Abstract
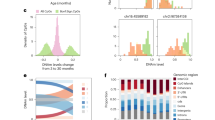
The aberrant aging hypothesis of schizophrenia (SCZ) and autism spectrum disorder (ASD) has been proposed, and the DNA methylation (DNAm) clock, which is a cumulative evaluation of DNAm levels at age-related CpGs, could serve as a biological aging indicator. This study evaluated epigenetic brain aging of ASD and SCZ using Horvath’s epigenetic clock, based on two public genome-wide DNA methylation datasets of post-mortem brain samples (NASD = 222; NSCZ = 142). Total subjects were further divided into subgroups by gender and age. The epigenetic age acceleration (AgeAccel) for each sample was calculated as the residual value resulting from the regression model and compared between groups. Results showed DNAm age has a strong correlation with chronological age in both datasets across multiple brain regions (P < 0.05). When divided into equally sized age groups, the AgeAccel of the cerebellum (CB) region from people over 45 years of age was greater compared to the control sample (AgeAccel of ASD vs control: 5.069 vs −6.249; P < 0.001). And a decelerated epigenetic aging process was observed in the CB region of individuals with SCZ aged 50–70 years (AgeAccel of SCZ vs control: −3.171 vs 2.418; P < 0.05). However, our results showed no significant difference in AgeAccel between ASD and control groups, and between SCZ and control groups in the total and gender-specific groups (P > 0.05). This study’s results revealed some evidence for aberrant epigenetic CB brain aging in old-aged patients with ASD and SCZ, indicating a different pattern of CB aging in older adults with these two diseases. However, further studies of larger ASD and SCZ cohorts are necessary to make definitive conclusions on this observation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Brain sample metadata are provided in Table S1–S7. Raw DNA methylation data for ASD and controls can be found in the PsychENCODE Knowledge Portal (https://www.synapse.org/#!Synapse:syn8263588). Raw DNA methylation data for SCZ and controls are available in the Gene Expression Omnibus (GEO) datasets database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE89707).
References
van ’t Hof M, Tisseur C, van Berckelear-Onnes I, van Nieuwenhuyzen A, Daniels AM, Deen M, et al. Age at autism spectrum disorder diagnosis: a systematic review and meta-analysis from 2012 to 2019. Autism. 2020;25:862–73.
Jutla A, Foss-Feig J, Veenstra-VanderWeele J. Autism spectrum disorder and schizophrenia: an updated conceptual review. Autism research : official journal of the International Society for Autism. Research .2022;15:384–412.
Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med. 2015;15:146–67.
Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Sci (N. Y, NY). 2018;359:693–7.
Du Y, Fu Z, Xing Y, Lin D, Pearlson G, Kochunov P, et al. Evidence of shared and distinct functional and structural brain signatures in schizophrenia and autism spectrum disorder. Commun Biol. 2021;4:1073.
Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011;69:26–33.
Grimm A, Eckert A. Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem. 2017;143:418–31.
Lu AT, Hannon E, Levine ME, Crimmins EM, Lunnon K, Mill J, et al. Genetic architecture of epigenetic and neuronal ageing rates in human brain regions. Nat Commun. 2017;8:15353.
Kaufmann T, van der Meer D, Doan NT, Schwarz E, Lund MJ, Agartz I, et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat Neurosci. 2019;22:1617–23.
Wertz J, Caspi A, Ambler A, Broadbent J, Hancox RJ, Harrington H, et al. Association of history of psychopathology with accelerated aging at midlife. JAMA Psychiatry. 2021;78:530–9.
Koutsouleris N, Davatzikos C, Borgwardt S, Gaser C, Bottlender R, Frodl T, et al. Accelerated brain aging in schizophrenia and beyond: a neuroanatomical marker of psychiatric disorders. Schizophrenia Bull. 2014;40:1140–53.
Pearson BL, Simon JM, McCoy ES, Salazar G, Fragola G, Zylka MJ. Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nat Commun. 2016;7:11173.
Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–67.
Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–54.
Guarasci F, D’Aquila P, Montesanto A, Corsonello A, Bellizzi D, Passarino G. Individual DNA methylation profile is correlated with age and can be targeted to modulate healthy aging and longevity. Curr Pharm Des. 2019;25:4139–49.
Zbieć-Piekarska R, Spólnicka M, Kupiec T, Parys-Proszek A, Makowska Ż, Pałeczka A, et al. Development of a forensically useful age prediction method based on DNA methylation analysis. Forensic Sci Int: Genet. 2015;17:173–9.
Okazaki S, Kimura R, Otsuka I, Funabiki Y, Murai T, Hishimoto A. Epigenetic clock analysis and increased plasminogen activator inhibitor-1 in high-functioning autism spectrum disorder. PloS One. 2022;17:e0263478.
McKinney BC, Lin H, Ding Y, Lewis DA, Sweet RA. DNA methylation evidence against the accelerated aging hypothesis of schizophrenia. NPJ Schizophr. 2017;3:13.
Dada O, Adanty C, Dai N, Jeremian R, Alli S, Gerretsen P, et al. Biological aging in schizophrenia and psychosis severity: DNA methylation analysis. Psychiatry Res. 2021;296:113646.
Viana J, Hannon E, Dempster E, Pidsley R, Macdonald R, Knox O, et al. Schizophrenia-associated methylomic variation: molecular signatures of disease and polygenic risk burden across multiple brain regions. Hum Mol Genet. 2017;26:210–25.
Davis S, Du P, Bilke S, Triche J, Bootwalla M. Methylumi: Handle Illumina Methylation Data. In R package, Vol. version 2.28.0. 2018.
Pidsley R, Wong CCY, Volta M, Lunnon K, Mill J, Schalkwyk LC. A data-driven approach to preprocessing Illumina 450K methylation array data. BMC Genom. 2013;14:293.
Chen YA, Lemire M, Choufani S, Butcher DT, Grafodatskaya D, Zanke BW, et al. Discovery of cross-reactive probes and polymorphic CpGs in the Illumina Infinium HumanMethylation450 microarray. Epigenetics .2013;8:203–9.
Price ME, Cotton AM, Lam LL, Farré P, Emberly E, Brown CJ, et al. Additional annotation enhances potential for biologically-relevant analysis of the Illumina Infinium HumanMethylation450 BeadChip array. Epigenet Chromatin. 2013;6:4.
Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:3156.
Horvath S. Erratum to: DNA methylation age of human tissues and cell types. Genome Biol. 2015;16:96.
Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26:146–53.
Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, et al. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol Autism. 2015;6:36.
Voisey J, Lawford BR, Morris CP, Wockner LF, Noble EP, Young RM, et al. Epigenetic analysis confirms no accelerated brain aging in schizophrenia. NPJ Schizophrenia. 2017;3:26.
McEwen LM, O’Donnell KJ, McGill MG, Edgar RD, Jones MJ, MacIsaac JL, et al. The PedBE clock accurately estimates DNA methylation age in pediatric buccal cells. Proc Natl Acad Sci USA. 2020;117:23329–35.
Yoon SH, Choi J, Lee WJ, Do JT. Genetic and epigenetic etiology underlying autism spectrum disorder. J Clin Med. 2020;9:966.
Magwai T, Shangase KB, Oginga FO, Chiliza B, Mpofana T, Xulu KR. DNA methylation and schizophrenia: current literature and future perspective. Cells .2021;10:2890.
Keil KP, Lein PJJEE. DNA methylation: a mechanism linking environmental chemical exposures to risk of autism spectrum disorders? Environ Epigenet. 2016;2:dvv012.
Siu MT, Butcher DT, Turinsky AL, Cytrynbaum C, Stavropoulos DJ, Walker S, et al. Functional DNA methylation signatures for autism spectrum disorder genomic risk loci: 16p11.2 deletions and CHD8 variants. Clin Epigenet. 2019;11:103.
Shadyab AH, McEvoy LK, Horvath S, Whitsel EA, Rapp SR, Espeland MA, et al. Association of blood-based epigenetic age acceleration with cognitive impairment and brain outcomes by cardiovascular disease among women. Alzheimer’s Dement. 2021;17:e051774.
Vaccarino V, Huang M, Wang Z, Hui Q, Shah AJ, Goldberg J, et al. Epigenetic age acceleration and cognitive decline: a twin study. J Gerontology: Ser A. 2021;76:1854–63.
McKinney BC, Lin H, Ding Y, Lewis DA, Sweet RA. DNA methylation age is not accelerated in brain or blood of subjects with schizophrenia. Schizophr Res. 2018;196:39–44.
Fransquet PD, Lacaze P, Saffery R, Shah RC, Vryer R, Murray A, et al. Accelerated epigenetic aging in peripheral blood does not predict dementia risk. Curr Alzheimer Res. 2021;18:443–51.
Li Z, He Y, Ma X, Chen X. Epigenetic age analysis of brain in major depressive disorder. Psychiatry Res. 2018;269:621–4.
Bernard JA, Seidler RD. Moving forward: age effects on the cerebellum underlie cognitive and motor declines. Neurosci Biobehav Rev. 2014;42:193–207.
Lu AT, Hannon E, Levine ME, Hao K, Crimmins EM, Lunnon K, et al. Genetic variants near MLST8 and DHX57 affect the epigenetic age of the cerebellum. Nat Commun. 2016;7:10561.
D’Mello AM, Stoodley CJJFIN. Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci. 2015;9:408.
Zhu J-D, Wu Y-F, Tsai S-J, Lin C-P, Yang AC. Investigating brain aging trajectory deviations in different brain regions of individuals with schizophrenia using multimodal magnetic resonance imaging and brain-age prediction: a multicenter study. Transl Psychiatry. 2023;13:82.
Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–8.
Horvath S, Mah V, Lu AT, Woo JS, Choi OW, Jasinska AJ, et al. The cerebellum ages slowly according to the epigenetic clock. Aging (Albany NY). 2015;7:294–306.
Crespi B, Badcock C. Psychosis and autism as diametrical disorders of the social brain. Behav Brain Sci. 2008;31:241–61. discussion 61-320
Cropley VL, Klauser P, Lenroot RK, Bruggemann J, Sundram S, Bousman C, et al. Accelerated gray and white matter deterioration with age in schizophrenia. Am J Psychiatry. 2016;174:286–95.
Finger CE, Moreno-Gonzalez I, Gutierrez A, Moruno-Manchon JF, McCullough LD. Age-related immune alterations and cerebrovascular inflammation. Mol psychiatry. 2022;27:803–18.
Constantinides C, Han LKM, Alloza C, Antonucci LA, Arango C, Ayesa-Arriola R, et al. Brain ageing in schizophrenia: evidence from 26 international cohorts via the ENIGMA Schizophrenia consortium. Mol Psychiatry. 2023;28:1201–9.
Harris E. Inflammation genes show age-dependent link with autism. JAMA 2023;329:1054.
Acknowledgements
We thank Shanghai NewCore Biotechnology Co., Ltd. (https://www.bioinformatics.com.cn, last accessed on 10 July 2023) for providing visualization support.
Funding
This work was supported by the Natural Science Basic Research Plan in Shaanxi Province of China [2021JCW-08], the Fundamental-clinical Research Program of the First Affiliated Hospital of Xi’an Jiaotong University [YXJLRH2022027]. This work was supported by US National Institutes of Health grant R01MH094714 to D.H.G. and is part of the PsychEncode Consortium. Data were generated as part of the PsychENCODE Consortium, supported by: U01DA048279, U01MH103339, U01MH103340, U01MH103346, U01MH103365, U01MH103392, U01MH116438, U01MH116441, U01MH116442, U01MH116488, U01MH116489, U01MH116492, U01MH122590, U01MH122591, U01MH122592, U01MH122849, U01MH122678, U01MH122681, U01MH116487, U01MH122509, R01MH094714, R01MH105472, R01MH105898, R01MH109677, R01MH109715, R01MH110905, R01MH110920, R01MH110921, R01MH110926, R01MH110927, R01MH110928, R01MH111721, R01MH117291, R01MH117292, R01MH117293, R21MH102791, R21MH103877, R21MH105853, R21MH105881, R21MH109956, R56MH114899, R56MH114901, R56MH114911, R01MH125516, and P50MH106934 awarded to: Alexej Abyzov, Nadav Ahituv, Schahram Akbarian, Alexander Arguello, Lora Bingaman, Kristin Brennand, Andrew Chess, Gregory Cooper, Gregory Crawford, Stella Dracheva, Peggy Farnham, Mark Gerstein, Daniel Geschwind, Fernando Goes, Vahram Haroutunian, Thomas M. Hyde, Andrew Jaffe, Peng Jin, Manolis Kellis, Joel Kleinman, James A. Knowles, Arnold Kriegstein, Chunyu Liu, Keri Martinowich, Eran Mukamel, Richard Myers, Charles Nemeroff, Mette Peters, Dalila Pinto, Katherine Pollard, Kerry Ressler, Panos Roussos, Stephan Sanders, Nenad Sestan, Pamela Sklar, Nick Sokol, Matthew State, Jason Stein, Patrick Sullivan, Flora Vaccarino, Stephen Warren, Daniel Weinberger, Sherman Weissman, Zhiping Weng, Kevin White, A. Jeremy Willsey, Hyejung Won, and Peter Zandi.
Author information
Authors and Affiliations
Contributions
LL and XQ drafted the manuscript. FZ, YJ, and YW designed the study. FZ provided the key datasets regarding our manuscript. SC, BC, and HL performed the statistical analyses. NZ, PM, XY, CP, YC, HZ, ZZ, JZ, and CL provided feasible advice on data analysis and drafting the manuscript. All authors read and approved the final manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Qi, X., Cheng, S. et al. Epigenetic analysis suggests aberrant cerebellum brain aging in old-aged adults with autism spectrum disorder and schizophrenia. Mol Psychiatry 28, 4867–4876 (2023). https://doi.org/10.1038/s41380-023-02233-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02233-6