skin bacteria have been shown to elicit distinct species-specific and even strain-specific profiles of immune activation67,78. This is especially true for the types of cytokines produced by skin resident T cells65,79. If regional differences in skin topography, chemical composition, and microbial ecology correspond to functional differences in cutaneous immunity across the skin surface, however, remains unclear.
Innate immune pathways in skin
Analogous to epithelial cells in the intestine, lung, mouth and vaginal mucosa, skin keratinocytes help calibrate the magnitude and flavor of immunity in response to various stimuli80. Keratinocytes and sebocytes are equipped with Toll-like receptors (TLRs) and NOD-like receptors (NLRs), making them poised to respond to lipopolysaccharides, peptidoglycans, and host or microbially-derived nucleic acids81,82. In skin, TLR2, TLR6 and NOD2 are particularly important for recognition and response to prevalent Staphylococcal and Streptococcal commensals and pathogens. TLRs 2, 3, 7, 8, and 9 help recognize skin trophic viruses such as herpesviruses, papillomaviruses and poxviruses. C type lectin receptors, in particular Dectin-1, recognize and control fungal pathogens such as Candida albicans83.
Bacterial sensing by keratinocytes also triggers production of IL-1 family cytokines that augment core downstream processes such as wound healing84 and T cell activation85,86. Keratinocytes, like aerodigestive epithelial cells87,88, can also be induced to express MHC class II, for example in response to TNFα, IFNγ, or IL-2289,90,91. Whereas keratinocytes are not thought to prime naïve T cells, their MHC class II expression can contribute to expansion and activation of skin memory T cells92. For example, in mice this capacity has been shown to promote epidermal accumulation of IFNγ-producing commensal-specific CD4+ and CD8+ T cells89.
Inflammasome sensors, such as NLRP3, also respond to signals of cellular damage or microbial invasion in skin. Cutaneous signals of adequate amplitude and in an appropriate context trigger inflammasome assembly, which then converts pro-interleukin-1β (pro-IL-1β) into biologically active IL-1β93. In addition to the role of inflammasomes in acute skin immune responses, they have also been implicated in prolonged inflammatory memory following acute inflammation via epigenetic changes to epithelial stem cells following stimulation of the inflammasome sensor AIM294. Aberrant activation of inflammasome machinery in skin contributes to prototypical rashes in monogenetic autoinflammatory disorders95. It is likewise implicated in the pathogenesis of many more common skin diseases, such as psoriasis, vitiligo, systemic lupus, and atopic dermatitis96.
Skin antimicrobial peptides and lipids
One downstream effect of innate immune stimulation is the generation of AMPs and antimicrobial lipids that defend the host against infectious agents97. AMPs limit pathogen colonization and shape composition of indigenous microbial communities98. They also serve as immunomodulatory molecules via their ability to recruit and directly activate dendritic cells, macrophages, mast cells, neutrophils and other sentinels of the cutaneous immune system99. A diverse group of epithelial AMPs have evolved to cope with the complex microbial communities in our environment (Fig. 2). The human β-defensins (hBD1-3) in skin exhibit antimicrobial activity against Escherichia coli and methicillin resistant Staphylococcus aureus (MRSA), while human cathelicidin (LL-37) can kill Group A Streptococcus and Candida albicans and inhibit biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa100. The skin also produces members of the resistin family, which have bactericidal function against coagulase negative Staphylococci101,102.
a The skin expresses AMPs constitutively, such as RNase7 and the β-defensins. Other AMPs are inducible in response to bacteria and other environmental triggers. Skin appendages also generate AMPs in response to stimuli. b Lysozyme and α-defensins are present constitutively, while other AMPs require immunological or bacterial stimuli for expression. AMPs Antimicrobial proteins, TLR Toll like receptor, PAMPS Pathogen-associated molecular patterns.
Recent work has also highlighted bactericidal capacity among Small proline rich proteins (SPRR) in the skin and gut. The skin expresses SPRR1 and SPRR2, which limit infection by Pseudomonas aeruginosa and Staphylococcus aureus103. Whereas intestinal SPPR2 production is modulated by microbial exposure, i.e., low levels are observed in germ free mice and restored with conventionalizion104, cutaneous SPRR1 and SPRR2 levels are comparable in germ free and conventionalized mice, with increased expression seen only after intradermal injection of LPS103. Thus, while cutaneous AMP biology parallels that seen in other barrier tissues, skin employs unique AMP repertoires and regulatory frameworks to bolster its defense.
Beyond AMPs, the skin also expresses antimicrobial lipids and fatty acids. Human sebaceous glands produce sapienic acid and linoleic acid, which can limit bacterial growth. These lipids exert their antimicrobial activity by triggering membrane depolarization and blocking macromolecular branching, which lead to bacterial cell dissolution and death105. The role of these antimicrobial lipids as effector molecules of innate immune defense is a key area of emerging interest.
Innate immune cell populations in skin
Skin contains a network of innate immune cells that readily respond to acute stimuli and recruit in other populations in response to tissue injury or alarmins (Fig. 3). Much like gut-resident CX3CR1-expressing mononuclear phagocytes that “sample” luminal microbial antigens106, Langerhans cells positioned in the epidermis extend their dendrites to capture antigens near the surface of the skin21. These embryonically seeded cells, which arise from macrophage precursors and acquire dendritic cell properties once in the epidermis, are marked in humans by expression of CD1a and CD1c (MHC-I related molecules involved in presentation of lipid antigens) as well as in mice and humans by the C-type lectin CD207 (also known as langerin)107,108. While generally a self-renewing population in the setting of homeostasis, bone-marrow derived monocytes help to augment the Langerhans cells niche during skin inflammation107. Langerhans cells have been demonstrated to promote both effector and regulatory T cell responses in skin109. Two subsets of Langerhans cells were recently reported in human skin110, though further work is needed to fully uncover how this relates to distinct functionality.
(a) Human and (b) murine skin immune cell types and common surface marks identifiable by flow cytometry. DETC dendritic epidermal T cell, DC dendritic cell, DN DC double negative DC, ILC2 type 2 innate lymphoid cell, LHC Langerhans cell, MAIT Mucosal Associated Invariant T cell, Mo-DC monocyte-derived DC, Mθ macrophage, NKT Natural Killer T cell, Treg Regulatory T cell, tet+ tetramer positive, TRM T resident memory cell.
Antigen presenting cells in the dermis include both conventional dendritic cells (cDCs) and monocyte-derived dendritic cells (moDCs). Dermal cDC1s are marked by expression of CD141 in human skin and CD103 in murine skin, whereas human and mouse cDC2s express CD1c and CD11b respectively111. In the murine dermis, there is also a population of CD103neg CD11bneg (so called ‘double-negative’) dendritic cells. CD123+ plasmacytoid DCs are minimally present in healthy skin but can increase significantly in the context of various diseases112. Classical monocytes, which express CD14 and/or CD16 in human skin and Ly6c, Ccr2 or CX3CR1 in the mouse, can acquire class II expression after entering the tissue and differentiate into moDCs113, a population marked by CD14 in the human and CD11b and CD64 in the mouse. A full discussion of the relative functional capacities of various skin DCs subsets is beyond the scope of this review but a very active area of research111,114.
Macrophage populations in the skin, like the intestine, are thought to comprise both embryonically seeded as well as recruited monocyte-derived subsets113. However, approaches to reliably distinguish these populations in skin and decipher their distinct functions are still rudimentary. Mast cells, marked histologically by their heterochromatic granules and production of tryptase, are also found in healthy human skin, especially at acral sites115. Cutaneous mast cells produce AMPs116 and other molecules that contribute to homeostatic innate immune defense as well early activation of allergic skin responses117,118. Innate lymphoid cells (ILCs) are another innate cell population integral to coordinating immune responses in skin. Natural killer cells, type 1, type 2 and type 3 ILCs are all present in skin, but ILC2s are particularly prevalent119, especially as compared to the relative ILC3 predominance seen in the intestinal mucosa and human lung120. While skin ILCs, like those in other mucosal tissues, are primarily a tissue resident population that is locally maintained and expanded, they express a distinct receptor pattern and are particularly dependent on IL-18 and TSLP for their activation and function121,122. Additional transcriptional profiles further differentiate subsets of ILCs found in the dermis versus the skin subcutis32. Skin inflammation is accompanied by marked changes in cutaneous innate immune cells. This includes expansion of existing populations, e.g., macrophages, dendritic cells, mast cells and ILCs121, recruitment of new ones, e.g., eosinophils and neutrophils, and cellular activation that leads to altered expression patterns of key surface markers111,123.
Skin lymphocytes
The skin is also a dense repository of T cells, containing an estimated 2 × 1010 across its entire surface, several fold the amount found in blood circulation124. Many of these cells are resident memory populations that spend the majority of their time in skin124,125. Analogous to intra-epithelial lymphocytes found in the intestinal lining126, human and murine skin both contain epidermotrophic T cell subsets. In murine skin, Vγ5Vδ1 dendritic epidermal T cells (DETCs) seed the epidermis during late gestation, where they form stable interactions with Langerhans cells and produce cytokines that contribute to homeostatic proliferation of keratinocytes and wound healing127. The human epidermis lacks DETCs but preferentially contains populations of CD8+ CD103+ αβ T cells which may perform analogous functions125,128. CD4+ T cells constitute the majority of αβ T cells in both human and murine skin. These cells are found preferentially in the dermis but can also be present in the epidermis and subcutis48,124.
Regulatory T cells (Tregs) with a resident memory phenotype comprise 10–30% of the cutaneous CD4+ T cell population in healthy adult human skin40 and 20–60% in murine skin129. In the small intestine, it is understood that food antigens promote Treg expansion and that microbial products, such as SCFA130 and secondary bile acids131, support colonic Treg populations132. Skin microbes are capable of producing SCFAs133,134, but germ-free adult mice do not demonstrate the same deficiency of Tregs in skin as is seen in the colon79. Thus, the exact signals that promote high levels of skin Tregs remain to be fully defined and may include other tissue-specific processes such as UV exposure135. Skin might be comprised of proportionally more thymic Tregs as compared to the colon43. Another distinction of cutaneous Tregs is their preferential expression of the transcription factor GATA3136. Notably, absence of Gata3 Treg expression or depletion of neonatal skin Tregs leads to increased type 2 inflammation in murine skin48,66.
The dermis also contains a wealth of unconventional lymphocyte subsets. These include dermal γδ T cell populations, which are more prevalent in murine than in human skin, as well as mucosa-associated invariant T (MAIT) cells. MAIT cells seed the tissue in early life via a thymic wave fueled by circulating microbe-derived riboflavin metabolites137. In later life, local production of the same metabolites by skin commensal bacteria such as S. epidermidis can expand these populations138. Whether other innate type lymphocytes, such as non-MAIT PLZF+ T cells are present in human skin, as has been shown for the intestine139, remains to be seen.
T-cell subsets, including dermal γδ T cell, cutaneous MAIT populations, and type 17 CD4+ T helper (Th17) cells, are major sources of cutaneous IL-17A. In contrast to the oral mucosa where frictional forces fuel homeostatic Th17 responses140, the abundance and function of IL-17-producing skin lymphocytes is largely supported by exposure to commensal microbes141. In murine as well as some primate models, skin colonization by fungal and bacterial commensals, such as Candida albicans, Malassezia furfur, or Staphylococcus epidermidis, leads to accumulation of Th1778,142. Likewise, Corynebacterium accolens elicits IL-17A production by murine Vγ4+ dermal γδ cells, a process dependent on the bacterium’s expression of surface mycolic acids68. Upon skin injury, Staphylococcus-induced Th17 cells contribute to tissue repair66. However, unlike in the intestines, use of IL-17 targeting biologics has not been associated with impairment of epithelial integrity and anti-IL-17 therapies are now a mainstay for the treatment of psoriasis143,144.
Emerging themes in skin immunology
Circadian rhythm
The circadian rhythm is a daily oscillation in behavior and physiology entrained by the 24 h day/night cycle along with other stimuli (e.g., feeding/fasting, oxygen). It affects the feeding rhythmicity of the host and has been shown to influence the resident microbiota, host metabolism and immune functions145,146. Intriguingly, critical interactions between the microbiota and intestinal mucosal epithelium are orchestrated by the circadian clock. Recent work revealed that rhythmic expression of intestinal antimicrobial proteins was driven by daily rhythms in gut epithelial attachment by segmented filamentous bacteria147. Similarly, emerging evidence has unearthed an important role for the circadian clock in regulating skin immunity. Several skin antimicrobial proteins including chemerin, cathelicidin and β-defensin 1 show circadian expression changes that affect survival of bacteria on the skin surface148. Another recent study also revealed time-of-day dependent activation of the interferon pathway in murine skin149. These findings demonstrate the importance of considering circadian rhythm as a key regulator of cutaneous immunity, although further studies are needed to understand the intensity and breadth of these effects as well as their mechanistic basis.
Hormones
Hormones secreted by mucosal surfaces such as secretin, gastrin, glucagon-like peptides and steroid hormones could affect host metabolism and immune responses at diverse mucosal sites150. Among them, glucocorticoids and sex hormones play a crucial role in the modulation of the mucosal barrier function as well as the susceptibility to infections. Steroid hormones from the adrenal gland are known to be systemically distributed by blood circulation with different effects on various bodily organs. In recent years, emerging evidence suggests a key role for local steroidogenesis in the intestine, lung and skin in modulating tissue immune responses during allergy or infection. For instance, type 2 skin inflammation induced by the vitamin D3 analog MC903 can promote local glucocorticoid synthesis in keratinocytes151. These same type 2 cytokines can also promote androgen production and drive lipid abnormalities in sebocytes152. Adding to this, differences in sex hormone levels contribute to the sexual dimorphism seen in response to infection at the skin surface. For example, female mice display more efficient defense against Staphylococcus aureus skin infection, likely due to enhanced activation of neutrophils153 on account of augmented type I IFN signaling in female versus male mice154. These studies highlight the importance of further research to comprehensively understand interactions between hormones and skin immune system.
Neuro-immunology
Commensurate with its role at the forefront of environmental sensing, skin is a highly innervated tissue with the capacity to integrate itch, pain, mechanical, and thermal stimuli. In the last several years, there have been considerable advances in our understanding of how the immune system impacts skin neurophysiology and conversely how cutaneous innervation affects skin immune function155. It has long been recognized that mast cell-derived molecules such as tryptase, leukotrienes and histamine can directly activate G-protein coupled receptors on cutaneous nerves to promote itch156.
We now understand that neurons also express an array of cytokine receptors, including those for TNFα, IL-17, IL-1, IL-4, IL-13, IL-31, IL-33 and TSLP. In the currently established framework, inflammatory states that augment skin production of TNFα, IL-17, or IL-1 can augment pain intensity, whereas those that produce Th2 cytokines preferentially increase cutaneous itch121,155,157. Even more recent work has identified a role for basophil-derived leukotriene C4 to drive acute itch in the setting of atopic dermatitis, a process that is independent of mast cells and driven by allergen-mediated IgE158.
Equally intriguing are the effects of cutaneous nerves on skin immune function. Release of calcitonin gene-related peptide (CGRP) by TRPV1+ neurons in response to skin infection by C. albicans has been shown to stimulate cDC2 production of IL-23, thereby augmenting Th17 responses159. Indeed, direct optogenetic stimulation of these same nerves in mice can elicit a robust Th17 skin response160. Notably, this local nerve activation leads to heightened Th17 tone and ‘anticipatory immune defense’ in surrounding ‘naïve’ skin as a result of neuronal reflex arc160. Cutaneous nerves can also contribute to skin immune homeostasis. For example, the neuropeptide TAFA4 produced by certain mechanoreceptors in response to UV injury can augment IL-10 production by skin macrophages, thereby limiting fibrosis and augmenting skin repair161. Additionally, a subset of MrgprD-expressing sensory nerves were recently shown to suppress hyperresponsive mast cell degranulation via their release of glutamate, which was sufficient to modulate intensity of inflammation in several murine models of allergic dermatitis162. Other neuropeptides, such as vasoactive intestinal peptide (VIP), that have been shown to have immunologic functions at other mucosal sites163, are also expressed by somatosensory neurons, although their effects on skin immunity remain to be determined164.
Skin-gut immune axis
Linked gut-skin immunobiology has long been a topic of interest based on clinical observations connecting inflammation at these two barrier sites165,166,167. Overlap among gut and skin homing receptors, e.g., CCR4 and α4β7, may contribute in part to such cross-talk168, for example facilitating gut-to-skin homing of allergen-specific T cells in murine epicutaneous allergen challenge167. Under homeostatic conditions, it has been shown that microbially-directed tuning of cutaneous immune function is dominated by the effects of local skin bacteria79. However, inflammation or microbial dysbiosis in either gut or skin appears to open the door to shared immunopathology. Microbially-driven intestinal Th17 responses can augment psoriasiform-like inflammation in murine skin169. Likewise, murine colitis can drive a shift away from Tregs among S. epidermidis-specific CD4+ T cells, contributing to Th17-dominant, neutrophil-rich skin inflammation170. This cross-tissue dialog can also run in the opposite direction, i.e., from skin to gut. Skin injury has been shown to augment anaphylaxis to oral antigens in mice via expansion of intestinal mast cells171, as well as to increase the severity of colitis via effects on intestinal stromal cells172. Given the multiple clinical contexts for potentially linked gut-skin inflammation, i.e., neutrophilic dermatoses in the setting of inflammatory bowel disease, food allergies and atopic dermatitis, or intestinal dysbiosis in psoriasis173, research is this area is bound to accelerate.
Diet
Dietary changes can have profound effects on the intestinal immune system and disease susceptibility174. High fat intake allows expansion of pathobionts that can compromise intestinal barrier function, triggering a chronic low-grade systemic inflammatory response175. In contrast, a diet high in fiber supports microbial production of SCFAs, promoting energy expenditure and protecting against inflammation and insulin resistance176. Independent of effects on gut microbes, caloric restriction can also directly augment intestinal innate immune function177. Notably, dietary composition can also impact the skin’s microbiota and modulate cutaneous immunity. For instance, elevated body mass index (BMI) is associated with altered composition of the skin microbiota, and mice placed on a high fat diet display increased relative abundance of lipid-loving Corynebacterium spp178,179. Dietary effects on skin microbial communities may occur via altered substrate availability, i.e., increased free fatty acids in the skin of mice on a high fat diet179, or effects on antimicrobial molecule expression, i.e., dietary vitamin A is required for optimal expression of the antimicrobial protein, RELMα, in murine skin180. Recent work has further demonstrated that a lipid-rich diet can increase expression of endogenous retroviruses in murine skin and amplify the inflammatory response to microbes181. These insights highlight new avenues for mucosal immunity research and therapeutic development. It is essential to identify beneficial and pathogenic bacterial species induced by specific dietary constituents and select nutrients or bacterial metabolites with potential immunomodulatory effects.
Conclusion
As highlighted above, immunologic mechanisms are critical to so many facets of skin biology. While aspects of cutaneous immune function are highly specific to skin as an external barrier organ, many others display shared features with immunity at the intestinal, lung and other mucosal surfaces. Emerging areas of interest in skin immunology further emphasize the connectivity of this barrier surface to systemic processes, such as endocrinology and neurobiology, and increasingly suggest the possibility of immune crosstalk between skin and other mucosal tissues. The fields of cutaneous and mucosal immunology have traditionally been considered parallel but distinct disciplines. However, there is much to be gleaned from their close, comparative study. We look forward to increasingly joint dialog between these two research communities under the umbrella of Mucosal Immunology’s readership.
References
J. Tobin, D. Biochemistry of human skin—our brain on the outside. Chem. Soc. Rev. 35, 52–67 (2006).
Lampe, M. A. et al. Human stratum corneum lipids: characterization and regional variations. J. Lipid Res. 24, 120–30 (1983).
Bouwstra, J. A. & Ponec, M. The skin barrier in healthy and diseased state. Biochim. Biophys. Acta Biomembranes 1758, 2080–95 (2006).
Ludovici, M. et al. Influence of the sebaceous gland density on the stratum corneum lipidome. Sci. Rep. https://doi.org/10.1038/s41598-018-29742-7 (2018).
Sjövall, P. et al. Imaging the distribution of skin lipids and topically applied compounds in human skin using mass spectrometry. Sci. Rep. https://doi.org/10.1038/s41598-018-34286-x (2018).
Pappas, A., Johnsen, S., Liu, J.-C. & Eisinger, M. Sebum analysis of individuals with and without acne. Derm. Endocrinol. 1, 157 (2009).
Elias, P. M. Stratum corneum acidification: how and why? Exp. Dermatol. 24, 179–80 (2015).
Konjar, Š., Pavšič, M. & Veldhoen, M. Regulation of oxygen homeostasis at the intestinal epithelial barrier site. Int. J. Mol. Sci. 22, 9170 (2021). 2021, Vol. 22, Page 9170.
Chen, Y. E., Fischbach, M. A. & Belkaid, Y. Skin microbiota–host interactions. Nature 553, 427 (2018).
Sanford, J. A., O’Neill, A. M., Zouboulis, C. C. & Gallo, R. L. Short-chain fatty acids from cutibacterium acnes activate both a canonical and epigenetic inflammatory response in human sebocytes. J. Immunol. https://doi.org/10.4049/jimmunol.1800893 (2019).
Sanford, J. A. et al. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aah4609 (2016).
Chinnappan, M. & Harris-Tryon, T. A. Novel mechanisms of microbial crosstalk with skin innate immunity. Exp Dermatol. https://doi.org/10.1111/exd.14429 (2021).
Pan, Y. et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543, 252–6 (2017).
Meyenn, L., von, Bertschi, N. L. & Schlapbach, C. Targeting T cell metabolism in inflammatory skin disease. Front. Immunol. 0, 2285 (2019).
Mahanty, S. & Setty, S. R. G. Epidermal lamellar body biogenesis: insight into the roles of golgi and lysosomes. Front. Cell Dev. Biol. https://doi.org/10.3389/fcell.2021.701950 (2021).
Raymond, A. A. et al. Lamellar bodies of human epidermis. Mol. Cell. Proteomics. https://doi.org/10.1074/mcp.m700334-mcp200 (2008).
Kahraman, E., Kaykın, M., Bektay, H. Ş. & Güngör, S. Recent advances on topical application of ceramides to restore barrier function of skin. Cosmetics, https://doi.org/10.3390/COSMETICS6030052 (2019).
Brandner, J. M. Importance of tight junctions in relation to skin barrier function. Curr. Probl. Dermatol. https://doi.org/10.1159/000441541 (2016).
Lee, B., Moon, K. M. & Kim, C. Y. Tight junction in the intestinal epithelium: its association with diseases and regulation by phytochemicals. J. Immunol. Res. https://doi.org/10.1155/2018/2645465 (2018).
Zhang, L. & Gallo, R. L. Antimicrobial peptides. Curr. Biol. 26, R14–R19 (2016).
Kubo, A., Nagao, K., Yokouchi, M., Sasaki, H. & Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 206, 2937–46 (2009).
Watanabe, R. et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 7, 279ra39 (2015).
Sutoh, Y., Mohamed, R. H. & Kasahara, M. Origin and evolution of dendritic epidermal T cells. Front. Immunol. 0, 1059 (2018).
Holmgren, S. & Olsson, C. Autonomic control of glands and secretion: a comparative view. Autonomic Neurosci. Basic Clin. https://doi.org/10.1016/j.autneu.2010.10.008 (2011).
Fischer, H. et al. Holocrine secretion of sebum is a unique DNase2-dependent mode of programmed cell death. J. Investig. Dermatol. https://doi.org/10.1016/j.jid.2016.10.017 (2017).
Ehrmann, C. & Schneider, M. R. Genetically modified laboratory mice with sebaceous glands abnormalities. Cell. Mol. Life Sci. https://doi.org/10.1007/s00018-016-2312-0 (2016).
Lovászi, M., Szegedi, A., Zouboulis, C. C. & Törőcsik, D. Sebaceous-immunobiology is orchestrated by sebum lipids. Derm. Endocrinol. 9, e1375636 (2017).
Schittek, B., Hipfel, R. & Sauer, B. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nature. http://www.kalbacher.uni-tuebingen.de/pdf/2001/hk20013.pdf (2001).
Nagy, I. et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 8, 2195–205 (2006).
Dai, X. et al. Eccrine sweat contains IL-1α, IL-1β and IL-31 and activates epidermal keratinocytes as a danger signal. PLoS ONE 8, 67666 (2013).
Choa, R. et al. Thymic stromal lymphopoietin induces adipose loss through sebum hypersecretion. Science 373, eabd2893 (2021).
Kobayashi, T. et al. Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell 176, 982–.e16 (2019).
Zhang, C. et al. Interleukins 4 and 13 drive lipid abnormalities in skin cells through regulation of sex steroid hormone synthesis. Proc. Natl. Acad. Sci. 118, 2021 (2021).
Tumbar, T. et al. Defining the epithelial stem cell niche in skin. Science 303, 359 (2004).
Lee, P. et al. Stimulation of hair follicle stem cell proliferation through an IL-1 dependent activation of γδT-cells. eLife 6, e28875 (2017).
Ali, N. et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 169, 1119–1129.e11 (2017).
Xiao, T., Yan, Z., Xiao, S. & Xia, Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res. Ther. 11, 1–9 (2020). 2020 11:1.
Nagao, K. et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 13, 744–52 (2012).
Adachi, T. et al. Hair follicle–derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat. Med. 21, 1272–9 (2015).
Rodriguez, R. S. et al. Memory regulatory T cells reside in human skin. J. Clin. Investig. 124, 1027–36 (2014).
Schulman, J. M. et al. The distribution of cutaneous metastases correlates with local immunologic milieu. J. Am. Acad. Dermatol. 74, 470–6 (2016).
Dhariwala, M. O. et al. Developing human skin contains lymphocytes demonstrating a memory signature. Cell Rep. Med. 1, 100132 (2020).
Scharschmidt, T. C. et al. Commensal microbes and hair follicle morphogenesis coordinately drive treg migration into neonatal skin. Cell Host Microbe 21, 467–.477.e5 (2017).
Kobayashi, T., Naik, S. & Nagao, K. Choreographing immunity in the skin epithelial barrier. Immunity 50, 552–65 (2019).
Zhang, L. et al. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 347, 67–71 (2015).
Zhang, L.J. et al. Age-related loss of innate immune antimicrobial function of dermal fat is mediated by transforming growth factor beta. Immunity 50, 121–136.e5 (2019).
Zhang, L. et al. Diet-induced obesity promotes infection by impairment of the innate antimicrobial defense function of dermal adipocyte progenitors. Sci. Transl. Med. 13, 5280 (2021).
Boothby, I. C. et al. Early-life inflammation primes a T helper 2 cell–fibroblast niche in skin. Nature 599, 667–72 (2021).
Correa-Gallegos, D., Jiang, D. & Rinkevich, Y. Fibroblasts as confederates of the immune system. Immunological Rev. 302, 147–62 (2021).
Reboldi, A. & Cyster, J. G. Peyer’s patches: organizing B-cell responses at the intestinal frontier. Immunol. Rev. https://doi.org/10.1111/imr.12400 (2016).
Harmsen, A. et al. Cutting edge: organogenesis of nasal-associated lymphoid tissue (NALT) occurs independently of lymphotoxin-α (LTα) and retinoic acid receptor-related orphan receptor-γ, but the organization of NALT is LTα dependent. J. Immunol. 168, 986–90 (2002).
Streilein, J. W. Lymphocyte traffic, T-cell malignancies and the skin. J. Investig. Dermatol. 71, 167–71 (1978).
Tschernig, T. & Pabst, R. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology 68, 1–8 (2000).
Kogame, T., Kabashima, K. & Egawa, G. Putative immunological functions of inducible skin-associated lymphoid tissue in the context of mucosa-associated lymphoid tissue. Front. Immunol. 0, 3387 (2021).
Kabashima, K., Honda, T., Ginhoux, F. & Egawa, G. The immunological anatomy of the skin. Nature Reviews. Nat. Rev. Immunol. 19, 19–30 (2019).
Natsuaki, Y. & Kabashima, K. Inducible lymphoid clusters, iSALTs, in contact dermatitis: a new concept of acquired cutaneous immune responses. Med. Mol. Morphol. 49, 127–32 (2016).
Honda, T. & Kabashima, K. Novel concept of iSALT (inducible skin-associated lymphoid tissue) in the elicitation of allergic contact dermatitis. Proc. Jpn. Acad. Ser. B, Phys. Biol. Sci. 92, 20 (2016).
Selwan, S. Microbiology and ecology of human skin. Practitioner 224, 1059–62 (1980).
Byrd, A. L., Belkaid, Y. & Segre, J. A. The human skin microbiome. Nat. Rev. Microbiol. 16, 143–55 (2018).
Oh, J. et al. Biogeography and individuality shape function in the human skin metagenome. Nature 514, 59–64 (2014).
Nakatsuji, T. et al. The microbiome extends to subepidermal compartments of normal skin. Nat. Commun. 4, 1431 (2013).
Lai, Y. et al. Activation of TLR2 by a small molecule produced by staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J. investig. Dermatol. 130, 2211 (2010).
Wanke, I. et al. Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J. Investig. Dermatol. 131, 382–90 (2011).
Chehoud, C. et al. Complement modulates the cutaneous microbiome and inflammatory milieu. Proc. Natl. Acad. Sci. 110, 15061–6 (2013).
Chen, Y. E. et al. Decoding commensal-host communication through genetic engineering of Staphylococcus epidermidis. bioRxiv 3, 664656 (2019).
Harrison, O. J. et al. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 363, eaat6280 (2019).
Linehan, J. L. et al. Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell 172, 784–96.e18 (2018).
Ridaura, V. K. et al. Contextual control of skin immunity and inflammation by Corynebacterium. J. Exp. Med. 215, 785–99 (2018).
Belkaid, Y. & Naik, S. Compartmentalized and systemic control of tissue immunity by commensals. Nat. Immunol. 14, 646–53 (2013).
Grice, E. A. & Segre, J. A. The skin microbiome. Nat. Rev. Microbiol. 9, 244–53 (2011).
Grice, E. A. et al. A diversity profile of the human skin microbiota. Genome Res. 18, 1043–50 (2008).
Findley, K. et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 498, 367–70 (2013).
Byrd, A. L., Belkaid, Y. & Segre, J. A. The human skin microbiome. Nat. Rev. Microbiol. https://doi.org/10.1038/nrmicro.2017.157 (2018).
Scharschmidt, T. C. & Fischbach, M. A. What lives on our skin: ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discov Today Dis. Mech. 10 (2013).
Kong, H. H., & Julia, O. State of residency: microbial strain diversity in the skin. J. Invest. Dermatol. https://doi.org/10.1016/J.JID.2021.10.005 (2021).
Zhou, W. et al. Host-specific evolutionary and transmission dynamics shape the functional diversification of Staphylococcus epidermidis in human skin. Cell 180, 454–70.e18 (2020).
Oh, J. et al. Temporal stability of the human skin microbiome. Cell 165, 854–66 (2016).
Naik, S. et al. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–8 (2015).
Naik, S. et al. Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–9 (2012).
Kobayashi, T., Naik, S. & Nagao, K. Choreographing immunity in the skin epithelial barrier. Immunity, https://doi.org/10.1016/j.immuni.2019.02.023 (2019).
Krutzik, S. R. et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. https://doi.org/10.1038/nm1246 (2005).
Sun, L., Liu, W. & Zhang, L. J. The role of toll-like receptors in skin host defense, psoriasis, and atopic dermatitis. J. Immunol. Res. https://doi.org/10.1155/2019/1824624 (2019).
Fukata, M. & Arditi, M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. https://doi.org/10.1038/mi.2013.13 (2013).
Wang, G. et al. Bacteria induce skin regeneration via IL-1β signaling. Cell host microbe 29, 777–91.e6 (2021).
Patrick, G. J. et al. Epicutaneous Staphylococcus aureus induces IL-36 to enhance IgE production and ensuing allergic disease. J. Clin. Invest. 131, e143334 (2021).
Liu, H. et al. Staphylococcus aureus epicutaneous exposure drives skin inflammation via IL-36-mediated T cell responses. Cell Host Microbe 22, 653 (2017).
Toulmin, S. A. et al. Type II alveolar cell MHCII improves respiratory viral disease outcomes while exhibiting limited antigen presentation. Nat. Commun. 12, 1–15 (2021).
Wosen, J. E., Mukhopadhyay, D., Macaubas, C. & Mellins, E. D. Epithelial MHC Class II expression and its role in antigen presentation in the gastrointestinal and respiratory tracts. Front. Immunol. 0, 2144 (2018).
Tamoutounour, S. et al. Keratinocyte-intrinsic MHCII expression controls microbiota-induced Th1 cell responses. Proc. Natl. Acad. Sci. USA 116, 23643–52 (2019).
Griffiths, C. E. M., Voorhees, J. J. & Nickoloff, B. J. Characterization of intercellular adhesion molecule-1 and HLA-DR expression in normal inflamed skin: Modulation by recombinant gamma interferon and tumor necrosis factor. J. Am. Acad. Dermatol. 20, 617–29 (1989).
Banerjee, G., Damodaran, A., Devi, N., Dharmalingam, K. & Raman, G. Role of keratinocytes in antigen presentation and polarization of human T lymphocytes. Scand. J. Immunol. 59, 385–94 (2004).
Black, A. P. B. et al. Human keratinocyte induction of rapid effector function in antigen-specific memory CD4+ and CD8+ T cells. Eur. J. Immunol. 37, 1485–93 (2007).
Nestle, F. O., Meglio, P. D. I., Qin, J. Z. & Nickoloff, B. J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. https://doi.org/10.1038/nri2622 (2009).
Naik, S. et al. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550, 475–80 (2017).
Figueras-Nart, I., Mascaró, J. M. Jr., Solanich, X. & Hernández-Rodríguez, J. Dermatologic and dermatopathologic features of monogenic autoinflammatory diseases. Front. Immunol. 10, 2448 (2019).
Tang, L. & Zhou, F. Inflammasomes in common immune-related skin diseases. Front. Immunol. 11, 882 (2020).
Mukherjee, S. & Hooper, L. V. Antimicrobial defense of the intestine. Immunity, https://doi.org/10.1016/j.immuni.2014.12.028 (2015).
Gallo, R. L. & Hooper, L. V. Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. https://doi.org/10.1038/nri3228 (2012).
Zhang, Q.-Y. et al. Antimicrobial peptides: mechanism of action, activity and clinical potential. Mil. Med. Res. 8, 1–25 (2021).
Herman, A. & Herman, A. P. Antimicrobial peptides activity in the skin. Skin Res. Technol. https://doi.org/10.1111/srt.12626 (2019).
Harris, T. A. et al. Resistin-like molecule a provides vitamin-A- dependent antimicrobial protection in the skin article resistin-like molecule a provides vitamin-A-dependent antimicrobial. Cell Host Microbe 25, 1–12 (2019).
Propheter, D. C., Chara, A. L., Harris, T. A., Ruhn, K. A. & Hooper, L. V. Resistin-like molecule beta is a bactericidal protein that promotes spatial segregation of the microbiota and the colonic epithelium. Proc. Natl. Acad. Sci. USA https://doi.org/10.1073/pnas.1711395114 (2017).
Zhang, C. et al. Small proline-rich proteins (SPRRs) are epidermally produced antimicrobial proteins that defend the cutaneous barrier by direct bacterial membrane disruption. bioRxiv, https://doi.org/10.1101/2021.09.01.458578 (2021).
Hooper, L. V. et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–4 (2001).
Parsons, J. B., Yao, J., Frank, M. W., Jackson, P. & Rock, C. O. Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from staphylococcus aureus. J. Bacteriol. https://doi.org/10.1128/JB.00743-12 (2012).
Niess, J. H. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–8 (2005).
Doebel, T., Voisin, B. & Nagao, K. Langerhans cells – the macrophage in dendritic cell clothing. Trends Immunol. 38, 817–28 (2017).
Deckers, J., Hammad, H. & Hoste, E. Langerhans cells: sensing the environment in health and disease. Front. Immunol. 0, 93 (2018).
Seneschal, J., Clark, R. A., Gehad, A., Baecher-Allan, C. M. & Kupper, T. S. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 36, 873 (2012).
Liu, X. et al. Distinct human Langerhans cell subsets orchestrate reciprocal functions and require different developmental regulation. Immunity 54, 2305–20.e11 (2021).
Haniffa, M., Gunawan, M. & Jardine, L. Human skin dendritic cells in health and disease. J. Dermatological Sci. 77, 85 (2015).
Psarras, A. et al. Functionally impaired plasmacytoid dendritic cells and non-haematopoietic sources of type I interferon characterize human autoimmunity. Nat. Commun. 11, 1–18 (2020).
Tamoutounour, S. et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39, 925–38 (2013).
Sumpter, T. L., Balmert, S. C. & Kaplan, D. H. Cutaneous immune responses mediated by dendritic cells and mast cells. JCI Insight 4, e123947 (2019).
Janssens, A. S. et al. Mast cell distribution in normal adult skin. J. Clin. Pathol. 58, 285 (2005).
Nardo, A., di, Yamasaki, K., Dorschner, R. A., Lai, Y. & Gallo, R. L. Mast cell cathelicidin antimicrobial peptide prevents invasive group A streptococcus infection of the skin. J. Immunol. 180, 7565 (2008).
Galli, S. J., Gaudenzio, N. & Tsai, M. Mast cells in inflammation and disease: recent progress and ongoing concerns. Annu. Rev. Immunol. 38, 49–77, https://doi.org/10.1146/annurev-immunol-071719-094903 (2020).
Gurish, M. F. & Austen, K. F. Developmental origin and functional specialization of mast cell subsets. Immunity 37, 25–33 (2012).
Kobayashi, T., Ricardo-Gonzalez, R. R. & Moro, K. Skin-resident innate lymphoid cells – cutaneous innate guardians and regulators. Trends Immunol. 41, 100 (2020).
Panda, S. K. & Colonna, M. Innate lymphoid cells in mucosal immunity. Front. Immunol. 0, 861 (2019).
Kim, B. S. et al. TSLP elicits IL-33–independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 5, 170ra16 (2013).
Ricardo-Gonzalez, R. R. et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 19, 1093–9 (2018).
Dudeck, J. et al. Mast cells acquire MHCII from dendritic cells during skin inflammation. J. Exp. Med. 214, 3791–811 (2017).
Clark, R. A. et al. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176, 4431–9 (2006).
Watanabe, R. et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 7, 279ra39 (2015).
Olivares-Villagómez, D. & Kaer, L. van Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends Immunol. 39, 264–75 (2018).
Thelen, F. & Witherden, D. A. Get in touch with dendritic epithelial T cells! Front. Immunol. 0, 1656 (2020).
Ho, A. W. & Kupper, T. S. T cells and the skin: from protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 19, 490–502 (2019). 2019 19:8.
Scharschmidt, T. C. et al. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 43, 1011–21 (2015).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science 341, 569–73 (2013).
Campbell, C. et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581, 475–9 (2020).
Muñoz-Rojas, A. R. & Mathis, D. Tissue regulatory T cells: regulatory chameleons. Nat. Rev. Immunol. 21, 597–611 (2021).
Schwarz, A., Bruhs, A. & Schwarz, T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J. Invest. Dermatol. 137, 855–64 (2017).
Agatha, S., Bruhs, A. & Schwarz, T. Commensal microbe-derived short-chain fatty acids regulate cutaneous immunity via IL-10 releasing regulatory T cells. J. Dermatological Sci. 86, e66 (2017).
Yamazaki, S. et al. Homeostasis of thymus-derived Foxp3+ regulatory T cells is controlled by ultraviolet B exposure in the skin. J. Immunol. 193, 5488–97 (2014).
Wohlfert, E. A. et al. GATA3 controls Foxp3+ regulatory T cell fate during inflammation in mice. J. Clin. Investig. 121, 4503–15 (2011).
Legoux, F. et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science 366, 494–9 (2019).
Constantinides, M. G. et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 366, eaax6624 (2019).
Halkias, J. et al. CD161 contributes to prenatal immune suppression of IFNγ-producing PLZF+ T cells. J. Clin. Invest. 130, 3562–3577 (2019).
Dutzan, N. et al. On-going mechanical damage from mastication drives homeostatic Th17 cell responses at the oral barrier. Immunity 46, 133–47 (2017).
Belkaid, Y. & Harrison, O. J. Homeostatic immunity and the microbiota. Immunity 46, 562 (2017).
Hurabielle, C. et al. Immunity to commensal skin fungi promotes psoriasiform skin inflammation. Proc. Natl. Acad. Sci. 117, 16465–74 (2020).
McGeachy, M. J., Cua, D. J. & Gaffen, S. L. The IL-17 family of cytokines in health and disease. Immunity 50, 892–906 (2019).
Zwicky, P., Unger, S. & Becher, B. Targeting interleukin-17 in chronic inflammatory disease: a clinical perspective. J. Exp. Med. 217, e20191123 (2020).
Frazier, K., Frith, M., Harris, D. & Leone, V. A. Mediators of host–microbe circadian rhythms in immunity and metabolism. Biology, https://doi.org/10.3390/biology9120417 (2020).
Zheng, D., Ratiner, K. & Elinav, E. Circadian influences of diet on the microbiome and immunity. Trends Immunol. https://doi.org/10.1016/j.it.2020.04.005 (2020).
Brooks, J. F. et al. The microbiota coordinates diurnal rhythms in innate immunity with the circadian clock. Cell, https://doi.org/10.1016/j.cell.2021.07.001 (2021).
Bilska, B. et al. Expression of antimicrobial peptide genes oscillates along day/night rhythm protecting mice skin from bacteria. Exp. Dermatol. https://doi.org/10.1111/exd.14229 (2021).
Greenberg, E. N. et al. Circadian control of interferon-sensitive gene expression in murine skin. Proc. Natl Acad. Sci. USA, https://doi.org/10.1073/pnas.1915773117 (2020).
Allaire, J. M., et al. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. https://doi.org/10.1016/j.it.2018.04.002 (2018).
Phan, T. S. et al. Keratinocytes control skin immune homeostasis through de novo–synthesized glucocorticoids. Sci. Adv. https://doi.org/10.1126/SCIADV.ABE0337 (2021).
Zhang, C. et al. Interleukins 4 and 13 drive lipid abnormalities in skin cells through regulation of sex steroid hormone synthesis. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2100749118 (2021).
Castleman, M. J. et al. Innate sex bias of staphylococcus aureus skin infection is driven by α-hemolysin. J. Immunol. https://doi.org/10.4049/jimmunol.1700810 (2018).
Gupta, S. et al. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc. Natl Acad. Sci. USA, https://doi.org/10.1073/pnas.2003603117 (2020).
Tamari, M., ver Heul, A. M. & Kim, B. S. Immunosensation: neuroimmune cross talk in the skin. Annu. Rev. Immunol. 39, 369–93, https://doi.org/10.1146/annurev-immunol-101719-113805 (2021).
Wang, F. & Kim, B. S. Itch: a paradigm of neuroimmune crosstalk. Immunity 52, 753–66 (2020).
Oetjen, L. K. et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 171, 217–28.e13 (2017).
Wang, F. et al. A basophil-neuronal axis promotes itch. Cell 184, 422–40.e17 (2021).
Kashem, S. W. et al. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous. Immun. Immun. 43, 515–26 (2015).
Cohen, J. A. et al. Cutaneous TRPV1+ neurons trigger protective innate type 17 anticipatory immunity. Cell 178, 919–.e14 (2019).
Hoeffel, G. et al. Sensory neuron-derived TAFA4 promotes macrophage tissue repair functions. Nature 594, 94–99 (2021). 2021 594:7861.
Zhang, S. et al. Nonpeptidergic neurons suppress mast cells via glutamate to maintain skin homeostasis. Cell 184, 2151–32.e16 (2021).
Souza-Moreira, L., Campos-Salinas, J., Caro, M. & Gonzalez-Rey, E. Neuropeptides as pleiotropic modulators of the immune response. Neuroendocrinology 94, 89–100 (2011).
Trier, A. M., Mack, M. R. & Kim, B. S. The neuroimmune axis in skin sensation, inflammation, and immunity. J. Immunol. 202, 2829–35 (2019).
Pessemier, Bde et al. Gut–skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms 9, 353 (2021).
Salem, I., Ramser, A., Isham, N. & Ghannoum, M. A. The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol. 0, 1459 (2018).
Oyoshi, M. K. et al. Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin. J. Clin. Investig. 121, 2210–20 (2011).
Fowell, D. J. & Kim, M. The spatio-temporal control of effector T cell migration. Nat. Rev. Immunol. 21, 582–96 (2021).
Zákostelská, Z. et al. Intestinal microbiota promotes psoriasis-like skin inflammation by enhancing Th17 response. PLOS ONE 11, e0159539 (2016).
Merana, G. R. et al. Intestinal inflammation breaks established immune tolerance to a skin commensal. SSRN Electronic J. https://doi.org/10.2139/SSRN.3863579 (2021).
Leyva-Castillo, J. M. et al. Mechanical skin injury promotes food anaphylaxis by driving intestinal mast cell expansion. Immunity 50, 1262–75.e4 (2019).
Dokoshi, T. et al. Skin inflammation activates intestinal stromal fibroblasts and promotes colitis. J. Clin. Invest. 131, e147614 (2021).
Chen, L. et al. Skin and gut microbiome in psoriasis: gaining insight into the pathophysiology of it and finding novel therapeutic strategies. Front. Microbiol. 0, 3201 (2020).
Zheng, D., Liwinski, T. & Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. https://doi.org/10.1038/s41422-020-0332-7 (2020).
Rohr, M. W., Narasimhulu, C. A., Rudeski-Rohr, T. A. & Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: a review. Adv. Nutr. https://doi.org/10.1093/advances/nmz061 (2020).
Zhao, L. et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science https://doi.org/10.1126/science.aao5774 (2018).
Meydani, S. N. et al. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging https://doi.org/10.18632/aging.100994 (2016).
Brandwein, M., Katz, I., Katz, A. & Kohen, R. Beyond the gut: skin microbiome compositional changes are associated with BMI. Human Microbiome J. https://doi.org/10.1016/j.humic.2019.100063 (2019).
Moestrup, K. S., Chen, Y., Schepeler, T., Schweiger, P. J. & Jensen, K. B. Dietary control of skin lipid composition and microbiome. J. Invest. Dermatol. https://doi.org/10.1016/j.jid.2017.12.005 (2018).
Harris, T. A. et al. Resistin-like molecule α provides vitamin-A-dependent antimicrobial protection in the skin. Cell Host Microbe, https://doi.org/10.1016/j.chom.2019.04.004 (2019).
Lima-Junior, D. S. et al. Endogenous retroviruses promote homeostatic and inflammatory responses to the microbiota. Cell 184, 3794–811.e19 (2021).
Acknowledgements
We thank our colleagues in the fields of skin immunology and apologize to any whose contributions we could not adequately highlight due to space constrains. THT is supported by a Harold Amos Award through the Robert Wood Johnson Foundation, by a Burroughs Wellcome Fund Career Award for Medical Scientists and grant K08 AR076459 from NIAMS. TCS is supported by a Sun Pharma Award from the Dermatology Foundation and grant DP2AI44968 from NIAID.
Author information
Authors and Affiliations
Contributions
CZ and GRM drafted portions of the manuscript. THT and TSC were responsible for the conceptual framework, writing and final editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
TCS serves as a member of the Scientific Advisory Board of Concerto Biosciences. CZ, CRM and THT declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, C., Merana, G.R., Harris-Tryon, T. et al. Skin immunity: dissecting the complex biology of our body’s outer barrier. Mucosal Immunol 15, 551–561 (2022). https://doi.org/10.1038/s41385-022-00505-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-022-00505-y
This article is cited by
-
Immune cell trafficking: a novel perspective on the gut-skin axis
Inflammation and Regeneration (2024)