Abstract
Obesity represents an escalating global health burden with profound medical and economic impacts. The conventional perspective on obesity revolves around its classification as a “pure” metabolic disorder, marked by an imbalance between calorie consumption and energy expenditure. Present knowledge, however, recognizes the intricate interaction of rare or frequent genetic factors that favor the development of obesity, together with the emergence of neurodevelopmental and mental abnormalities, phenotypes that are modulated by environmental factors such as lifestyle. Thirty years of human genetic research has unveiled >20 genes, causing severe early-onset monogenic obesity and ~1000 loci associated with common polygenic obesity, most of those expressed in the brain, depicting obesity as a neurological and mental condition. Therefore, obesity’s association with brain function should be better recognized. In this context, this review seeks to broaden the current perspective by elucidating the genetic determinants that contribute to both obesity and neurodevelopmental and mental dysfunctions. We conduct a detailed examination of recent genetic findings, correlating them with clinical and behavioral phenotypes associated with obesity. This includes how polygenic obesity, influenced by a myriad of genetic variants, impacts brain regions associated with addiction and reward, differentiating it from monogenic forms. The continuum between non-syndromic and syndromic monogenic obesity, with evidence from neurodevelopmental and cognitive assessments, is also addressed. Current therapeutic approaches that target these genetic mechanisms, yielding improved clinical outcomes and cognitive advantages, are discussed. To sum up, this review corroborates the genetic underpinnings of obesity, affirming its classification as a neurological disorder that may have broader implications for neurodevelopmental and mental conditions. It highlights the promising intersection of genetics, genomics, and neurobiology as a foundation for developing tailored medical approaches to treat obesity and its related neurological aspects.
Similar content being viewed by others
Introduction
Obesity, characterized by an excessive accumulation of adipose tissue, has emerged as a major worldwide public health burden together with its associated co-morbidities. The global rise in obesity prevalence with an estimated 4 billion affected by 2035 has prompted extensive research into its underlying causes with the aim for better prevention and more effective medical care [1]. Obesity is a multifactorial disease as both genetic framework and environmental factors contribute to the obesity epidemic. Studies based on twins, families, and adoption data indicate that heritability factors may account for up to 80% of obesity predisposition [2, 3]. However, only less than 10% of severe obesity have been attributed to known genetic causes, so far [4, 5]. This underscores the significant potential of genetic research to identify novel genetic determinants and enhance our understanding of the molecular and physiological pathways regulating body weight. The environmental determinants such as diet, physical activity and stress, overtly influence molecular pathways involved in the energy balance. They interact with genetic variants that modulate individual susceptibility to obesity risk [6]. A relatively new dimension in this context is the field of epigenetics. Here, environmental triggers induce alterations in gene expression without altering the genetic code, acting on the function of the genes but not their structure, positioning epigenetics as an important component in the discourse on obesity [7].
Approximately three decades ago, the discovery of the first human genes involved in monogenic obesity, i.e. LEP encoding leptin [8] and LEPR encoding leptin ireceptor [9] marked a turning point in our understanding of obesity causes and highlighted the irregularities in the brain function to contribute to this condition. As the genetic basis of obesity expanded with the discovery of additional genes linked to severe, early-onset obesity, the characterization of obesity as a neurological disease has gained clarity. Current knowledge recognizes the brain as essential in regulating energy balance through its orchestration of various functions. This includes the management of neural pathways responsible for appetite and satiety, the modulation of neurotransmitter activity that conveys signals related to energy status, and the integration of neuronal and hormonal signals that inform the brain of the body’s nutritional needs [10].
The central nervous system (CNS), particularly the hypothalamic region, is essential in regulating appetite, satiety, and energy balance. It processes both short and long-term body energy signals, coordinating the secretion of hormones like leptin and ghrelin that modulate hunger (Fig. 1). Neurons in the hypothalamus are closely linked with both neuronal subclusters within this region and with various extrahypothalamic brain regions, enabling a harmonized behavioral response [11].
ADCY3 adenylate cyclase 3, AgRP agouti-related peptide, BDNF brain derived neurotrophic factor, GLP1 glucagon like peptide 1, GLP1R glucagon like peptide 1 receptor, KSR2 kinase suppressor of ras 2, LHA lateral hypothalamu, MC4R melanocortin-4 receptor, MRAP2 melanocortin 2 receptor accessory Ppn 2, MSH melanocyte-stimulating hormone, NTS nucleus tractus solitarius, PCSK1 proprotein convertase subtilisin/kexin type 1, POMC proopiomelanocortin, SIM1 SIM BHLH transcription factor 1, TRKB tropomyosin-related kinase B, VMH ventromedial nucleus of the hypothalamus.
Available evidence indicates that brain changes causing severe obesity can also lead to significant declines in mental and intellectual abilities. Such alterations adversely affect various cognitive areas, such as decision-making, memory retention, and information processing. In essence, monogenic and polygenic obesity does not just directly impact body weight but can also hinder one’s cognitive performance and overall brain health [12]. However, there are also several secondary mechanisms explaining the strong link between established obesity and CNS abnormalities including sleep apnea, elevated pro-inflammatory cytokines, dysregulated metabolism, oxidative stress, and stigma and discrimination associated with obesity that may lead to stress and mental health issues, including severe depression, eventually affecting cognitive abilities [13].
An understanding of these multiple factors and their interactions provides insights into the complex relationship between obesity and neurological abnormalities, cognitive impairment, and hence the need of a holistic approach to address these issues. The present review focuses on recent progress in the genetics of obesity and seeks to draw attention to the significant yet underappreciated connection between obesity and neurodevelopmental disorders. It advocates for a multifaceted examination of obesity, considering various perspectives to fully understand CNS regulation of energy balance.
Leptin-melanocortin pathway (LMP) and obesity
Obesity results from an imbalance in the energy metabolism. The hypothalamus is the nexus for maintaining energy equilibrium. It interprets signals from peripheral organs and accordingly fine-tunes our hunger levels [14] through the leptin-melanocortin pathway, the key mechanism for appetite regulation. Any disruption in ligands or receptors involved in melanocortin signaling, such as rare, pathogenic mutations in LEP, LEPR, or MC4R encoding melanocortin 4 receptor, lead to early-onset severe obesity [4, 15]. Upon its synthesis in adipocytes, leptin undergoes systemic transport to the brain, where it specifically binds to its receptors located within the arcuate nucleus of the hypothalamus. This binding event serves as the triggering point for the activation of the leptin-melanocortin pathway.
Leptin, in this pathway, acts as a stimulator of proopiomelanocortin (POMC) neurons, which are integral in the production of alpha-melanocyte-stimulating hormone (α-MSH). Proprotein convertase subtilisin/kexin type 1 (PCSK1) plays a crucial role in this process by facilitating the cleavage of POMC into its active peptides, including α-MSH. Concurrently, leptin also exerts an inhibitory effect on neurons responsible for generating Agouti-related peptide (AgRP). Notably, AgRP functions as an antagonist to α-MSH, effectively opposing its actions. α-MSH, released from POMC neurons binds to the melanocortin receptor MC4R. This equilibrium between the two neuropeptides, AgRP and POMC is important for maintaining energy homeostasis resulting in a reduction in food intake and an increase in energy expenditure, particularly when energy stores in the form of fat are sufficient [16]. Therefore, both AgRP and POMC neurons exhibit sensitivity to metabolic status [16, 17]. Notably, rare, pathogenic mutations in all the genes involved in leptin driven melanocortin pathway (LEP, LEPR, POMC, PCSK1, MC4R, primarily [Table 1]) lead to severe early-onset obesity accompanied by eating disorders. These effects are pronounced when mutations occur in a bi-allelic manner for LEP, LEPR, PCSK1 and POMC, or in a homozygous or heterozygous state for MC4R. We have recently shown that total loss-of-function heterozygous mutations in PCSK1 were also able to cause monogenic obesity [18]. These mutations are commonly associated with additional metabolic and endocrine abnormalities, in addition to severe obesity [5, 19].
Leptin-melanocortin pathway (LMP) and eating disorder
Over 30 years of research have demonstrated that mutations in the leptin-melanocortin pathway (LMP) cause various forms of eating disorders. Specifically, homozygous loss-of-function (LoF) mutations in genes such as LEP, LEPR, and homozygous and heterozygous in MC4R result in uncontrollable hyperphagia, an eating disorder characterized by excessive hunger and food intake, leading to extreme early-onset obesity [5, 20].
Surprisingly, some studies have identified a significant link between MC4R gain-of-function (GoF) variants and another eating disorder—binge eating disorder (BED)—that may affect obese individuals (Qasim, Mayhew et al. 2019). On the other hand, findings from the UK Biobank (UKBB) general population and another analysis of approximately 17,000 individuals of European origin from nine independent cohorts revealed that carriers of GoF variants in MC4R exhibit lower BMI and a reduced risk of metabolic diseases, thereby contributing to a lower prevalence of obesity [21, 22]. These behavioral outcomes are similar to some forms of bulimia (which is often associated with normal weight due to vomiting habits), indicating a complex impact of genetic factors on eating behaviors and their consequences. The differences observed can be attributed to the signaling bias of these variants, where increased β-arrestin recruitment rather than cAMP production results in protective metabolic effects while potentially altering eating behaviors differently [21].
Hypoleptinemia has been shown to trigger various psychological and behavioral adaptations to starvation, which are central to anorexia nervosa (AN) [23, 24]. In AN, reduced leptin levels are a hallmark, critically contributing to the psychological and behavioral manifestations of the disorder implicated in numerous mental health issues, including depression, anxiety, and mood disturbances [25]. Recent study by us highlighted that obese children with genetically driven leptin deficiency exhibit profound behavioral complications including agressive/disruptive behaviors beyond hyperphagia, severely impacting their social interactions and schooling [26]. These children face significant challenges with motivation, reward processing, and stress response due to impaired melanocortin signaling, affecting brain regions outside the hypothalamus. These findings underscore the crucial link between genetic mutations in the LMP and brain circuit dysfunctions, highlighting its significant role in both obesity and opposite eating disorders [23, 24, 27, 28].
Indirect genetic influences on the LMP and association with obesity
In addition to the main molecular components driving the melanocortin signaling, various modulatory elements and regulatory factors have been elucidated to modify this LMP driven signaling. Indeed, a multitude of signals originating either from the periphery or the CNS are recognized for their roles in modulating feeding behavior and energy expenditure. Melanocortin receptor accessory protein 2 (MRAP2) is responsible for both the trafficking and signaling of MC4R. Loss of MRAP2 function leads to decreased MC4R signaling and obesity in mouse models [29]. Furthermore, monoallelic, pathogenic mutations in MRAP2 cause monogenic obesity associated with metabolic syndrome in humans [30]. Another example is Adenylate cyclase 3 (ADCY3) that serves as intermediaries for intracellular transmission of the MC4R activation signal [31]. The protein it encodes appears to be co-located with MC4R in the primary cilia of some neurons within the hypothalamus. Furthermore, it has been observed that the suppression of adenylyl cyclase signaling in these cilia leads to an increase in body weight [32]. Biallelic, loss-of-function mutations in ADCY3 were found to cause severe, early-onset obesity associated with anosmia, cognitive impairment, developmental delay, seizures and severe pneumonia [31]. SH2B Adaptor Protein 1 (SH2B1) acts as a crucial adaptor or scaffolding protein within the LMP and monoallelic, loss-of-function mutations in SH2B1 could lead to hyperphagia [33]. Initial discovery of this gene’s significance was linked to its presence within a copy number variation (CNVs) on chromosome 16p11.2 among patients with severe early-onset obesity [34].
Furthermore, it has been identified additional genes associated with obesity that influence the LMP, modulating either the development of specific brain regions or the regulation and sensitivity of key components within the pathway (Table 1). For instance, SIM BHLH Transcription Factor 1 (SIM1) plays a role in the development and function of the paraventricular nucleus (PVN) of the hypothalamus. Monoallelic, loss-of-function mutations in SIM1 were found to be associated with severe, early-onset obesity, possibly associated with a Prader Willi-like syndrome [35, 36]. Therefore, abnormalities in SIM1 can disrupt the normal development and function of the PVN, leading to dysregulation of appetite and energy balance. Kinase Suppressor of Ras 2 (KSR2), modulates the sensitivity of MC4R, and potentially other receptors, to melanocortin hormones. Monoallelic, loss-of-function mutations in KSR2 were found to be associated with obesity, although the Mendelian mode is still under scrutinity [37]. Agouti Signaling Protein (ASIP) represses MC4R activation and increases food intake. A tandem duplication at ASIP, leading to aberrant ASIP expression pattern, was found to cause obesity associated with red hair [38]. GNAS encodes the stimulatory G-protein alpha subunit protein (Gαs), which mediates G protein-coupled receptor signaling. A recent study showed that monoallelic, loss-of-function mutation of GNAS led to obesity by impeding MC4R signaling via defective interaction between Gαs and MC4R [39]. Similarly, recent studies have identified transient receptor potential channel 5 (TRPC5) as a significant contributor to obesity and associated behavioral phenotypes by identifying microdeletions on chromosome Xq23 encompassing this gene. TRPC5 is a brain-expressed cation channel that acts on hypothalamic Pomc and oxytocin neurons, playing a crucial role in regulating instinctive behaviors vital for survival, including feeding, arousal, social interaction, and maternal care. Disruption of TRPC5 leads to food seeking behavior, anxiety, autism-like behaviors, and in female mutation carriers, postpartum depression [40].
Syndromic and non-syndromic obesity: an overlap
Monogenic obesity usually results from pathogenic mutations in a single gene. However, such obesity can also be due to copy-number variations (CNVs) that may span multiple genes, like the 600 kb deletion at chr16p11.2 [41]. Until now, monofactorial obesity has been rigidly classified as syndromic or non-syndromic. The non-syndromic form of monogenic obesity is characterized by the presence of obesity as the sole clinical feature, without the accompaniment of additional symptoms or syndromes. In contrast, syndromic obesity is not only associated with obesity per se, but also with other clinical disorders or features including craniofacial dysmorphism, neurodevelopmental disabilities and different forms/levels of cognitive deficiencies or mental disorders (Table 1). Prominent instances of syndromic obesity, such as Bardet–Biedl syndrome (BBS), exemplify this complexity. BBS is a genetically heterogeneous disorder - caused by biallelic pathogenic mutations in 26 different genes, marked by a set of variable, symptoms/phenotypes including retinal dystrophy, postaxial polydactyly, learning difficulties, hypogonadism, and renal anomalies, alongside obesity [42]. Alström syndrome, caused by biallelic pathogenic mutations in ALMS1, encompasses obesity as well as sensory impairments, dilated cardiomyopathy, and metabolic disturbances [43]. Prader-Willi syndrome on the other hand, resulting from aberrations on chromosome 15q11-q13, is characterized by insatiable hunger leading to obesity, intellectual impairment, and endocrine dysfunctions, including growth hormone deficiency and hypogonadism [44].
However, as our understanding of obesity-linked genes and their associated phenotypes expands, these conventional categories become less sharply defined. For instance, monoallelic, loss-of-function mutations in SH2B1 are associated with obesity and insulin resistance, but they also give rise to neurological and behavioral issues [33]. Patients with a monoallelic, pathogenic SIM1 mutations often exhibit neurodevelopmental disorders alongside obesity, mirroring the diverse symptoms observed with chromosome 6q deletions [35, 36]. ADCY3 deficiency is involved in diverse physiological processes and health problems - from anosmia and respiratory problems to learning impairment and metabolic disorders, as mentioned above. Furthermore, GNAS deficiency manifests a range of clinical symptoms, including growth, endocrine, and metabolic issues [45]. Patients carrying complete loss-of-function mutations in P4HTM have severe hypotonia, cognitive impairment, and developmental anomalies not associated with obesity, although non synonymous mutations cause childhood obesity, also associated with CNS abnormalities [46]. Furthermore, patients with the 600 kb deletion at chr16p11.2, often show autism in addition to fully penetrant obesity, although the reverse duplication of the same region show excessive leanness and schizophrenia [41, 47].
Apart from that, a high mortality or morbidity rates is also observed among the affected children with pathogenic mutations in the LMP. Indeed, the comprehensive clinical evaluations of children deficient for LEP or LEPR from consanguineous families of Pakistan have unveiled a broader health impact of these mutations beyond obesity. As mentioned above, a significant number of these children also exhibit cognitive and behavioral challenges, including an increased tendency to drop out of school, difficulties integrating with peers (social isolation), and agressive/disruptive behaviors. Moreover, these children exhibited very high rates of life-threatening morbidity and of premature mortality, mainly due to lung or gut infections [26]. This multifaceted impact of leptin signaling deficiency underlines the inadequacy of classifying such conditions as strictly non-syndromic obesity. Instead, these findings highlight a spectrum where a single-gene disorder, traditionally associated with a straightforward phenotype, manifests a range of clinical and cognitive complications, indicating a more complex syndromic-like nature.
Obesity and brain function
The aforementioned examples of monogenic obesity and associated phenotypes (Table 1) underscore the link between obesity and cognitive/intellectual deficits. Both conditions might be influenced by an overlap of interconnected regulatory pathways thus introducing the concept of shared genetic and epigenetic vulnerabilities. In support of this hypothesis, in a study on consanguineous Pakistani families with childhood obesity, 49% of the probands carried pathogenic point mutations or CNVs in 13 genes/loci responsible for both non-syndromic and syndromic monofactorial obesity in this population. Importantly, the study also identified 28 rare or novel CNVs associated with intellectual disability and/or autism in an additional 22 obese subjects accounting for 10% of cases in this cohort [48].
Damage of brain cells may be secondarily due to an increase in the release of inflammatory cytokines by the adipose tissue that impairs cognitive function over time [49]. Specifically, pro-inflammatory cytokines like interleukin 1 inhibits neuronal activity [13]. This inhibition adversely affects synaptic plasticity and cognitive function. Sleep disorders, such as sleep apnea may also negatively impact cognitive performance. Obesity has also been linked to vascular changes, promoting atherosclerosis. Reduced blood flow to the brain can lead to cognitive decline and an increased risk of neurodegenerative changes. Obesity is associated with chronic low-grade inflammation throughout the body, including the brain. This systemic neuroinflammation may disrupt neural circuits and contribute to cognitive impairments [50]. This chronic low-grade inflammation, can dysregulate the Hypothalamic–pituitary–adrenal axis (HPA axis), impacting cortisol rhythms and potentially contributing to mood disorders, metabolic syndrome, and cognitive impairments. This obesity-induced disruption of the HPA axis, coupled with changes in cortisol secretion, is thought to affect brain structures like the hippocampus, further influencing cognitive function and potentially increasing the risk of neurodegenerative diseases [51].
Neuroimaging studies have revealed structural and functional changes in the brain of individuals with obesity, pointing to potential neural vulnerabilities. The research highlights that childhood obesity has been linked to alterations in brain structure, especially in the prefrontal cortex (PFC) [52], affecting decision-making, response inhibition, working memory, and cognitive flexibility [52]. These findings are in line with changes in gray matter volume, connectivity, and reduced cortical thickness, predominantly in prefrontal regions [53, 54].
Common polygenic obesity and the neural regulation
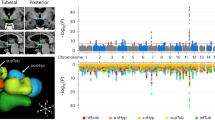
Comprehensive explorations of the human genome via the meta-analysis of 60 genome-wide association studies (GWAS) have identified more than 1,100 independent loci modulating a range of obesity related traits including body mass index (BMI), and increasing risk for common obesity [19, 55]. Importantly, the vast majority of adiposity-associated single nucleotide polymorpisms (SNPs) are located in genes that are expressed in the CNS, bringing the hypothesis that they act on body weight through their impact on brain function. To respond to this question, PCR-free expression assays targeting genes located nearby the GWAS-identified SNPs associated with BMI/obesity were performed in a large panel of human tissues. The study found that the expression of BMI/obesity susceptibility genes was strongly enriched in different regions of the brain, especially in the insula and substantia nigra, which are two brain regions involved in addiction and reward [56]. This pattern of expression of polygenic obesity genes is strikingly different from the one found in monogenic obesity where most of the gene (with the exception of LEP expressed in adipocytes) are expressed predominantly in the hypothalamus. Therefore, if both monogenic and polygenic obesity are linked to changes in brain function the mechanisms involved may be different: in monogenic obesity the central circuity of appetite regulation in the hypothalamus is impaired causing hyperphagia, although in polygenic obesity food addiction and other abnormalities in food behavior are involved, with a high sensibility to environment stress (Fig. 2).
Importantly, polygenic susceptibility to obesity can influence monogenic obesity penetrance and the phenotypes of rare variants carriers: for instance, carriers of monoallelic, pathogenic mutations in MC4R with a low polygenic risk score (bottom quartile) have an approximately 5-kg/m² lower BMI (approximately 14 kg of body weight for a 1.7-m-tall person) than those with a high PRS (top quartile) [57].
From genetics to more personalized treatments
Obesity genetic research has led to novel therapeutic options that have revolutionized the conventional lifestyle interventions and are alternative to bariatric surgery that does not work well in most patients with monogenic obesity (in reason of the irrepressible hyperphagia and bigne eating episodes) [58]. Metreleptin, a recombinant leptin analog, has been approved by the FDA for the treatment of congenital leptin deficiency and acquired lipodystrophy. It reduces hunger and body weight among affected individuals and restores normal development and growth in children. In addition, case studies have demonstrated that metreleptin can be effective in treating anorexia nervosa by normalizing low circulating leptin levels. This normalization improves cognitive, emotional, and behavioral effects, alleviating symptoms such as hyperactivity, repetitive thoughts of food, inner restlessness, and weight phobia [23, 59]. Metreleptin can also significantly enhance mood, sleep, and cognitive functions, suggesting that leptin substitution therapy could be a valuable addition to traditional treatments [27]. Setmelanotide, a melanocortin-4 receptor agonist, has shown promising results in treatment of obesity as caused by recessive mutation in POMC, PCSK1, and LEPR deficiencies [60, 61]. Setmelanotide’s effectiveness was also explored in individuals with BBS, yielding noteworthy results in weight loss [62] and decrease in hunger scores.
Glucagon-like peptide-1 (GLP-1) receptor agonists are increasingly used, world-wide in both monogenic [63, 64] and common polygenic obesity [65, 66]. This is not surprising given the physiological effect of GLP-1 on appetite, and the impact of rare variants of GLP1R on the variation of BMI [67].
Liraglutide has been approved for the treatment of obesity in adults and adolescents aged 12 years and older. More recently, Semaglutide has demonstrated a higher efficacy compared to other GLP-1 analogs [68].
Emerging strategies, now involve the use of combination drug therapies that target various gut increting signaling mechanisms. Tirzepatide has recently received FDA approval as a treatment for obesity, marking a significant achievement due to its innovative approach of simultaneously targeting GIP and GLP-1 receptors (Fig. 1).
These aformentioned drugs interact with central pathways that regulate appetite and possibly energy expenditure. Moreover, metreleptin, setmelanotide, and liraglutide have been associated with improved cognitive function and mood. This is not surprising as these drugs act on regions of the CNS linked to these brain functions regulation [69]. These findings confirm that obesity is not merely a disorder of energy imbalance but also involves complex neurobehavioral components. It also emphasizes the importance of evaluating the neurobehavioral and mental effects of obesity treatments during the clinical trial process and beyond.
The advent of CRISPR gene-editing technologies holds promise for future treatments that could directly correct the underlying genetic mutations while minimizing adverse effects. The FDA approval of Casgevy, a therapy utilizing the CRISPR–Cas9 gene-editing tool, for sickle-cell disease [70] may open further avenues for monogenic obesity too. The integration of these novel pharmacological agents and technologies into clinical practice signifies a pivotal shift towards precision medicine in the management of obesity. This trend underscores the increasing importance of genetic diagnosis as a prerequisite for selecting the most effective, individualized treatment regimen [71].
Conclusion
In conclusion, our understanding of obesity as a pure metabolic dysfunction has evolved rapidly, moving away from simplistic notions of ‘calories in’ vs ‘calories out’ to a more sophisticated appreciation of the brain’s central role. Obesity appears to be associated with abnormal brain function and mental disorders. Similar to other aspects of obesity, brain abnormalities is an integral part of obesity and needs to be more widely acknowledged in order to facilitate the development of targeted therapies.
References
World Obesity Atlas. World Obesity Atlas 2023. London WC2A 1EN: World Obesity Atlas; 2023.
Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr. 2008;87:398–404.
Silventoinen K, Jelenkovic A, Sund R, Hur Y-M, Yokoyama Y, Honda C, et al. Genetic and environmental effects on body mass index from infancy to the onset of adulthood: an individual-based pooled analysis of 45 twin cohorts participating in the COllaborative project of Development of Anthropometrical measures in Twins (CODATwins) study. Am J Clin Nutr. 2016;104:371–9.
El-Sayed Moustafa JS, Froguel P. From obesity genetics to the future of personalized obesity therapy. Nat Rev Endocrinol. 2013;9:402–13.
Saeed S, Arslan M, Froguel P. Genetics of obesity in consanguineous populations: toward precision medicine and the discovery of novel obesity genes. Obesity. 2018;26:474–84.
Wen X, Zhang B, Wu B, Xiao H, Li Z, Li R, et al. Signaling pathways in obesity: mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2022;7:298.
Wu F-Y, Yin R-X. Recent progress in epigenetics of obesity. Diabetol Metab Syndr. 2022;14:1–30.
Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–8.
Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401.
Alcantara IC, Tapia APM, Aponte Y, Krashes MJ. Acts of appetite: neural circuits governing the appetitive, consummatory, and terminating phases of feeding. Nat Metab. 2022;4:836–47.
Gautron L, Elmquist JK, Williams KW. Neural control of energy balance: translating circuits to therapies. Cell. 2015;161:133–45.
Lee EB, Mattson MP. The neuropathology of obesity: insights from human disease. Acta Neuropathol. 2014;127:3–28.
Sui SX, Pasco JA. Obesity and brain function: the brain-body crosstalk. Medicina. 2020;56:499.
Dietrich MO, Horvath TL. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci. 2013;36:65–73.
Saeed S, Bonnefond A, Manzoor J, Shabbir F, Ayesha H, Philippe J, et al. Genetic variants in LEP, LEPR, and MC4R explain 30% of severe obesity in children from a consanguineous population. Obesity. 2015;23:1687–95.
Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–8.
Yulyaningsih E, Zhang L, Herzog H, Sainsbury A. NPY receptors as potential targets for anti‐obesity drug development. Br J Pharmacol. 2011;163:1170–202.
Folon L, Baron M, Toussaint B, Vaillant E, Boissel M, Scherrer V, et al. Contribution of heterozygous PCSK1 variants to obesity and implications for precision medicine: a case-control study. Lancet Diab Endocrinol. 2023;11:182–90.
Loos RJ, Yeo GS. The genetics of obesity: from discovery to biology. Nat Rev Genet. 2022;23:120–33.
Hinney A, Korner A, Fischer-Posovszky P. The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits. Nat Rev Endocrinol. 2022;18:623–37.
Lotta LA, Mokrosinski J, Mendes de Oliveira E, Li C, Sharp SJ, Luan J, et al. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell. 2019;177:597–607 e599.
Stutzmann F, Vatin V, Cauchi S, Morandi A, Jouret B, Landt O, et al. Non-synonymous polymorphisms in melanocortin-4 receptor protect against obesity: the two facets of a Janus obesity gene. Hum Mol Genet. 2007;16:1837–44.
Hebebrand J, Hinney A, Antel J. Could leptin substitution therapy potentially terminate entrapment in anorexia nervosa? Nat Rev Endocrinol. 2023;19:435–6.
Micioni Di Bonaventura E, Botticelli L, Tomassoni D, Tayebati SK, Micioni Di Bonaventura MV, Cifani C. The melanocortin system behind the dysfunctional eating behaviors. Nutrients. 2020;12:3502.
Hebebrand J, Hildebrandt T, Schlogl H, Seitz J, Denecke S, Vieira D, et al. The role of hypoleptinemia in the psychological and behavioral adaptation to starvation: Implications for anorexia nervosa. Neurosci Biobehav Rev. 2022;141:104807.
Saeed S, Khanam R, Janjua QM, Manzoor J, Ning L, Hanook S, et al. High morbidity and mortality in children with untreated congenital deficiency of leptin or its receptor. Cell Rep Med. 2023;4:101187.
Hebebrand J, Plieger M, Milos G, Peters T, Hinney A, Antel J. Does hypoleptinemia trigger entrapment in anorexia nervosa? Etiological and clinical considerations. Eur Eat Disord Rev. 2024;32:557–74.
Qasim A, Mayhew AJ, Ehtesham S, Alyass A, Volckmar AL, Herpertz S, et al. Gain-of-function variants in the melanocortin 4 receptor gene confer susceptibility to binge eating disorder in subjects with obesity: a systematic review and meta-analysis. Obes Rev. 2019;20:13–21.
Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341:275–8.
Baron M, Maillet J, Huyvaert M, Dechaume A, Boutry R, Loiselle H, et al. Loss-of-function mutations in MRAP2 are pathogenic in hyperphagic obesity with hyperglycemia and hypertension. Nat Med. 2019;25:1733–8.
Saeed S, Bonnefond A, Tamanini F, Mirza MU, Manzoor J, Janjua QM, et al. Loss-of-function mutations in ADCY3 cause monogenic severe obesity. Nat Genet. 2018;50:175–9.
Siljee JE, Wang Y, Bernard AA, Ersoy BA, Zhang S, Marley A, et al. Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat Genet. 2018;50:180–5.
Doche ME, Bochukova EG, Su H-W, Pearce LR, Keogh JM, Henning E, et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J Clin Investig. 2012;122:4732–6.
Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463:666–70.
Ramachandrappa S, Raimondo A, Cali AM, Keogh JM, Henning E, Saeed S, et al. Rare variants in single-minded 1 (SIM1) are associated with severe obesity. J Clin Investig. 2013;123:3042–50.
Bonnefond A, Raimondo A, Stutzmann F, Ghoussaini M, Ramachandrappa S, Bersten DC, et al. Loss-of-function mutations in SIM1 contribute to obesity and Prader-Willi–like features. J Clin Investig. 2013;123:3037–41.
Pearce LR, Atanassova N, Banton MC, Bottomley B, van der Klaauw AA, Revelli J-P, et al. KSR2 mutations are associated with obesity, insulin resistance, and impaired cellular fuel oxidation. Cell. 2013;155:765–77.
Kempf E, Landgraf K, Stein R, Hanschkow M, Hilbert A, Abou Jamra R, et al. Aberrant expression of agouti signaling protein (ASIP) as a cause of monogenic severe childhood obesity. Nat Metab. 2022;4:1697–712.
Mendes de Oliveira E, Keogh JM, Talbot F, Henning E, Ahmed R, Perdikari A, et al. Obesity-associated GNAS mutations and the melanocortin pathway. N. Engl J Med. 2021;385:1581–92.
Li Y, Cacciottolo TM, Yin N, He Y, Liu H, Liu H, et al. Loss of transient receptor potential channel 5 causes obesity and postpartum depression. Cell. 2024;187:4176–92.
Walters RG, Jacquemont S, Valsesia A, de Smith AJ, Martinet D, Andersson J, et al. A new highly penetrant form of obesity due to deletions on chromosome 16p11. 2. Nature. 2010;463:671–5.
Forsythe E, Beales PL. Bardet–biedl syndrome. Eur J Hum Genet. 2013;21:8–13.
Tahani N, Maffei P, Dollfus H, Paisey R, Valverde D, Milan G, et al. Consensus clinical management guidelines for Alström syndrome. Orphanet J Rare Dis. 2020;15:1–22.
Elena G, Bruna C, Benedetta M, Stefania DC, Giuseppe C. Prader-Willi syndrome: clinical aspects. J Obes. 2012; 2012.
Hendricks AE, Bochukova EG, Marenne G, Keogh JM, Atanassova N, Bounds R, et al. Rare variant analysis of human and rodent obesity genes in individuals with severe childhood obesity. Sci Rep. 2017;7:4394.
Saeed S, Ning L, Badreddine A, Mirza MU, Boissel M, Khanam R, et al. Biallelic mutations in P4HTM cause syndromic obesity. Diabetes. 2023;72:1228–34.
Jacquemont S, Reymond A, Zufferey F, Harewood L, Walters RG, Kutalik Z, et al. Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11. 2 locus. Nature. 2011;478:97–102.
Saeed S, Arslan M, Manzoor J, Din SM, Janjua QM, Ayesha H, et al. Genetic causes of severe childhood obesity: a remarkably high prevalence in an inbred population of Pakistan. Diabetes. 2020;69:1424–38.
Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, et al. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci. 2014;34:2618–31.
O’Brien PD, Hinder LM, Callaghan BC, Feldman EL. Neurological consequences of obesity. Lancet Neurol. 2017;16:465–77.
Martins LB, Monteze NM, Calarge C, Ferreira AVM, Teixeira AL. Pathways linking obesity to neuropsychiatric disorders. Nutrition. 2019;66:16–21.
Ronan L, Alexander-Bloch A, Fletcher PC. Childhood obesity, cortical structure, and executive function in healthy children. Cereb Cortex. 2020;30:2519–28.
Gupta A, Mayer EA, Sanmiguel CP, Van Horn JD, Woodworth D, Ellingson BM, et al. Patterns of brain structural connectivity differentiate normal weight from overweight subjects. NeuroImage: Clin. 2015;7:506–17.
Maayan L, Hoogendoorn C, Sweat V, Convit A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity. 2011;19:1382–7.
Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in∼ 700000 individuals of European ancestry. Hum Mol Genet. 2018;27:3641–9.
Ndiaye FK, Huyvaert M, Ortalli A, Canouil M, Lecoeur C, Verbanck M, et al. The expression of genes in top obesity-associated loci is enriched in insula and substantia nigra brain regions involved in addiction and reward. Int J Obes. 2020;44:539–43.
Chami N, Preuss M, Walker RW, Moscati A, Loos RJ. The role of polygenic susceptibility to obesity among carriers of pathogenic mutations in MC4R in the UK Biobank population. PLoS Med. 2020;17:e1003196.
Faccioli N, Poitou C, Clément K, Dubern B. Current treatments for patients with genetic obesity. J Clin Res Pediatr Endocrinol. 2023;15:108.
Milos G, Antel J, Kaufmann LK, Barth N, Koller A, Tan S, et al. Short-term metreleptin treatment of patients with anorexia nervosa: rapid on-set of beneficial cognitive, emotional, and behavioral effects. Transl Psychiatry. 2020;10:303.
Clément K, van den Akker E, Argente J, Bahm A, Chung WK, Connors H, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8:960–70.
Kühnen P, Clément K. Long-term MC4R agonist treatment in POMC-deficient patients. N Engl J Med. 2022;387:852–4.
Haqq AM, Chung WK, Dollfus H, Haws RM, Martos-Moreno GÁ, Poitou C, et al. Efficacy and safety of setmelanotide, a melanocortin-4 receptor agonist, in patients with Bardet-Biedl syndrome and Alström syndrome: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial with an open-label period. Lancet Diab Endocrinol. 2022;10:859–68.
Iepsen EW, Zhang J, Thomsen HS, Hansen EL, Hollensted M, Madsbad S, et al. Patients with obesity caused by melanocortin-4 receptor mutations can be treated with a glucagon-like peptide-1 receptor agonist. Cell Metab. 2018;28:23–32. e23.
Diene G, Angulo M, Hale PM, Jepsen CH, Hofman PL, Hokken-Koelega A, et al. Liraglutide for weight management in children and adolescents with Prader–Willi syndrome and obesity. J Clin Endocrinol Metab. 2023;108:4–12.
Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382:2117–28.
Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22.
Gao W, Liu L, Huh E, Gbahou F, Cecon E, Oshima M, et al. Human GLP1R variants affecting GLP1R cell surface expression are associated with impaired glucose control and increased adiposity. Nat Metab. 2023;5:1673–84.
Christou GA, Katsiki N, Blundell J, Fruhbeck G, Kiortsis DN. Semaglutide as a promising antiobesity drug. Obes Rev. 2019;20:805–15.
Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: a systematic review. BMC Med. 2016;14:191.
Wong C. UK first to approve CRISPR treatment for diseases: what you need to know. Nature. 2023;623:676–7.
Jayachandran M, Fei Z, Qu S. Genetic advancements in obesity management and CRISPR–Cas9-based gene editing system. Mol Cell Biochem. 2023;478:491–501.
Acknowledgements
This work is supported by the grant from the National Center for Precision Diabetic Medicine – PreciDIAB, which is jointly supported by the French National Agency for Research (ANR-18-IBHU-0001), by the European Union (FEDER), by the Hauts-de-France Regional Council and by the European Metropolis of Lille (MEL). Figure 2 was created with BioRender software, ©biorender.com
Author information
Authors and Affiliations
Contributions
The conception and initial draft of the review were the joint efforts of PF and SS. The figures were designed by SS, AB, and PF, who also took part in the reviewing and editing of all subsequent versions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saeed, S., Bonnefond, A. & Froguel, P. Obesity: exploring its connection to brain function through genetic and genomic perspectives. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02737-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02737-9