Abstract
Cannabis is the most frequently used illicit drug in the United States with more than 45 million users of whom one-third suffer from a cannabis use disorder (CUD). Despite its high prevalence, there are currently no FDA-approved medications for CUD. Patients treated with semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1RA) approved for treating type 2 diabetes (T2D) and for weight management have reported reduced desire to drink and smoke. Preclinical studies have shown that semaglutide decreased nicotine and alcohol consumption. Preclinical and preliminary clinical evidence of semaglutide’s potential beneficial effects on various substance use disorders led us to evaluate if it pertained to CUD. In this retrospective cohort study of electronic health records (EHRs) from the TriNetX Analytics Network, a global federated health research network of approximately 105.3 million patients from 61 large healthcare organizations in the US, we aimed to assess the associations of semaglutide with both incident and recurrent CUD diagnosis compared to non-GLP-1RA anti-obesity or anti-diabetes medications. Hazard ratio (HR) and 95% confidence intervals (CI) of incident and recurrent CUD were calculated for 12-month follow-up by comparing propensity-score matched patient cohorts. The study population included 85,223 patients with obesity who were prescribed semaglutide or non-GLP-1RA anti-obesity medications, with the findings replicated in 596,045 patients with T2D. In patients with obesity (mean age 51.3 years, 65.6% women), semaglutide compared with non-GLP-1RA anti-obesity medications was associated with lower risk for incident CUD in patients with no prior history CUD (HR: 0.56, 95% CI: 0.42–0.75), and recurrent CUD diagnosis in patients with a prior history CUD (HR: 0.62, 95% CI: 0.46–0.84). Consistent reductions were seen for patients stratified by gender, age group, race and in patients with and without T2D. Similar findings were replicated in the study population with T2D when comparing semaglutide with non-GLP-1RA anti-diabetes medications for incident CUD (HR: 0.40, 95% CI: 0.29–0.56) and recurrent CUD (HR: 0.66, 95% CI: 0.42–1.03). While these findings provide preliminary evidence of the potential benefit of semaglutide in CUD in real-world populations, further preclinical studies are warranted to understand the underlying mechanism and randomized clinical trials are needed to support its use clinically for CUD.
Similar content being viewed by others
Introduction
Cannabis is the most widely used illicit substance worldwide, with estimates from the United Nations Office on Drugs and Crime (UNODC) that in 2020, 209 million people or 4% of the global adult population, had used cannabis (range: 141 million–256 million) [1]. In the United States, the increased legalization of recreational and medical cannabis by the States has expanded the number of users who consume cannabis regularly and at higher doses [2], which is driven both by the availability of higher potency cannabis products [3] and access to diverse formulations (e.g., edibles, beverages, vaping, and dabbing) [4]. Indeed, in the United States between 2008 and 2019, the number of past-year cannabis users increased from 22.6 million to 45.0 million, that of daily or near-daily users increased from 3.6 to 9.8 million, and the number of adults with cannabis use disorder (CUD) increased from 3.4 to 4.1 million [5]. Nearly a quarter of cannabis users who meet the DSM-5 (the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition) criteria for CUD have a severe disorder that interferes with their everyday life [6]. Moreover, CUD has been associated with a higher risk for psychotic disorders [7, 8], self-harm, and suicidal behaviors [9,10,11]. Despite the high prevalence of CUD and its negative consequences, there are currently no FDA-approved medications. Treatment of CUD is managed by tapering marijuana use and providing support to combat withdrawal symptoms alongside behavioral interventions. However, behavioral therapies such as contingency management, cognitive-behavioral, and motivational enhancement therapies, are only moderately effective. Thus, there is an urgency to develop medications that can help treat CUD.
Clinical anecdotes that patients treated with semaglutide, a glucagon-like peptide-1 receptor (GLP1R) agonist approved for treating type 2 diabetes (T2D) in 2017 and for weight management in 2021 reported reduced desire to drink and smoke have attracted attention regarding its potential to treat addiction [12]. Currently, several registered clinical trials are ongoing to evaluate the effect of semaglutide on alcohol consumption [13,14,15,16,17] and on smoking cessation [18, 19]. Preclinical studies have investigated the effects of semaglutide or other GLP1R agonizts on nicotine, alcohol, cocaine or opioids [20,21,22,23]. However, little attention has been placed on the effects of semaglutide or other GLP1R agonizts on cannabis consumption even though the prevalence of cannabis use is even higher than that of tobacco use among certain demographics. Preclinical and preliminary clinical evidence of semaglutide’s potential beneficial effects in various substance use disorders led us to evaluate if it could extend to CUD. Here we used a large electronic health record (EHR) database to conduct a nationwide multicenter retrospective cohort study in patients who were obese with or without a pre-existing CUD and who were prescribed semaglutide vs. non-GLP-1RA anti-obesity medications to determine whether semaglutide was associated with changes in both the incidence and recurrence of CUD. Outcomes were separately assessed by age groups, sex, and race. Findings were replicated in a separate study population of patients with T2D during a non-overlapping period.
Method
Database
We used TriNetX, a global federated health research network providing analytics to access and analyze de-identified and aggregated electronic medical records (diagnoses, procedures, medications, laboratory values, genomic information) from approximately 105.3 million patients in 61 large healthcare organizations. These healthcare organizations cover diverse geographic regions, age, race/ethnic, income and insurance groups, and clinical setting [24]. The MetroHealth System, Cleveland OH, IRB determined research using TriNetX as described in this study was not Human Subject Research, and IRB approval is not required (more details in the Supplementary Appendix). The TriNetX platform has been used in retrospective cohort studies [25,26,27,28,29,30,31,32,33,34,35,36,37,38] including evaluating risk and health outcomes of patients with substance use disorders including CUD [25, 32, 35] and the associations of GLP-1RAs with cancer [37] and semaglutide with suicidal ideations [38].
Study populations
The study populations with obesity
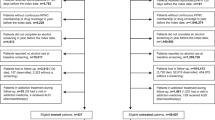
The analyses in patients with obesity were restricted to a starting date of 6/2021 and an ending date of 12/2022. The starting date was when semaglutide was approved in the US for weight management as Wegovy. The ending date of 12/2022 was chosen to allow for a 12-month follow-up period by the time of data analysis on January 21, 2024. To assess the associations of semaglutide with incident CUD (first time diagnosis of CUD), the study population included 83,189 patients who had active medical encounters for the diagnosis of obesity in 6/2021–12/2022, were for the first time (new users design) prescribed semaglutide or non-GLP-1RA anti-obesity medications (bupropion, naltrexone, orlistat, topiramate, phentermine) [39] during 6/2021-12/2022 (time zero or index event), had no diagnosis of CUD on or before the index event and had a diagnosis of at least one of obesity-associated comorbidities (T2D, hypertension, hypercholesterolemia, hyperlipidemia, heart diseases, stroke) on or before the index event. Patients who were prescribed other GLP-1RAs or had bariatric surgery on or before the index event were excluded. This study population was then divided into two cohorts: (1) Semaglutide cohort – 45,445 patients prescribed semaglutide, and (2) non-GLP1-RA anti-obesity medication cohort – 37,744 patients prescribed non-GLP-1RA anti-obesity medications but not semaglutide. We used new-user design to mitigate prevalent user bias and confounding associated with the drug itself [40, 41].
To assess the associations of semaglutide with recurrent CUD, the study population included 2034 patients who had active medical encounters for the diagnosis of obesity in 6/2021–12/2022, were for the first time (new users design) prescribed semaglutide or non-GLP-1RA anti-obesity medications during 6/2021–12/2022 (time zero or index event), had a diagnosis of CUD on or before the index event, and had a diagnosis of at least one of obesity-associated comorbidities on or before the index event. Patients who were prescribed other GLP-1RAs or had bariatric surgery on or before the index event were excluded. The outcome recurrent CUD in patients with a prior history of CUD was defined as a medical encounter for CUD diagnosis after the index event. This study population was then divided into two cohorts: (1) Semaglutide cohort – 688 patients prescribed semaglutide, and (2) non-GLP1-RA anti-obesity medication cohort – 1,346 patients prescribed non-GLP-1RA anti-obesity medications but not semaglutide.
The study populations with T2D
The analyses on the associations of semaglutide with both incident and recurrent CUD among patients with T2D had a starting time of 12/2017 when semaglutide was approved in the US as Ozempic for treating T2D and an ending date of 5/2021 to allow us to separately examine the effects of semaglutide on CUD as Ozempic from those as Wegovy in the study population with obesity. Since patients in the study population with obesity were for the first time prescribed semaglutide after 6/2021, there was no overlap in the exposure cohorts for these two study populations.
To assess the association of semaglutide with incident CUD, the study population included 587,849 patients with T2D who had active medical encounters for T2D during 12/2017–5/2021, were for the first time prescribed semaglutide (Ozempic) or non-GLP1-1RA anti-diabetes medications (new-user design) during 12/2017–5/2021(index event), had no diagnosis of CUD on or before the index event and had a diagnosis of at least one of obesity-associated comorbidities (T2D, hypertension, hypercholesterolemia, hyperlipidemia, heart diseases, stroke) on or before the index event. The status of non-GLP1RA anti-diabetes medications was determined by the Anatomical Therapeutic Chemical or ATC code A10 “Drugs used in diabetes” with GLP1R agonizts (ATC code A10BJ “ Glucagon-like peptide-1 (GLP-1) analogs”) excluded. The list of non-GLP1RA anti-diabetes medications included insulins, metformin, sulfonylureas, alpha glucosidase inhibitors, thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, sodium-glucose co-transporter 2 (SGLT2) inhibitors. Patients who were prescribed other GLP-1RAs or had bariatric surgery on or before the index event were excluded. This study population was divided into two cohorts: (1) Semaglutide cohort – 25,843 patients prescribed semaglutide, and (2) Non-GLP-1RA anti-diabetes medication cohort – 562,006 patients prescribed non-GLP-1RA anti-diabetes medications but not semaglutide.
To assess the associations of semaglutide with recurrent CUD, the study population included 8196 patients with T2D who had active medical encounters for T2D diagnosis in 12/2017–5/2021, were for the first time prescribed semaglutide or non-GLP1-1RA anti-diabetes medications during 12/2017–5/2021(index event), had a diagnosis of CUD on or before the index event, and had a diagnosis of at least one of obesity-associated comorbidities on or before the index event. Patients who were prescribed other GLP-1RAs or had bariatric surgery on or before the index event were excluded. This study population was then divided into two cohorts: (1) Semaglutide cohort – 254 patients prescribed semaglutide, and (2) non-GLP1-RA anti-diabetes medication cohort – 7942 patients prescribed non-GLP-1RA anti-diabetes medications but not semaglutide.
Statistical analysis
For each study population, the Semaglutide cohort and the comparison cohort were propensity-score matched (1:1 using nearest neighbor greedy matching with a caliper of 0.25 times the standard deviation) on covariates that are potential risk factors for CUD [42, 43] including demographics, adverse socioeconomic determinants of health (e.g., problems related to education and literacy, employment and unemployment, housing and economic circumstances, social environment, upbringing, primary support group including family circumstances and various psychosocial circumstances), problems with lifestyle (e.g., tobacco use, lack of physical exercise, inappropriate diet and eating habits, high-risk sexual behavior, gambling and betting, and other problems related to lifestyle including antisocial behaviors and sleep deprivation), pre-existing medical conditions and medications. Obesity sub-categories were also matched to control obesity severity which included 3 ICD-10 diagnosis codes and 15 BMI categories ranging from BMI 30 to BMI 70 or greater (more details in Tables 1 and 2 and Supplementary Tables S1–3 in the Supplementary Appendix). The outcome –incident or recurrent diagnosis of CUD (International Classification of Diseases, Tenth Revision (ICD-10) code F12 “Cannabis related disorders”) – that occurred within the 12-month time window after the index event) were compared between matched cohorts. Recurrent CUD in patients with a prior history of CUD was defined as a medical encounter for CUD diagnosis (ICD-10 F12) after the index event. Kaplan–Meier analysis was used to estimate the probability of outcome at daily time intervals with censoring applied. When the last fact (the outcome of interests or other medical encounters) in the patient’s record is in the time window for analysis, the patient was censored on the day after the last fact in their record. Hazard ratio (HR) and 95% confidence intervals were used to describe the relative hazard of the outcomes based on a comparison of time to event rates.
Separate analyses were performed in patients stratified by sex (women, men), age groups (≤55, >55 years), and race (Black, White). For the study population with obesity, a separate analysis was performed in patients with T2D and patients without T2D. For the study population with T2D, a separate analysis was performed in patients with obesity and patients without obesity.
To examine longer-term associations of semaglutide with CUD, the outcome –incident and recurrent diagnosis of CUD– in patients with T2D was followed for 1-, 2-, and 3-year following the index event (the first prescription of semaglutide vs. non-GLP-1 RA anti-diabetes medications occurred during 12/2017-5/2021). Similar analyses were not performed for patients with obesity since semaglutide was approved as Wegovey for weight management in 6/2021.
The data were collected and analyzed on January 21, 2024, within the TriNetX Analytics Platform. Details of clinical codes for eligibility criteria, exposure, outcomes, and confounders are in Supplementary Table S1 in the Supplementary Appendix.
Results
Associations of semaglutide with incident and recurrent CUD diagnosis in patients with obesity
The study population for the analyses of incident CUD diagnosis in patients with obesity consisted of 83,189 patients with obesity who had no prior recorded diagnosis of CUD. The Semaglutide cohort compared with the Non-GLP-1RA anti-obesity medications cohort was older and had a higher prevalence of morbid obesity, T2D and obesity-associated comorbidities, and a lower prevalence of mental disorders and substance use disorders. After propensity-score matching, the two cohorts (26,784 in each cohort, mean age 51.3 years, 65.6% women, 16.1% Black, 66.5% White, 6.7% Hispanic) were balanced (Table 1).
The study population for the analysis of recurrent CUD diagnosis or medical encounter for CUD diagnosis in patients with obesity who had a prior diagnosis of CUD consisted of 2,034 patients. The Semaglutide cohort compared to the non-GLP-1RA anti-obesity medications cohort was older, had a lower prevalence of adverse socioeconomic determinants of health, mental disorders, and substance use disorders, and a higher prevalence of morbid obesity, T2D and obesity-associated comorbidities. After propensity-score matching, the two cohorts (504 in each cohort, mean age 46.1 years, 57.5% women, 26.4% black, 54.7% white, 6.7% Hispanic) were balanced (see Supplementary Table S2 in the Supplementary Appendix).
Among patients with no prior history of CUD, semaglutide was associated with a significantly lower risk for incident CUD diagnosis compared with non-GLP-1RA anti-obesity medications for a 12-month follow-up period (0.28% vs. 0.48%; HR: 0.56, 95% CI: 0.42–0.75). Consistent reductions with semaglutide were seen in patients stratified by gender, age group, and race, except in Black patients, with the biggest reduction in men and patients aged >55 years old. A similar lower risk for incident CUD was observed in patients with and without T2D (Fig. 1A).
A Comparison of incident CUD diagnosis (new diagnosis) in patients with obesity who had no prior history of CUD between propensity-score matched Semaglutide and Non-GLP-1RA anti-obesity medications cohorts, stratified by gender, age group, race and the status of type 2 diabetes. B Comparison of medical encounters for CUD diagnosis (recurrent CUD diagnosis) in patients with obesity who had a prior history of CUD between propensity-score matched Semaglutide and Non-GLP-1RA anti-obesity medications cohorts, stratified by gender, age group, race and the status of type 2 diabetes. Outcomes were followed for 12 months following the index event (first prescription of semaglutide or non-GLP-1 RA anti-obesity medications during 6/2021–12/2022). Hazard rates were calculated using Kaplan–Meier analysis to estimate the probability of outcome at daily time intervals with censoring applied. Overall risk = number of patients with outcomes during the 12-month time window/number of patients in the cohort at the beginning of the time window.
Among patients with a prior history of CUD, semaglutide was associated with a significantly lower risk for recurrent CUD diagnosis (medical encounters for CUD diagnosis) compared with non-GLP-1RA anti-obesity medications for a 12-month follow-up period (13.0% vs. 20.4%; HR: 0.62, 95% CI: 0.46–0.84). Consistent reductions with semaglutide were seen in patients stratified by gender, age group, and race, except in Black patients, with the biggest reduction in men and patients aged >55 years old. A similar lower risk for recurrent CUD was observed in patients with and without T2D (Fig. 1B).
Associations of semaglutide with incident and recurrent CUD diagnosis in patients with type 2 diabetes
The study population for the analyses of incident CUD diagnosis in patients with T2D consisted of 587,849 patients with T2D who had no prior recorded diagnosis of CUD. The Semaglutide cohort compared with the Non-GLP-1RA anti-diabetes medications cohort was younger and had a higher prevalence of obesity, obesity-associated comorbidities and mental disorders. After propensity-score matching, the two cohorts (25,820 in each cohort, mean age 58.0 years, 45.4% women, 14.8% Black, 61.2% White, 6.7% Hispanic) were balanced (Table 2).
The study population for the analysis of recurrent CUD diagnosis in patients with T2D who had a prior diagnosis of CUD consisted of 8,196 patients. The Semaglutide cohort compared to the non-GLP-1RA anti-diabetes medications cohort included more women, had a higher prevalence of problems with lifestyle, obesity, obesity-associated comorbidities, mental disorders, and substance use disorders. After propensity-score matching, the two cohorts (241 in each cohort, mean age 51.8 years, 39.0% women, 32.6% black, 44.4% white, 5.8% Hispanic) were balanced (see Supplementary Table S3 in the Supplementary Appendix).
Among patients with T2D who had no prior history of CUD, semaglutide was associated with a significantly lower risk for incident CUD diagnosis compared with non-GLP-1RA anti-diabetes medications for a 12-month follow-up period (0.21% vs. 0.48%; HR: 0.40, 95% CI: 0.29–0.56). Consistent reductions with semaglutide were seen in patients stratified by gender, age group, and race, with the biggest reduction in men, white, and patients aged >55 years old. A similar lower risk for incident CUD was observed in patients with and without obesity (Fig. 2A).
A Comparison of incident CUD diagnosis (new diagnosis) in patients with type 2 diabetes who had no prior history of CUD between propensity-score matched Semaglutide and Non-GLP-1RA anti-diabetes medications cohorts, stratified by gender, age group, race and the status of obesity. B Comparison of medical encounters for CUD diagnosis in patients with type 2 diabetes who had a prior history of CUD between propensity-score matched Semaglutide and Non-GLP-1RA anti-diabetes medications cohorts, stratified by gender, age group, race and the status of obesity. Outcomes were followed for 12 months following the index event (first prescription of semaglutide or non-GLP-1 RA anti-diabetes medications during 12/2017–5/2021). Hazard rates were calculated using Kaplan–Meier analysis to estimate the probability of outcome at daily time intervals with censoring applied. Overall risk = number of patients with outcomes during the 12-month time window/number of patients in the cohort at the beginning of the time window.
Among patients with T2D who had a prior history of CUD, semaglutide was associated with a lower risk for recurrent CUD diagnosis (medical encounters for CUD diagnosis) compared with non-GLP-1RA anti-diabetes medications for a 12-month follow-up period (13.7% vs. 19.1%; HR: 0.66, 95% CI: 0.42–1.03). Consistent reductions with semaglutide were seen in patients stratified by gender, age group, and race. A similar lower risk for recurrent CUD was observed in patients with and without obesity, though not statistically significant in patients without obesity (Fig. 2B).
We then examined the longer-term association of semaglutide with both incident and recurrent diagnosis of CUD in patients with T2D. Compared with non-GLP-1RA anti-diabetes medications, semaglutide was associated with a significantly lower risk of incident CUD, though the association attenuated over time. Among patients with a prior history of CUD, semaglutide was associated with a lower, but not statistically significant, risk of recurrent CUD and the strength of the association attenuated over time (Fig. 3).
Discussion
Here we document a potential beneficial effect of semaglutide on both the incidence and recurrence of CUD in two separate real-world populations, one with obesity and the other with T2D. These results are consistent with reports that patients treated with semaglutide notice reduced interest in consuming addictive substances including alcohol or tobacco [12]. Currently, several registered clinical trials are ongoing to evaluate the effect of semaglutide on alcohol consumption [13,14,15,16,17] and on smoking cessation [18, 19]. However, little attention has been placed on the effects of semaglutide or other GLP1-RAs on cannabis consumption even though the prevalence of cannabis use is even higher than that of tobacco use among certain demographics. While preclinical studies have investigated the effects of GLP-1RAs on nicotine, alcohol, cocaine, or opioids [20,21,22,23], to our knowledge no studies have been reported on their effects of tetrahydrocannabinol (THC), the major psychoactive component in cannabis. The potential beneficial effects of semaglutide (and presumably other GLP1R agonizts) in the treatment of CUD deserve attention in that currently there are no approved medications for its treatment [44]. As such the only available proven effective therapeutic alternatives are psychosocial interventions, but their effects tend to be limited [45]. Thus, our results support conducting preclinical studies of semaglutide (and other GLP1R agonist) on THC and randomized clinical trials to evaluate the therapeutic benefits of semaglutide in CUD in individuals with obesity or T2D as well as in patients with CUD who don’t have these co-morbid conditions.
Though the mechanism(s) underlying the decreased risk of incident and recurrent CUD with semaglutide is unclear, several mechanisms could be involved. Since our results document not just a reduction in relapse but also in the incidence of CUD, one could question the mechanism by which semaglutide prevents the transition from cannabis use to CUD. Though the majority of cannabis users do not proceed to develop a CUD, approximately 10% do, and among young users, the risk can increase to 30% [46]. This transition is typically associated with an increase in the regularity and frequency of use [47]. In the case of nicotine, preclinical studies showed evidence that the rewarding effects of nicotine are reduced by stimulation of GLP-1 receptors in the medial habenula, which is a brain region that mediates nicotine’s aversive effects [48]; whereas exenatide a GLP-1R agonist drug attenuated nicotine’s activation of dopamine reward circuitry [49]. Whether similar mechanisms pertain to cannabis is unclear since there are no studies that have evaluated the effects of GLP1 signaling in the aversive or rewarding effects of THC. Cannabinoid receptor 1 (CB1R) is expressed in presynaptic and postsynaptic neuronal sites in the lateral habenula, where they modulate behavioral responses to stress exposure, such that CB1R blockade reduced anxiety-like behaviors [50] and also in GABAergic cells in the ventral tegmental area (VTA) and their stimulation increases firing of dopamine neurons, which modulate reward [51]. Indeed endocannabinoid signaling through CB1R regulates the activity of dopamine (DA) neurons in the VTA [52], which projects to the nucleus accumbens, a key brain reward region activated by addictive drugs including THC [53, 54]. CB1R signaling also inhibits the excitatory stimulation from the infralimbic cortex in response to stressful stimuli relayed via the bed nucleus of the stria terminalis (BNST) [55]. Since the BNST also expresses GLP1R and their ablation reduces anxiety-like behaviors [56], this could provide another potential convergence mechanism by which semaglutide or other GLP1R could affect cannabis consumption. Though to our knowledge there are no studies on interactions between GLP-1 and CB1 receptors in the habenula, VTA or other brain regions, there is evidence of reciprocal functional interactions between these two receptors peripherally [57].
In juxtaposition, the high prevalence of cannabis use alongside the rise in prescriptions of semaglutide for diabetes and obesity has led to concerns among cannabis users that their cannabis use could interfere with semaglutide therapeutic effects. Even though there is no evidence of interactions between semaglutide and cannabis nor of reports of reduced efficacy, this is an area that requires monitoring. In particular, since CB1Rs, the main target for the rewarding effects of cannabis, are expressed on incretin-secreting cells in rodents and may play a role in regulating GLP-1 secretion [58, 59]. In fact, CB1R antagonist drugs increased GLP-1 release and improved the response to GLP-1RA in rats with diet-induced obesity [58, 60]. Since THC is a partial CB1R agonist, a concern could be that it might inhibit GLP-1 release and attenuate the effects of semaglutide. On the other hand, and seemingly paradoxically in rats the CB1R agonist WIN 55,212-2 enhanced the anorexigenic effects of subthreshold doses of the GLP1-RA exendin-4 resulting in a significant decrease in food intake and body weight in rats [61]. Similar effects for an agonist and antagonist on food intake could reflect the dose dependency of cannabinoids effects on food intake, such that decreased intake is reported after high doses whereas low doses increase food consumption [61].
In interpreting the findings of our study, it is important to consider them within its limitations: First, this is a retrospective observational study, so no causal inferences can be drawn. Second, patients in our study represented those who had medical encounters with healthcare systems contributing to the TriNetX Platform. Though both the exposure and comparison cohorts were drawn from the same platform, which should not significantly impact the relative hazard rate ratios, results from the TriNetX platform need to be validated in other populations and platforms of EHRs. Third, retrospective observational studies have inherent limitations, including unmeasured or uncontrolled confounders and biases. Though we controlled for an extensive list of variables and the findings were replicated in two separate populations with different characteristics at two non-overlapping study periods, these limitations could not be fully eliminated. Future controlled trials are necessary to assess the associations of semaglutide with CUD. Fourth, in our study the follow-up time for the main analyses was 12 months. Though we conducted a longer-term follow-up analysis in the T2D population, future studies are necessary to evaluate long-term associations of semaglutide in patients with obesity and patients with T2D. Fifth, the higher dose format of semaglutide as Wegovy was approved for weight management, and the lower dose format of semaglutide as Ozempic was approved for treating T2D. Since Wegovy and Ozempic were approved for different indications, we could not directly assess the dosage effect of semaglutide on CUD in the same study population. Sixth, we could not directly control for patient adherence to medications due to limited medical adherence information captured in patient EHRs. Our study population included patients who had recent medical encounters for the diagnosis of obesity or T2D and were subsequently prescribed semaglutide or other anti-obesity or anti-diabetes medications, suggesting that patients had active obesity or T2D that needed medical attention and treatments. However, patients may discontinue medications for reasons such as financial burden, drug side effects, or lack of efficacy.
In summary, our results show that semaglutide was associated with a lower risk for both incident and relapse of CUD compared to non-GLP-1 RA anti-obesity and anti-diabetes medications. While these findings provide preliminary evidence of the potential benefit of semaglutide in CUD in real-world populations further preclinical studies are warranted to understand the underlying mechanism and randomized clinical trials are needed to support its use clinically for CUD.
Data availability
This study used population-level aggregate and de-identified data collected by the TriNetX Platform and are available from TriNetX, LLC (https://trinetx.com/) but third-party restrictions apply to the availability of these data. The data were used under license for this study with restrictions that do not allow for the data to be redistributed or made publicly available. To gain access to the data, a request can be made to TriNetX (join@trinetx.com), but costs might be incurred, and a data-sharing agreement would be necessary. Data specific to this study including diagnosis codes and cohort characteristics in aggregated format are included in the manuscript as tables, figures, and supplementary files.
Code availability
All the statistical analyses in this study including propensity-score matching and Kaplan–Meier Survival analyses were conducted within the TriNetX platform by using its built-in functions. Data and code to reproduce the analyses can be accessed at https://github.com/bill-pipi/semaglutide_cannabis.
References
United Nations Office on Drugs and Crime. Global overview: Drug demand, drug supply. World Drug Report 2022. 2022. United Nations: Office on Drugs and Crime. https://www.unodc.org/unodc/en/data-and-analysis/wdr-2022_booklet-2.html. Accessed October 22, 2023.
Hasin DS. US Epidemiology of Cannabis Use and Associated Problems. Neuropsychopharmacology. 2018;43:195–212.
Chandra S, Radwan MM, Majumdar CG, Church JC, Freeman TP, ElSohly MA. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci. 2019;269:5–15.
Hall W, Stjepanović D, Caulkins J, Lynskey M, Leung J, Campbell G, et al. Public health implications of legalising the production and sale of cannabis for medicinal and recreational use. Lancet. 2019;394:1580–90.
Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Results from the 2019 National Survey on Drug Use and Health: Detailed Tables. 2019. https://www.samhsa.gov/data/report/2019-nsduh-detailed-tables. Accessed October 22, 2023.
Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, et al. Prevalence and correlates of DSM-5 cannabis use disorder, 2012-2013: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Am J Psychiatry. 2016;173:588–99.
Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, et al. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: A review. JAMA Psychiatry. 2016;73:292–7.
Hjorthøj C, Compton W, Starzer M, Nordholm D, Einstein E, Erlangsen A, et al. Association between cannabis use disorder and schizophrenia stronger in young males than in females. Psychol Med. 2023;53:7322–8.
Fontanella CA, Steelesmith DL, Brock G, Bridge JA, Campo JV, Fristad MA Association of Cannabis Use With Self-harm and Mortality Risk Among Youths With Mood Disorders. JAMA Pediatr. 2021. https://doi.org/10.1001/jamapediatrics.2020.5494.
Kung H-C, Pearson JL, Liu X. Risk factors for male and female suicide decedents ages 15-64 in the United States. Results from the 1993 National Mortality Followback Survey. Soc Psychiatry Psychiatr Epidemiol. 2003;38:419–26.
Arendt M, Munk-Jørgensen P, Sher L, Jensen SOW. Mortality following treatment for cannabis use disorders: predictors and causes. J Subst Abus Treat. 2013;44:400–6.
Reardon S. Could New Weight-Loss Drugs like Ozempic Treat Addiction? Scientific American. https://www.scientificamerican.com/article/could-new-weight-loss-drugs-like-ozempic-treat-addiction1/. July 12, 2023.
Semaglutide Therapy for Alcohol Reduction (STAR). 2023. https://clinicaltrials.gov/study/NCT06015893. Accessed October 5, 2023.
Semaglutide Therapy for Alcohol Reduction - Tulsa (STAR-T). 2023. https://clinicaltrials.gov/study/NCT05891587. Accessed October 5, 2023.
Semaglutide for Alcohol Use Disorder. 2022. https://clinicaltrials.gov/study/NCT05520775. Accessed October 5, 2023.
Clinical Trial of Rybelsus (Semaglutide) Among Adults With Alcohol Use Disorder (AUD). 2023. https://clinicaltrials.gov/study/NCT05892432. Accessed October 5, 2023.
Does Semaglutide Reduce Alcohol Intake in Patients With Alcohol Use Disorder and Comorbid Obesity? (SEMALCO). 2023. https://clinicaltrials.gov/study/NCT05895643. Accessed October 5, 2023.
Lengsfeld S, Burkard T, Meienberg A, Jeanloz N, Coynel D, Vogt DR, et al. Glucagon-like peptide-1 analogues: a new way to quit smoking? (SKIP)-a structured summary of a study protocol for a randomized controlled study. Trials. 2023;24:284.
Yammine L, Verrico CD, Versace F, Webber HE, Suchting R, Weaver MF, et al. Exenatide as an adjunct to nicotine patch for smoking cessation and prevention of postcessation weight gain among treatment-seeking smokers with pre-diabetes and/or overweight: study protocol for a randomised, placebo-controlled clinical trial. BMJ Open. 2023;13:e072707.
Chuong V, Farokhnia M, Khom S, Pince CL, Elvig SK, Vlkolinsky R, et al. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight. 2023. https://doi.org/10.1172/jci.insight.170671.
Aranäs C, Edvardsson CE, Shevchouk OT, Zhang Q, Witley S, Blid Sköldheden S, et al. Semaglutide reduces alcohol intake and relapse-like drinking in male and female rats. EBioMedicine. 2023;93:104642.
Evans B, Stoltzfus B, Acharya N, Nyland JE, Arnold AC, Freet CS, et al. Dose titration with the glucagon-like peptide-1 agonist, liraglutide, reduces cue- and drug-induced heroin seeking in high drug-taking rats. Brain Res Bull. 2022;189:163–73.
Klausen MK, Thomsen M, Wortwein G, Fink-Jensen A. The role of glucagon-like peptide 1 (GLP-1) in addictive disorders. Br J Pharm. 2022;179:625–41.
TriNetX - The World’s Largest, Living Ecosystem of Real-World Data and Evidence. TriNetX. 2021. https://trinetx.com/. Accessed May 6, 2023.
Wang L, Wang Q, Davis PB, Volkow ND, Xu R. Increased risk for COVID‐19 breakthrough infection in fully vaccinated patients with substance use disorders in the United States between December 2020 and August 2021. World Psychiatry. 2022;21:124–32.
Wang L, Berger NA, Kaelber DC, Davis PB, Volkow ND, Xu R Incidence Rates and Clinical Outcomes of SARS-CoV-2 Infection With the Omicron and Delta Variants in Children Younger Than 5 Years in the US. JAMA Pediatr. 2022. https://doi.org/10.1001/jamapediatrics.2022.0945.
Wang L, Davis PB, Kaelber DC, Volkow ND, Xu R Comparison of mRNA-1273 and BNT162b2 Vaccines on Breakthrough SARS-CoV-2 Infections, Hospitalizations, and Death During the Delta-Predominant Period. JAMA. 2022. https://doi.org/10.1001/jama.2022.0210.
Wang L, Davis PB, Kaelber DC, Xu R COVID-19 breakthrough infections and hospitalizations among vaccinated patients with dementia in the United States between December 2020 and August 2021. Alzheimers Dement. 2022. https://doi.org/10.1002/alz.12669.
Wang W, Kaelber DC, Xu R, Berger NA. Breakthrough SARS-CoV-2 Infections, Hospitalizations, and Mortality in Vaccinated Patients With Cancer in the US Between December 2020 and November 2021. JAMA Oncol. 2022;8:2022 https://doi.org/10.1001/jamaoncol.2022.1096.
Wang L, Kaelber DC, Xu R, Berger NA. COVID-19 breakthrough infections, hospitalizations and mortality in fully vaccinated patients with hematologic malignancies: A clarion call for maintaining mitigation and ramping-up research. Blood Rev. 2022;54:100931.
Wang L, Berger NA, Xu R. Risks of SARS-CoV-2 Breakthrough Infection and Hospitalization in Fully Vaccinated Patients With Multiple Myeloma. JAMA Netw Open. 2021;4:e2137575.
Wang L, Volkow ND, Berger NA, Davis PB, Kaelber DC, Xu R Association of COVID-19 with endocarditis in patients with cocaine or opioid use disorders in the US. Mol Psychiatry. 2022. https://doi.org/10.1038/s41380-022-01903-1.
Gao Z, Winhusen TJ, Gorenflo M, Ghitza UE, Davis PB, Kaelber DC, et al. Repurposing ketamine to treat cocaine use disorder: integration of artificial intelligence-based prediction, expert evaluation, clinical corroboration and mechanism of action analyses. Addiction. 2023. https://doi.org/10.1111/add.16168.
Olaker VR, Kendall EK, Wang CX, Parran TV, Terebuh P, Kaelber DC, et al. Association of recent SARS-CoV-2 infection with new-onset alcohol use disorder, January 2020 through January 2022. JAMA Netw Open. 2023;6:e2255496.
Wang L, Volkow ND, Berger NA, Davis PB, Kaelber DC, Xu R. Cardiac and mortality outcome differences between methadone, buprenorphine and naltrexone prescriptions in patients with an opioid use disorder. J Clin Psychol. 2023;79:2869–83.
Wang L, Davis PB, Volkow ND, Berger NA, Kaelber DC, Xu R. Association of COVID-19 with new-onset Alzheimer’s disease. J Alzheimers Dis. 2022. https://doi.org/10.3233/JAD-220717.
Wang L, Wang W, Kaelber DC, Xu R, Berger NA. GLP-1 Receptor Agonists and Colorectal Cancer Risk in Drug-Naive Patients With Type 2 Diabetes, With and Without Overweight/Obesity. JAMA Oncol. 2023. https://doi.org/10.1001/jamaoncol.2023.5573.
Wang W, Volkow ND, Berger NA, Davis PB, Kaelber DC, Xu R. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat Med. 2024;30:168–76.
Prescription Medications to Treat Overweight & Obesity. National Institute of Diabetes and Digestive and Kidney Diseases. 2023. https://www.niddk.nih.gov/health-information/weight-management/prescription-medications-treat-overweight-obesity. Accessed July 23, (2023).
Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–20.
Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol. 2016;183:758–64.
Dugas EN, Sylvestre M-P, Ewusi-Boisvert E, Chaiton M, Montreuil A, O’Loughlin J. Early Risk Factors for Daily Cannabis Use in Young Adults. Can J Psychiatry. 2019;64:329–37.
van den Bree MBM, Pickworth WB. Risk factors predicting changes in marijuana involvement in teenagers. Arch Gen Psychiatry. 2005;62:311–9.
Brezing CA, Levin FR. The Current State of Pharmacological Treatments for Cannabis Use Disorder and Withdrawal. Neuropsychopharmacology. 2018;43:173–94.
Sabioni P, Le Foll B. Psychosocial and pharmacological interventions for the treatment of cannabis use disorder. Focus (Am Psychiatr Publ). 2019;17:163–8.
Leung J, Chan GCK, Hides L, Hall WD. What is the prevalence and risk of cannabis use disorders among people who use cannabis? a systematic review and meta-analysis. Addict Behav. 2020;109:106479.
Robinson T, Ali MU, Easterbrook B, Coronado-Montoya S, Daldegan-Bueno D, Hall W, et al. Identifying risk-thresholds for the association between frequency of cannabis use and development of cannabis use disorder: A systematic review and meta-analysis. Drug Alcohol Depend. 2022;238:109582.
Tuesta LM, Chen Z, Duncan A, Fowler CD, Ishikawa M, Lee BR, et al. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat Neurosci. 2017;20:708–16.
Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS One. 2013;8:e77284.
Berger AL, Henricks AM, Lugo JM, Wright HR, Warrick CR, Sticht MA, et al. The lateral habenula directs coping styles under conditions of stress via recruitment of the endocannabinoid system. Biol Psychiatry. 2018;84:611–23.
Rashidy-Pour A, Pahlevani P, Vaziri A, Shaigani P, Zarepour L, Vafaei AA, et al. Involvement of CB1 receptors in the ventral tegmental area in the potentiation of morphine rewarding properties in acquisition but not expression in the conditioned place preference model. Behav Brain Res. 2013;247:259–67.
French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–52.
Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8.
French ED. Δ9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett. 1997;226:159–62.
Massi L, Elezgarai I, Puente N, Reguero L, Grandes P, Manzoni OJ, et al. Cannabinoid receptors in the bed nucleus of the stria terminalis control cortical excitation of midbrain dopamine cells in vivo. J Neurosci. 2008;28:10496–508.
Zheng H, Reiner DJ, Hayes MR, Rinaman L. Chronic suppression of glucagon-like peptide-1 receptor (GLP1R) mRNA translation in the rat bed nucleus of the stria terminalis reduces anxiety-like behavior and stress-induced hypophagia, but prolongs stress-induced elevation of plasma corticosterone. J Neurosci. 2019;39:2649–63.
Zizzari P, He R, Falk S, Bellocchio L, Allard C, Clark S, et al. CB1 and GLP-1 receptors cross talk provides new therapies for obesity. Diabetes. 2021;70:415–22.
González-Mariscal I, Krzysik-Walker SM, Kim W, Rouse M, Egan JM. Blockade of cannabinoid 1 receptor improves GLP-1R mediated insulin secretion in mice. Mol Cell Endocrinol. 2016;423:1–10.
Moss CE, Marsh WJ, Parker HE, Ogunnowo-Bada E, Riches CH, Habib AM, et al. Somatostatin receptor 5 and cannabinoid receptor 1 activation inhibit secretion of glucose-dependent insulinotropic polypeptide from intestinal K cells in rodents. Diabetologia. 2012;55:3094–103.
Patel KN, Joharapurkar AA, Patel V, Kshirsagar SG, Bahekar R, Srivastava BK, et al. Cannabinoid receptor 1 antagonist treatment induces glucagon release and shows an additive therapeutic effect with GLP-1 agonist in diet-induced obese mice. Can J Physiol Pharm. 2014;92:975–83.
Radziszewska E, Bojanowska E. Effects of glucagon-like peptide-1 receptor stimulation and blockade on food consumption and body weight in rats treated with a cannabinoid CB1 receptor agonist WIN 55,212-2. Med Sci Monit Basic Res. 2013;19:6–11.
Acknowledgements
We acknowledge support from National Institute on Alcohol Abuse and Alcoholism (AA029831), National Institute on Aging (AG057557, AG061388, AG062272, AG07664), from National Cancer Institute Case Comprehensive Cancer Center (CA221718, CA043703, CA2332216).
Author information
Authors and Affiliations
Contributions
RX conceived the study. RX and NDV designed the study. RX and NDV drafted the manuscript. WW performed data analysis. NAB, PBD, and DCK critically contributed to study design, result interpretation, and manuscript preparation. We confirm the originality of the content. RX had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, W., Volkow, N.D., Berger, N.A. et al. Association of semaglutide with reduced incidence and relapse of cannabis use disorder in real-world populations: a retrospective cohort study. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02498-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02498-5