Abstract
Well-differentiated papillary mesothelial tumor (WDPMT, formerly called well-differentiated papillary mesothelioma) is a morphologically distinctive lesion composed of expansile papillae with a myxoid core covered by a single layer of generally bland mesothelial cells. Whether some WDPMT are precursors of invasive mesothelioma is uncertain, and this question is confounded by shallow biopsies of ordinary diffuse mesotheliomas that have superficial areas resembling WDPMT as well as by misinterpretation of some cases of mesothelioma in situ. Genetic analyses on a very small number of published cases of peritoneal WDPMT have shown a variety of mutations/copy number losses that do not overlap at all with those that are found recurrently in invasive mesotheliomas. The newly described entity of mesothelioma in situ usually appears as a single layer of mesothelial cells that have lost BAP1 by immunostaining, but sometimes is papillary and produces a morphologic mimic of WDPMT. We propose that, at least in the peritoneal cavity where most WDPMT occur, there are two morphologically identical but functionally distinct lesions: one is true WDPMT, a process that is probably benign, and the other is papillary mesothelioma in situ with the configuration of WDPMT. For that reason immunostaining for BAP1, and if necessary MTAP or CDKN2A FISH, should always be performed on cases with the appearance of WDPMT. It is possible, but speculative, that the small number of reports in the literature which describe invasive mesothelioma arising from WDMPT are actually describing invasive mesothelioma arising from mesothelioma in situ that looks like WDPMT.
Similar content being viewed by others
Well-differentiated papillary mesothelial tumor (WDPMT), formerly known as well-differentiated papillary mesothelioma, is a histologically distinctive mesothelial tumor of uncertain malignant potential found in the pleura, peritoneum, and tunica vaginalis. Both the latest Thoracic Tumors and Female Genital Tumors WHO books1,2 recommend using WDPMT rather than well-differentiated papillary mesothelioma as a diagnostic term because surgeons and oncologists frequently view the “mesothelioma” part of “well-differentiated papillary mesothelioma” as an indication that these are overtly malignant neoplasms that should be treated in the same fashion as ordinary invasive diffuse mesotheliomas (MM) (for example, ref. 3). However, changing the nomenclature only partially addresses the clinical problem, because morphologic overlaps with invasive mesotheliomas, the significance of an invasive component in WDPMT, genetic separation from invasive mesotheliomas, the relationship of WDPMT to the newly described entity of mesothelioma in situ, and, most important, how WDPMT behave remain unresolved questions. This article will briefly highlight these issues and recommend some approaches for pathologists.
Clinical and gross pathologic findings
The largest individual WDPMT series comes from Sun et al.4 who published 75 cases of their own and found a total of 180 cases in the literature as of 2019; of these 135 were peritoneal, 37 pleural, 6 in the tunica vaginalis, and 2 in hernia sacs. In the same year Vogin et al.3 reported another 56 peritoneal cases (without pathologic details), although 9 of these were described as having “fatty infiltration” raising a suspicion that they represent misdiagnosed ordinary invasive MM. In the summary prepared by Sun et al., peritoneal cases in women outnumbered those in men by 6:1, whereas pleural cases showed a roughly equal female:male distribution.
Clinically, pleural cases generally are symptomatic and are associated with a pleural effusion5,6; at thoracoscopy there typically are multiple small nodules over the affected pleura. In contrast, in the peritoneal cavity WDPMT is usually found incidentally during surgery for another process. Only a very small fraction of patients present with abdominal pain or ascites and are found to have WDPMT as the cause. Such patients sometimes have bowel wall or mesenteric thickening on imaging6. In a minority of cases peritoneal WDPMT are associated with multicystic mesotheliomas, adenomatoid tumors, and endometriosis. On direct examination, peritoneal cases may have single nodules, a few nodules, or occasionally innumerable nodules.
Microscopic findings
WDPMT is typically composed of papillae with expansile myxoid cores covered by a single layer of generally bland, flattened to cuboidal mesothelial cells (Fig. 1). Sometimes the expansile papillary stroma of WDPMT is fibrotic/hyalinized, presumably a finding in old lesions6. WDPMT stain with traditional mesothelial markers such as calretinin, WT-1, cytokeratin 5/6, and D2-40, and should be negative with traditional carcinoma markers. An important pitfall to note is that PAX-8, a marker frequently positive in ovarian and other gynecologic tumors, is frequently positive in WDPMT, typically in peritoneal but occasionally in pleural lesions. PAX-8 positivity was observed in 94% of WDPMT in the series of Sun et al.4 and 61% of WDPMT in the series of Xing et al.7). These numbers suggest that PAX-8 positivity in WDPMT is more frequent than in MM4, although in our experience PAX-8 staining of both WDPMT and peritoneal mesotheliomas is dependent on antibody clone and antibody type (polyclonal stains more frequently than monoclonal).
Another marker that has sometimes been proposed as positive in WDPMT but negative in mesotheliomas is L1CAM (CD171)8. However, Inaguma et al.9 reported positive L1CAM staining in 70% of mesotheliomas and Itami et al.10 in 53% of mesotheliomas, so L1CAM probably has little diagnostic value in this setting.
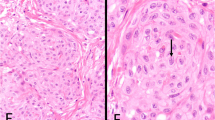
While the appearance of WDPMT is characteristic, there are nonetheless three important microscopic confounders. One is a superficial sample of an ordinary invasive diffuse mesothelioma; MM sometimes have papillary areas near free surfaces and such areas can closely mimic WDPMT5,6 (Fig. 2). Cytologic detail can be helpful: prominent nucleoli and, particularly, cytologic pleomorphism should raise a suspicion of invasive mesothelioma rather than WDPMT, although prominent nucleoli can, rarely, be seen in tumors that are (apparently) WDPMT. The operator’s description or CT scan reports may be informative if there is unequivocal evidence of malignancy.
A, B Two different areas of an ordinary invasive diffuse mesothelioma in which there are superficial expansile papillae with myxoid cores. A shallow biopsy might sample only the papillary area, leading to a misdiagnosis of well differentiated papillary mesothelial tumor. Review of the operator’s report can be very helpful in this situation if the description is that of a diffuse serosal malignancy.
A second confounder is ovarian type serous tumors, including ovarian serous surface papillomas, serous borderline tumors, and, occasionally, low grade serous carcinomas, the vast majority in the peritoneum cavity but rarely intratesticular/paratesticular1,11,12. All of these lesions can have a papillary structure that mimics WDPMT, but typically have columnar cells with considerable cytologic atypia in the borderline and low grade serous carcinomas. Most important, they stain strongly with broad spectrum carcinoma markers such as MOC-31, claudin-4, and BerEP4. They are usually calretinin negative but WT-1 and PAX-8 positive.
The third, and crucial, confounder is mesothelioma in situ, which can take on the microscopic appearance of WDPMT (see section 5 below)
Significance of invasive foci in WDPMT
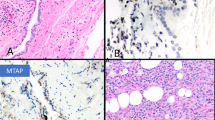
In most WDPMT the proliferating mesothelial cells are confined to the surface of the papillae, but occasional tumors demonstrate invasion of mesothelial cells into the fibrovascular stalks that support the papillae or sometimes below the level of the stalks (Fig. 3). These lesions are different from the type of ordinary mesothelioma in which there is surface formation of structures that mimic WDPMT (such as Fig. 2), because WDPMT with invasive foci have the overall structure of WDPMT, not diffuse mesothelioma.
A The low power view shows a typical pattern of well differentiated papillary mesothelial tumor, but (B) a focal region in another area of the stalk (not shown in A) demonstrates locally invasive tumor (box). This lesion is from a hydrocoele; patient was treated with hemiscrotectomy and was alive and well without evidence of recurrence 6 years later.
Churg et al.13 described 20 WDPMT with invasion confined to the stalks which they labeled “well differentiated papillary mesothelioma with invasive foci.” In that series tumors with invasive foci were associated with a distinct tendency toward multifocality and recurrence, the latter seen in eight patients. No patient developed metastatic disease, and only one patient was reported to have died of disease, but no histologic confirmation of the type of tumor present at death was available.
Trpkov et al. reported14 a case of tunica vaginalis WDPMT that by illustration had invasive foci, and the patient was well without recurrence 6 years later. Butnor et al.6 found superficial invasion in two pleural cases; one did not have followup and one had persisting disease at 1 year. In the peritoneal series reported by Sun et al.4, six cases had invasion of mesothelial cells deep to the stalk but none of these patients developed disseminated mesothelioma. Malpica et al.15 noted that 1 of 26 peritoneal WDPMT showed shallow invasion into the underlying (ovarian) tissue but that this was not an adverse prognostic finding.
Reported cases in which clearly documented and unequivocal diffuse invasive mesotheliomas arose in a WDPMT are few. Torii et al.16 reported a case of multifocal pleural WDPMT that was treated with extrapleural pneumonectomy; deep invasive disease was seen in the resection specimen. Of interest, this patient had a pleural effusion and the illustrated cytologic appearance of the effusion was that of a mesothelioma, suggesting that the WDPMT was really mesothelioma in situ because mesothelioma in situ has been reported to produce a picture identical to invasive mesothelioma in effusion samples17,18.
Shimizu et al.19 described a patient with an incidentally discovered WDPMT lesion in a lung cancer resection specimen; there was invasion of mesothelial cells from the WDPMT into the underlying stroma and the invasive process morphologically looked like a mesothelioma. Costanzo et al.20 illustrated a case that morphologically started as pleural WDPMT and ended as metastatic mesothelioma 13 years later. Burrig et al.21 described a similarly well illustrated case in which mesothelioma developed 5 years after diagnosis of a peritoneal WDPMT. Galateau-Salle et al.5 reported two patients with pleural WDPMT and no evidence of invasion at biopsy, in whom invasive lethal mesothelioma was diagnosed after 10 years. Sun et al.4 show a nicely illustrated case in which invasive mesothelioma developed 15 years after the diagnosis of WDPMT. Washini et al.22 described a case in which invasive mesothelioma developed 7 years after the diagnosis of WDPMT; however, while the invasive tumor looks like a mesothelioma, from their illustrations the original tumor is suspicious for mesothelioma in situ rather than WDPMT.
Genetics
There is very little in the literature on genomic analyses of WDPMT, and all of this is confined to peritoneal tumors. Stevers et al.23 reported ten cases and found only mutually exclusive CDC42 (two cases) or TRAF7 (eight cases) missense mutations. Their TRAF7 mutations all localized at the C-terminus of the protein. Of interest, to get adequate sequencing depth Stevers et al. combined multiple separate WDPMT tumors in two patients but still found only a single CDC42 or TRAF7 mutation in each patient, which suggests that multifocal WDPMT represents clonal spread. Nine cases did not have copy number losses or gains.
In contrast, Shrestha et al.24 analyzed 5 cases and found recurrent missence mutations of EHD1, ATM, FBXO10, SH2D2A, CDH5, MAGED1, and TP73, all present in either 4/5 or 5/5 cases. No case had a mutation of either TRAF7 or CDC42, although one showed copy number loss of TRAF7. The WDPMT was strongly enriched for C>A transversion substitutions, a pattern that is not seen in MM. There were a variety of copy number losses and gains but no consistent pattern across the 5 tumors.
None of the WDPMT in either the Stevers or Shrestha paper had abnormalities in genes found to be recurrently mutated or deleted in MM such as BAP1, SETD2, NF2, LATS1/2, CDKN2A/B)25, although it should be noted that TRAF7 mutations have been described in a few peritoneal mesotheliomas24. Nemoto et al.26 reported a lesion claimed to be WDPMT in which loss of heterozygosity in NF2 was present; however, their illustrations do not look at all like WDPMT and probably represent an ordinary MM.
WDPMT as a manifestation of mesothelioma in situ
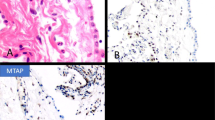
Mesothelioma in situ (MIS) was recently defined as single layer of flattened to cuboidal mesothelial cells that had lost BAP1, and sometimes MTAP, by immunochemical staining27. A single case of MIS with CDKN2A deletion but BAP1 retention has also been described28. However, the original description27 of ten cases of MIS included one (pleural) case with both flat mesothelium and a process morphologically very similar to WDPMT. We have subsequently seen a number of cases of peritoneal MIS that have both flat single-layered mesothelium and areas identical to WDPMT (Fig. 4).
Low and medium power views of mesothelioma in situ mimicking a well differentiated papillary mesothelial tumor (A, B). Lesion is peritoneal. Stain for BAP1 (C) shows nuclear loss in both the papillary areas and the flat mesothelium (arrow), indicating that the correct diagnosis is mesothelioma in situ.
Other authors have described “WDPMT” with BAP1 loss. Lee et al.29 stained eight peritoneal cases that microscopically looked like WDPMT. BAP1 was lost in three and these three had synchronous (but spatially separated) or metachronous invasive mesothelioma; genetic analysis showed an identical pattern between the WDPMT and the invasive tumor in each case. We believe that these cases really are examples of MIS that morphologically look like WDPMT.
Dacic et al.30 performed whole-exome sequencing on two cases of MIS which immunohistochemically showed BAP1 loss; in both cases, the analyzed areas were partially flat and partially papillary, with the latter areas having the appearance of WDPMT. The pleural case had copy number loss and LOH in the 3p21 region that harbors BAP1. The peritoneal tumor showed a somatic BAP1 splice site mutation and BAP1 copy number loss. No other somatic mutations, indels, or somatic copy number abnormalities were identified; in particular, none of the recurrent mutations or recurrent copy number losses seen in ordinary MM were present. Dacic et al. suggested that BAP1 mutations/deletions may be the earliest abnormality in invasive mesothelioma. Since BAP1 loss/mutation is always a marker of malignancy in mesothelial proliferations31 these data strongly imply that some WDPMT, or at least papillary processes that look like WDPMT, are precursors of invasive mesothelioma, an idea that contradicts the (very limited) published genomic analyses of WDPMT described above.
It appears likely that MIS always becomes invasive, although the time from biopsy diagnosis of MIS to invasive disease is unclear and can be quite long. In the series reported by Churg et al.27, invasive disease developed in 7/10 patients with an 8th patient showing diffuse pleural thickening on imaging thought suspicious for an invasive mesothelioma at 57 months. For the seven cases with eventual overt MM, the median time to invasion was 60 months with the longest time 92 months, but one patient still had not developed invasive disease at 120 months. In the report of Klebe et al.17, invasive tumor developed after 4 years 10 months. Fels Elliott32 described a patient with invasive disease diagnosed at 10 months after biopsy, and Minami et al.28 a patient in whom no invasive disease could be detected at 9 months. The metachronous case described by Lee et al.29 had a 10 yr interval between the diagnosis of WDPMT (really MIS) and the appearance of invasive mesothelioma. In the multi-author position paper by Klebe et al.33, 40% of pathologists reported seeing cases with a greater than 4 year interval between the diagnosis of MIS and the appearance of invasive mesothelioma, although it is not entirely clear whether all authors of that paper were using the same definition of MIS. It should be noted that the papers reviewed in this paragraph largely describe flat and not papillary lesions; in fact, Klebe et al. position paper33 suggests that WDPMT morphology is not a feature of MIS.
Conclusions: the nature of WDPMT
The data just described raise a contradictory question: is WDPMT a generally innocuous finding, at least in the peritoneal cavity where most such tumors occur, or it is a form of mesothelioma in situ and a precursor of invasive mesothelioma? The WHO Female Genital Tumors book1 proposes that WDPMT in the peritoneum are benign and that aggressive behavior is indicative of a missed diagnosis of MM, whereas the Thoracic Tumors WHO book2 concludes that most pleural WDPMT behave as slowly growing recurrent lesions. A major confounder is that, in many instances, tumors that are called recurrent or (eventually) clinically malignant are not biopsied so whether they are MM is usually not documented.
Detailed followup on the largest series4,15,34 suggests that, in the peritoneal cavity, the vast majority of tumors that look like WDPMT do appear to be functionally benign, even if multifocal. In the pleural cavity the prognosis is more guarded5,6, although as detailed above, it is hard to find many published examples of invasive mesothelioma developing from WDPMT lesions in either site.
Genomic analyses, albeit based on few and only peritoneal cases, imply that WDPMT are distinct neoplasms that have no relationship to ordinary diffuse mesotheliomas. However, this idea is contradicted by the observation of BAP1 loss by immunochemistry and BAP1 mutations/deletions in cases of mesothelioma in situ that are morphologically identical to WDPMT27,29,30 and by the occasional case in which tumors that morphologically appear to be MM have been documented to arise in WDPMT, as described above.
We propose that, in fact, there are two different lesions that can look microscopically like WDPMT: true (probably benign, at least in the peritoneal cavity) WDPMT, and WDPMT-like mesothelioma in situ, but it’s not possible to separate these entities on routine morphology. The relative frequency of these two different lesions is unclear and the number of reported cases is too small to draw definite conclusions. As noted, Lee et al.29 found 3/8 cases with WDPMT morphology that had BAP1 loss by immunochemistry, whereas 3 other reports35,36,37 totaling 18 cases did not find BAP1 loss, although in one series35 the comment was made that BAP1 retention was often patchy in WDPMT; this phenomenon can occasionally be seen in mesothelioma in situ (Churg A, Galateau-Salle, unpublished data) and probably represents spread of a malignant clone.
What is not known at this point is what fraction of WDPMT actually represent MIS, nor is it known what fraction of MIS look like WDPMT. The authors of this article have seen around 30 examples of the latter, but because these are consultation cases, there is extreme selection bias and one can’t use these numbers to estimate the real frequency of MIS that looks like WDPMT. However, it is possible that such lesions are more common than anyone realizes because the frequently long lead time for MIS to develop into invasive mesothelioma exceeds the followup time for some fraction of reported patients. For example, in the report of Malpica et al.15, there was only 1 recurrence (incidentally discovered) in a series of 25 peritoneal WDPMT, but the median followup time was 32 months. In the series of Sun et al.4 the median followup time for 46 patients was 29 months and the one apparent transformation of WDPMT into invasive mesothelioma took 15 years. In the 22 patients described by Daya and McCaughey34, one patient died at 7 years with what appears to be diffuse peritoneal tumor by description. A number of other individual cases with very long times between the diagnosis of WDPMT and invasive mesothelioma have been listed above in “Significance of invasive foci in WDPMT”. It is conceivable that, in fact, all of the cases reported as WDPMT that eventually “turned into” or recurred as invasive mesothelioma are really examples of mesothelioma in situ mimicking WDPMT. At the moment this idea is speculative but is potentially testable using archival material.
Conclusions: recommendations for diagnosis
Given this situation, we suggest that at a minimum, a BAP1 immunostain should be performed on any lesion that looks like WDPMT; if BAP1 is retained, then MTAP immunohistochemistry or CDKN2A FISH should be the next step. It should also be remembered that the combination of BAP1/MTAP/CDKN2A investigation will not detect all invasive mesotheliomas38 and the same conclusion presumably applies to mesothelioma in situ, particularly since the sensitivity of CDKN2A FISH (and by implication MTAP immunochemistry) is known to be fairly low in the peritoneal cavity39. A potentially helpful finding is that all the MIS cases we have seen that look like WDPMT also have flat mesothelioma in situ which usually looks like a single layer of bland cuboidal mesothelial cells (see Fig. 4C); however, benign reactions can also look like a single layer of bland cuboidal mesothelial cells, so appropriate immunohistochemical or FISH detection is still required.
This approach may appear to be overkill, but even if MIS constitutes only a few percent of lesions that look like WDPMT, MIS probably requires aggressive therapy before it becomes invasive. On the other hand, truly benign WDPMT probably requires no therapy at all beyond just local excision, and in fact, radical therapy for such tumors is often associated with adverse outcomes3. What we suggest, when dealing with a lesion that looks like WDPMT and has retained BAP1 and retained MTAP or no CDKN2A deletion, is that the case be signed out as WDPMT. However, a comment should be added that in all likelihood the lesion is benign and requires no further therapy, but followup is warranted because there is a small risk that such lesions still could represent MIS.
The significance of stalk invasion that originates in a WDPMT remains unclear and is not necessarily an adverse finding, but in such cases BAP1/MTAP/CDKN2A FISH analysis is crucial. Even if this analysis is negative, a statement that patients with WDPMT with invasion should be carefully followed would probably be appropriate in a surgical pathology report.
Data availability
There are no data beyond those described in the manuscript.
References
WHO Classification of Tumors Editorial Board: Female Genital Tumors. Lyon, International Agency for Research on Cancer, 2020.
WHO Classification of Tumors Editorial Board: Thoracic Tumors. Lyon, International Agency for Research on Cancer, 2021.
Vogin G, Hettal L, Vignaud JM, Dartigues P, Goere D, Ferron G, et al; RENAPE Network. Well-differentiated papillary mesothelioma of the peritoneum: a retrospective study from the RENAPE Observational Registry. Ann Surg Oncol. 26:852–860 (2019).
Sun M, Zhao L, Weng Lao I, Yu L, Wang J. Well-differentiated papillary mesothelioma: a 17-year single institution experience with a series of 75 cases. Ann Diagn Pathol. 38:43–50 (2019).
Galateau-Sallé F, Vignaud JM, Burke L, Gibbs A, Brambilla E, Attanoos R, et al; Mesopath group. Well-differentiated papillary mesothelioma of the pleura: a series of 24 cases. Am J Surg Pathol. 28:534–540 (2004).
Butnor KJ, Sporn TA, Hammar SP, Roggli VL. Well-differentiated papillary mesothelioma. Am J Surg Pathol. 25:1304–1309 (2001).
Xing D, Banet N, Sharma R, Vang R, Ronnett BM, Illei PB. Aberrant Pax-8 expression in well-differentiated papillary mesothelioma and malignant mesothelioma of the peritoneum: a clinicopathologic study. Hum Pathol. 72:160–166 (2018).
Goode B, Joseph NM, Stevers M, Van Ziffle J, Onodera C, Talevich E, et al. Adenomatoid tumors of the male and female genital tract are defined by TRAF7 mutations that drive aberrant NF-kB pathway activation. Mod Pathol. 31:660–673 (2018).
Inaguma S, Wang Z, Lasota JP, Miettinen MM. Expression of neural cell adhesion molecule L1 (CD171) in neuroectodermal and other tumors: an immunohistochemical study of 5155 tumors and critical evaluation of CD171 prognostic value in gastrointestinal stromal tumors. Oncotarget. 7:55276–55289 (2016).
Itami H, Fujii T, Nakai T, Takeda M, Kishi Y, Taniguchi F, et al. TRAF7 mutations and immunohistochemical study of uterine adenomatoid tumor compared with malignant mesothelioma. Hum Pathol. 111:59–66 (2021).
Bürger T, Schildhaus HU, Inniger R, Hansen J, Mayer P, Schweyer S, Bremmer F, et al. Ovarian-type epithelial tumours of the testis: immunohistochemical and molecular analysis of two serous borderline tumours of the testis. Diagn Pathol. 10:118 (2015).
Ulbright TM, Young RH. Tumors of the Testis and Adjacent Structures. American Registry of Pathology, Silver Spring, Maryland, 2013.
Churg A, Allen T, Borczuk AC, Cagle PT, Galateau-Sallé F, Hwang H et al. Well-differentiated papillary mesothelioma with invasive foci. Am J Surg Pathol. 38:990–998 (2014)
Trpkov K, Barr R, Kulaga A, Yilmaz A. Mesothelioma of tunica vaginalis of “uncertain malignant potential” - an evolving concept: case report and review of the literature. Diagn Pathol. 6:78 (2011).
Malpica A, Sant’Ambrogio S, Deavers MT, Silva EG. Well-differentiated papillary mesothelioma of the female peritoneum: a clinicopathologic study of 26 cases. Am J Surg Pathol. 36:117–127 (2012).
Torii I, Hashimoto M, Terada T, Kondo N, Fushimi H, Shimazu K, et al. Well-differentiated papillary mesothelioma with invasion to the chest wall. Lung Cancer. 2010 67:244–247 (2010).
Klebe S. Progression of mesothelioma in situ to invasive disease 4 years and 10 months after initial diagnosis. Pathology. 2021 Sep 23:S0031–3025(21) 00460–8. https://doi.org/10.1016/j.pathol.2021.06.124. Epub ahead of print.
Churg A, Galateau-Salle F, Tan L, Qing G. Cytological diagnosis of mesothelioma in situ versus invasive mesothelioma. Pathology. 54:133–136 (2022).
Shimizu S, Yoon HE, Ito N, Tsuji T, Funakoshi Y, Utsumi T, et al. A case of solitary well-differentiated papillary mesothelioma with invasive foci in the pleura. Pathol Int. 67:45–49 (2017).
Costanzo L, Scarlata S, Perrone G, Rossi L, Papa A, Di Matteo FM, et al. Malignant transformation of well-differentiated papillary mesothelioma 13 years after the diagnosis: a case report. Clin Respir J. 8:124–129 (2014)
Bürrig KF, Pfitzer P, Hort W. Well-differentiated papillary mesothelioma of the peritoneum: a borderline mesothelioma. Report of two cases and review of literature. Virchows Arch A Pathol Anat Histopathol. 417:443–447 (1990).
Washimi K, Yokose T, Amitani Y, Nakamura M, Osanai S, Noda H et al. Well-differentiated papillary mesothelioma, possibly giving rise to diffuse malignant mesothelioma: a case report. Pathol Int. 63:220–225 (2013).
Stevers M, Rabban JT, Garg K, Van Ziffle J, Onodera C, Grenert JP et al. Well-differentiated papillary mesothelioma of the peritoneum is genetically defined by mutually exclusive mutations in TRAF7 and CDC42. Mod Pathol. 32:88–99 (2019).
Shrestha R, Nabavi N, Volik S, Anderson S, Haegert A, McConeghy B, et al. Well-differentiated papillary mesothelioma of the peritoneum is genetically distinct from malignant mesothelioma. Cancers12:1568 (2020).
Bueno R, Stawiski EW, Goldstein LD, Durinck S, De Rienzo A, Modrusan Z, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 48:407–416 (2016).
Nemoto H, Tate G, Kishimoto K, Saito M, Shirahata A, Umemoto T, et al. Heterozygous loss of NF2 is an early molecular alteration in well-differentiated papillary mesothelioma of the peritoneum. Cancer Genet. 205:594–598 (2012).
Churg A, Galateau-Salle F, Roden AC, Attanoos R, von der Thusen JH, Tsao MS, et al. Malignant mesothelioma in situ: morphologic features and clinical outcome. Mod Pathol. 33:297–302 (2020).
Minami K, Jimbo N, Tanaka Y, Hokka D, Miyamoto Y, Itoh T, et al. Malignant mesothelioma in situ diagnosed by methylthioadenosine phosphorylase loss and homozygous deletion of CDKN2A: a case report. Virchows Arch. 476:469–473 (2020).
Lee HE, Molina JR, Sukov WR, Roden AC, Yi ES. BAP1 loss is unusual in well-differentiated papillary mesothelioma and may predict development of malignant mesothelioma. Hum Pathol. 79:168–176 (2018).
Dacic S, Roy S, Lyons MA, von der Thusen JH, Galateau-Salle F, Churg A. Whole exome sequencing reveals BAP1 somatic abnormalities in mesothelioma in situ. Lung Cancer. 2020 149:1–4 (2020).
Wang LM, Shi ZW, Wang JL, Lv Z, Du FB, Yang QB, et al. Diagnostic accuracy of BRCA1-associated protein 1 in malignant mesothelioma: a meta-analysis. Oncotarget. 8:68863–68872 (2017)
Fels Elliott DR, Travieso JL, As-Sanie S, Hrycaj SM, Lieberman RW, Myers JL, et al. Progression oF peritoneal mesothelioma in situ to invasive mesothelioma arising in the setting of endometriosis with germline BAP1 mutation: a case report. Int J Gynecol Pathol. 2021 29. https://doi.org/10.1097/PGP.0000000000000832. Epub ahead of print.
Klebe S, Nakatani Y, Dobra K, Butnor KJ, Roden AC, Nicholson AG. The concept of mesothelioma in situ, with consideration of its potential impact on cytology diagnosis. Pathology. 53:446–453 (2021).
Daya D, McCaughey WT. Well-differentiated papillary mesothelioma of the peritoneum. A clinicopathologic study of 22 cases. Cancer. 65:292–296 (1990).
McGregor SM, Dunning R, Hyjek E, Vigneswaran W, Husain AN, Krausz T. BAP1 facilitates diagnostic objectivity, classification, and prognostication in malignant pleural mesothelioma. Hum Pathol. 46:1670–1678 (2015).
Joseph NM, Chen YY, Nasr A, Yeh I, Talevich E, Onodera C, et al. Genomic profiling of malignant peritoneal mesothelioma reveals recurrent alterations in epigenetic regulatory genes BAP1, SETD2, and DDX3X. Mod Pathol. 30:246–254 (2017).
Cigognetti M, Lonardi S, Fisogni S, Balzarini P, Pellegrini V, Tironi A, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol. 28:1043–1057 (2015).
Churg A, Naso JR. The separation of benign and malignant mesothelial proliferations: new markers and how to use them. Am J Surg Pathol. 44:e100–e112 (2020).
Singhi AD, Krasinskas AM, Choudry HA, Bartlett DL, Pingpank JF, Zeh HJ et al. The prognostic significance of BAP1, NF2, and CDKN2A in malignant peritoneal mesothelioma. Mod Pathol. 29:14–24 (2016).
Funding
This work was supported in part by The French National Cancer Institute (INCA) core grant and the National Health Institute (Santé Publique) France.
Author information
Authors and Affiliations
Contributions
Study design: AC, FGS. Data acquisition and analysis: AC, FGS. Manuscript drafting: AC, FGS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Ethics approval was from the Research Ethics Board of the University of British Columbia.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Churg, A., Galateau-Salle, F. Well differentiated papillary mesothelial tumor: a new name and new problems. Mod Pathol 35, 1327–1333 (2022). https://doi.org/10.1038/s41379-022-01082-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01082-y
This article is cited by
-
Benigne Tumoren des Mesothels
Die Pathologie (2024)
-
Mesothelioma: morphologic and immunohistochemical findings
Die Pathologie (2024)
-
Peritoneal papillary mesothelioma in situ: BAP1 mutation with indolent behavior for 15 years
Virchows Archiv (2023)