Abstract
Third-generation chimeric antigen receptor T cells (CARTs) for relapsed or refractory (r/r) chronic lymphocytic leukemia (CLL) may improve efficacy compared to second-generation CARTs due to their enhanced CAR design. We performed the first phase 1/2 investigator-initiated trial evaluating escalating doses of third-generation CARTs (HD-CAR-1) targeting CD19 in patients with r/r CLL and B-cell lymphoma. CLL eligibility criteria were failure to two therapy lines including at least one pathway inhibitor and/or allogeneic hematopoietic cell transplantation. Nine heavily pretreated patients received HD-CAR-1 at dose levels ranging from 1 × 106 to 200 × 106 CART/m2. In-house HD-CAR-1 manufacturing was successful for all patients. While neurotoxicity was absent, one case of grade 3 cytokine release syndrome was observed. By day 90, six patients (67%) attained a CR, five of these (83%) with undetectable MRD. With a median follow-up of 27 months, 2-year PFS and OS were 30% and 69%, respectively. HD-CAR-1 products of responders contained significantly more CD4 + T cells compared to non-responders. In non-responders, a strong enrichment of effector memory-like CD8 + T cells with high expression of CD39 and/or CD197 was observed. HD-CAR-1 demonstrated encouraging efficacy and exceptionally low treatment-specific toxicity, presenting new treatment options for patients with r/r CLL. Trial registration: #NCT03676504.
Similar content being viewed by others
Introduction
The use of second-generation CD19-directed chimeric antigen receptor T-cell (CART) therapy has revolutionized the treatment of several B-cell malignancies [1,2,3,4,5,6,7,8,9]. However, although chronic lymphocytic leukemia (CLL) has been the disease used for proof of principle of CART efficacy in humans [10], the clinical development of CLL CARTs has been hampered by inferior response rates and shorter response duration compared to other indolent B-cell lymphomas (ZUMA5, ELARA) [7, 11,12,13]. It has been hypothesized that this is at least in part due to the inherent dysfunction and alterations of T cells in this disease [10, 14,15,16,17,18,19]. However, liso-cel has recently been approved by the U.S. Food and Drug Administration as the first second-generation CART product for adults with relapsed or refractory (r/r) CLL or small lymphocytic lymphoma.
CARTs derived from patients with CLL typically display signs of exhaustion that lead to restricted expansion and decreased cytokine production resulting in insufficient leukemia suppression [20]. One way of improving CART efficacy in CLL involves modifications of the CAR vector. Third-generation CARs harbor two costimulatory domains mediating enhanced and faster expansion as well as longer persistence of CARTs [21,22,23,24,25]. However, clinical data on third-generation CARTs for CLL are scarce [26].
Here, we report initial findings of third-generation CARTs manufactured academically, as part of the investigator-initiated trial (IIT) Heidelberg CAR T-cell trial 1 (HD-CAR-1) [27], for the treatment of r/r CLL.
Methods
Trial design and manufacturing of CARTs
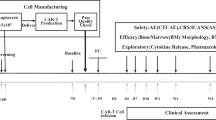
This was a 2-strata basket trial in patients with r/r acute lymphoblastic leukemia (ALL, stratum 1), and B-cell lymphoma and CLL (stratum 2) aiming to assess feasibility and to identify dose-limiting toxicities of HD-CAR-1 CARTs. To be eligible, patients with CLL needed to have failed ≥2 lines of therapy, including at least one pathway inhibitor and/or allogeneic hematopoietic cell transplantation (alloHCT), and an Eastern Cooperative Oncology Group performance score of 0 or 1. In a 3 + 3 design (across all eligible entities), patients received increasing doses of autologous T cells retrovirally transduced with a third-generation CD19-directed CAR (RV-SFG.CD19.CD28.4-1BBzeta) [27]. HD‐CAR‐1 CARTs were manufactured as previously described [27,28,29] at the institutional Good Manufacturing Practice Core Facility. For transduction, RV-SFG.CD19.CD28.4-1BBzeta retroviral vector supernatant was provided by Prof. Malcolm Brenner from Baylor College of Medicine in Houston, Texas, USA. This CAR harbors the costimulatory domains CD28 and 4-1BB. The trial was approved by the institutional review board as well as by the German federal regulatory authority for immunotherapy (Paul-Ehrlich-Institut, Langen, Germany), and written informed consent was obtained from all participants. The trial was conducted in compliance with the principles of the Declaration of Helsinki.
CART therapy and endpoint evaluation
Patients received HD-CAR-1 CARTs 2 days following administration of lymphodepletion with fludarabine 30 mg/m2/d and cyclophosphamide 500 mg/m2/d for 3 days. Cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and immune effector cell-associated hematotoxicity (ICAHT) were graded according to consensus guidelines [30, 31]. CRS and ICANS were managed according to institutional guidelines as published [32]. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. For lymphodepletion, CART infusion and monitoring patients were hospitalized from day −6 through day +14. The clinical efficacy of HD-CAR-1 treatment was assessed using the 2018 International Workshop on Chronic Lymphocytic Leukemia (iwCLL2018) criteria [33]. Minimal or measurable residual disease (MRD) was assessed by MRD-flow using one CLL cell in 10.000 events as detection threshold [34]. HD-CAR-1 CART frequencies were assessed as described [35].
Analysis of cellular composition of CART products with flow cytometry
HD-CAR-1 CART products from seven CLL patients (#1–7) were analyzed with spectral flow cytometry using a 36-marker panel on two different days (day 1: samples of #1-5 and #7, day 2: sample of #6). In brief, samples were thawed, washed and stained with the antibody mix. Staining was conducted over three consecutive rounds, with each round consisting of a 20-min incubation at 4 °C (antibodies used summarized in Supplementary Table S1). Samples were measured on Cytek Aurora flow cytometer (Cytek Biosciences, Fremont, CA, USA).
Computational analysis
Spectral unmixing was performed using SpectroFlo (Cytek Biosciences). To detect and remove anomalies based on common flow cytometry parameters, .fcs files were further processed using the R package flowAI (version 1.24.0). The function flow_auto_qc was run, and anomalies identified based on the flow rate were removed. These .fcs files were then imported into FlowJo (version 10.8.1; BD Biosciences, Franklin Lakes, NJ, USA) to assess unmixing quality and for gating on single viable cells. The data was then transformed using the logicle transform function of FlowJo (export channel values) and exported as .csv files for downstream analysis in R (version 4.3.0). Since batch effects between the samples of #1-5 and #7 and the sample of #6 were observed, downstream analyses involving clustering and Uniform Manifold Approximation and Projection (UMAP) [36] visualization were performed for samples of #1-5 and #7 only. Cell type labels of the sample of #6 were determined by linear discriminant analysis (LDA) from the MASS package (version 7.3–60) [37] using cell type annotations of the samples of #1-5 and #7 as reference.
All downstream analyses were performed using the R package Seurat (version 5.0.3) [38]. In brief, this involved atomic sketching (a non-uniform downsampling approach), a dimensional reduction using principal component analysis (PCA) followed by Louvain clustering and visualization of the data in a UMAP embedding. Cluster labels of cells not included in the initial sketching were determined by LDA using the R package MASS. Clusters were manually annotated based on surface marker expression and expert knowledge. Cell type frequencies were calculated per sample and compared between non-responders (#1, #2 and #7) and responders (#3-6). In accordance with the iwCLL2018 criteria [33], responders were defined as patients who achieved a CR after CART infusion that was sustained at least for 2 months. All non-responders had undergone prior alloHCT, while none of the responders had. Significance levels were determined by a two-sided Welch’s t-test. After overall cluster annotation, CD4+ and CD8 + T cells were separated and clustered independently using the same approach as described above. Differential abundance of cells within the respective UMAP space was highlighted by density plots. For quantification of differentially abundant CD4+ or CD8 + T-cell subtypes between responders and non-responders, the frequency of each T-cell subtype was calculated per sample. For each subtype, the frequency of responders was divided by the corresponding mean frequency of non-responders. These responder-specific fold-changes were log2 transformed and visualized in boxplots.
Statistical analysis
Statistics were calculated using Prism Software (version 10.2.0; Graphpad Software Inc., Boston, MA, USA). Progression-free survival (PFS) was determined measuring the duration from the date of CART administration until clinical progression, relapse, or death. Survival curves were compared using log-rank testing. A p-value less than 0.05 was considered statistically significant.
Results
Patients
Eight patients with r/r CLL were enrolled between October 2018 and May 2023. As the trial cohort was already fully recruited, another patient with r/r CLL was treated compliant with the trial but formally off-study in July 2023 after having given fully informed consent (#9). Baseline characteristics of all nine patients are listed in Table 1. Patients had a median age of 60 years (range 45 to 68) and had received a median of 5 (range 2 to 10) prior treatment lines. Four patients (44%) had received prior alloHCT. Seven patients (78%) harbored TP53 abnormalities. All patients had failed Bruton’s tyrosine kinase inhibitors (BTKi), and all had received at least one venetoclax-based regimen. Eight of the nine patients were refractory to venetoclax-based treatment as well. Nevertheless, bridging therapy was administered to all patients, mostly with venetoclax-antibody combinations, resulting in CR and partial remission (PR) in three and two patients, respectively. However, all patients had flow-detectable MRD at lymphodepletion. One patient (#8) had a history of Richter transformation which however was not present at the time of enrollment.
Manufacturing and dosing of HD-CAR-1 CARTs
HD-CAR-1 manufacturing was successful in all patients with a median transduction efficiency of 52.3% (range 41.7% to 67.7%) and a median CART viability of 95.2% (range 86.2% to 95.8%). Median duration of manufacturing was 13 days (range 10 to 13). A second leukapheresis had to be performed in two patients (#3 and #5) due to an unfavorable T-cell:B-cell ratio in the first leukapheresis products. Characteristics and cellular composition of HD-CAR-1 CART products are listed in Supplementary Table S2.
All patients received at least one dose of HD-CAR-1 CARTs (Table 2). Dose levels (DL) were DL1 (1 × 106 CARTs/m2) in one patient, DL2 (5 × 106 CARTs/m2) in one patient, DL5 (10 × 107 CARTs/m2) in two patients and DL6 (20 × 107 CARTs/m2) in five patients.
Safety
HD-CAR-1 CARTs were well tolerated (Table 2). Although seven of nine patients experienced CRS, higher grade CRS was observed only in a single patient (11%), and ICANS was completely absent. Early ICAHT occurred in eight patients (89%), but was grade 4 only in a single patient. Late ICAHT was observed as grade 1 in one patient (#9) and as grade 2 in two patients (#2 and #7) without the need for granulocyte colony-stimulating factor support.
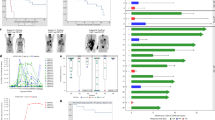
All six patients with CR as best response presented B-cell aplasia at end-of-study (EOS) on day 90 after CART administration (#3-8; Fig. 1A). Two patients showed absolute T-cell counts above the lower limit of normal (LLN) according to [39] at EOS, but presented counts below LLN at day 200 after CART administration (#5 and #6; Fig. 1B). In one patient, B-cell and T-cell recovery took place on day 450 besides ongoing CR with undetectable MRD (uMRD; #6). B-cell aplasia and lymphopenia were still present in patients #3, #4, #5, and #9 at their latest assessment on day 771, 548, 198 and 301 after CART administration, respectively (Fig. 1).
A Absolute B-cell count in cells/μl. B Absolute T-cell count in cells/μl. PB of patients was assessed with flow cytometry directly before and up to 890 days after CART administration. Normal ranges of absolute values are displayed according to [39].
Hypogammaglobulinemia was observed in eight of nine patients at lymphodepletion. One of these patients showed recovery of gamma globulin levels after HD-CAR-1 treatment (#5). One patient (#2) did not exhibit hypogammaglobulinemia at lymphodepletion or after day 90 post CART infusion. Intravenous immunoglobulins were administered to three patients following HD-CAR-1 treatment (#1, #3 and #5).
The only early infections post-CART infusion occurred in patient #5 who presented herpes simplex virus type 1 infection of the lower lip and respiratory infection without pathogen identification. Late infections (beyond day 30) occurred in four patients (#1-3 and #7; Table 2).
Expansion and persistence of HD-CAR-1
Rapid CART expansion in the peripheral blood (PB) was observed in eight patients (89%) with a median CART peak level of 82,358 CART/µg peripheral blood mononuclear cell (PBMC) DNA (range 37,792 to 369,756; Table 2). In all seven patients evaluated, CARTs were persistent at EOS. Five patients were evaluated beyond 100 days after HD-CAR-1 infusion, with detectable CARTs in the PB of all patients (#3-6 and #8; Fig. 2). Patient #3 still showed 202 CART/µg PBMC DNA even at day 994 after HD-CAR-1 infusion, and in patient #8 a particularly high CART concentration was observed with 18,199 and 13,521 CART/µg PBMC DNA at day 171 and 295, respectively.
Rapid expansion of CARTs was observed in eight of nine patients. CART expansion in PB was measured by single‐copy gene duplex quantitative PCR (SCG‐DP‐PCR) as described [35].
Outcomes
All patients reached EOS on day 90 after CART administration (Table 2). Whereas clinically meaningful responses were not observed at dose levels 1 and 2, CR as best response was achieved with higher CART dose levels in six of seven patients (86%), with uMRD in five of them (72%). In #7, CR could not be confirmed. Although it has to be noted that five patients already had responded to bridging therapies at lymphodepletion, all of these deepened response after HD-CAR-1 treatment, either from CR MRD+ to CR uMRD, or from PR to CR MRD + /uMRD (Table 2). Median duration of response (DOR) was 6.4 months.
PFS was significantly longer in patients who achieved a CR versus those who did not (median PFS, 12.1 months vs. 3.8 months, p 0.024; Fig. 3A). With a median follow-up of 27 months, 2-year PFS and OS for all patients were 30% and 69%, respectively (Fig. 3B). Course of the disease after CART administration of all patients treated at dose level 5 or higher is presented in Fig. 3C. All cases of CLL relapse or progression after HD-CAR-1 treatment were found to be CD19-positive. Three patients died due to PD, four patients are alive after disease progression, and two patients are in ongoing MRD-negative CR (#6 and #8).
A Progression-free survival (PFS) of all patients achieving a complete remission (CR) after CART administration vs. non-CR patients. B Overall survival (OS) and PFS of all patients after CART treatment. C Swimmer plots of all patients after CART administration treated at dose level 5 or higher.  : CART therapy;
: CART therapy;  : progressive disease;
: progressive disease;  : stable disease;
: stable disease;  : partial remission;
: partial remission;  : MRD-positive CR;
: MRD-positive CR;  : MRD-negative CR;
: MRD-negative CR;  : death;
: death;  : 2nd allogeneic hematopoietic cell transplantation;
: 2nd allogeneic hematopoietic cell transplantation;  : radiation therapy; A acalabrutinib, P pirtobrutinib, V venetoclax, Z zanubrutinib.
: radiation therapy; A acalabrutinib, P pirtobrutinib, V venetoclax, Z zanubrutinib.
Cellular composition of CART products of responders vs. non-responders
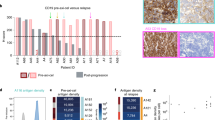
To deeply characterize HD-CAR-1 CART products, we performed 36-plex spectral flow cytometry of CART products from four responders (#3-6) and three non-responders (#1, #2 and #7; Fig. 4A). Notably, all non-responders had previously received alloHCT, unlike the responders. As expected, CART products comprised mostly CD4+ and CD8 + T cells, albeit minor fractions of natural killer cells (NK), natural killer T cells (NKT) and γδ T cells were also found (Fig. 4B). Quantification of cell types revealed a significant enrichment of CD4 + T cells in responders compared to non-responders. Correspondingly, CD8 + T cells were more abundant in non-responding patients (Fig. 4C). Unsupervised clustering and dimensionality reduction of the CD8+ and CD4 + T-cell compartments (Fig. 4D, G) revealed additional differences in the composition of CART products between responders and non-responders as indicated by the shifts in cellular density within the respective T-cell compartment (Fig. 4E, H). Quantification of cell type abundances indicated a strong enrichment of effector memory (EM)-like CD8 + T cells with high expression of CD39 and/or CD197 in non-responders compared to responders (Fig. 4F). Similarly, non-responders displayed a higher fraction of EM-like cells in the CD4 + T-cell compartment with expression of exhaustion markers including CD39 and programmed cell death protein 1 (PD1) (Fig. 4I).
A HD-CAR-1 CART products of seven CLL patients (#1-7) were analyzed with spectral flow cytometry using a 36-marker panel and computational analysis (see methods). B The downsampled subset of cells from all seven CART products is presented with uniform manifold approximation and projection (UMAP) visualization. Clusters are labeled depending on surface marker expression and displayed in different colors. C Frequencies of CD4+ (left) and CD8+ (right) T cells within the CART products of responders (R) vs. non-responders (NR) are displayed as bloxplots. Significance levels were determined by a two-sided Welch’s t-test. CD8+ D and CD4+ G T-cell subsets extracted from all seven CART products are presented with UMAP visualization and clusters are annotated based on surface marker expression and displayed in different colors. Differential compositions between R and NR of CD8+ E and CD4+ H T-cell subsets are displayed as density plots. Differential frequencies between R and NR of specific CD8+ F and CD4+ I T-cell subtypes are presented as boxplots of log2 fold-changes. Higher abundance in R is indicated as positive log2 fold change and negative log2 fold changes visualize higher frequencies in NR. CM central memory, EM effector memory, NK natural killer, NKT natural killer T cells, ns not significant.
Discussion
Over the past two decades, the landscape of CLL therapy has undergone a significant transformation, moving away from chemoimmunotherapy approaches towards the adoption of targeted therapies, notably BTK and B-cell lymphoma 2 inhibitors [10, 40]. However, challenges persist as in particular patients with genetically high-risk CLL tend to develop resistance to these targeted therapies with a consecutive dismal outlook [41,42,43]. It has been advised to consider these patients for alloHCT or exploratory cellular therapies [10, 40, 44].
Although CD19-directed CARTs have been studied in r/r CLL by various groups using various second-generation CART constructs [45,46,47,48,49,50], only few entered advanced phases of clinical development. This was largely due to comparably low rates of complete responses and durable disease control. Of the CD19 CARTs currently labeled for clinical use in other B-malignancies, only brexu-cel and liso-cel have been explored in CLL. Brexu-cel was administered to 15 heavily pretreated patients in the phase 1 ZUMA-8 clinical trial and resulted in an ORR of 47% (CR 13%) [51]. Liso-cel, a CART product that features a consistent 1:1 CD4 + :CD8 + CART ratio, was evaluated in the larger phase 1/2 TRANSCEND CLL 004 trial, which was the basis for the recent U.S. Food and Drug Administration approval of liso-cel for the treatment of adults with r/r CLL or small lymphocytic lymphoma. Similar to brexu-cel, non-T-cell elements are removed from the leukapheresis product prior to liso-cel manufacturing [9]. In TRANSCEND CLL 004, 117 patients, most of them resistant to both BTKi and venetoclax, were treated with liso-cel at two different DL. In the primary efficacy set at the optimum DL 2 (100 × 106 CARTs; n = 49), ORR was 48% with a CR rate of 18%, resulting in a median PFS of 12 months. However, most of the complete responses were durable. In contrast, achieving a status of uMRD, which occurred in 76% of all patients evaluable for MRD, did not translate into superior PFS [52].
The low CR rate observed in these trials prompted us to attempt debulking of leukemia cell load prior to HD-CAR-1 treatment to facilitate CART efficacy and at the same time to reduce toxicity risks. In most heavily pretreated patients this was successfully achieved by using venetoclax-CD20 antibody combinations despite prior failure of fixed-duration venetoclax. Our data suggests that this can indeed result in high rates of CR or CR deepening with MRD clearance in the majority of patients treated at higher HD-CAR-1 dose levels. The CRs obtained or deepened with HD-CAR-1, however, mostly appeared to be less durable than those observed in the TRANSCEND CLL 004 trial. Application of HD-CAR-1 earlier during the treatment course and accompanying CLL-directed treatments, such as antibody or venetoclax maintenance and/or concomitant use of bispecific antibodies may be options for consolidating responses to HD-CAR-1 [53, 54].
Of note, no case of ICANS and only a single case of higher-grade CRS was observed with HD-CAR-1. This is in contrast to other CART trials in r/r CLL, where higher grade CRS and ICANS were observed much more frequently [51, 52, 55]. Although this might be partly related to the comparably low tumor burden of our patients at the time of CART infusion, it is in keeping with the favorable safety profile of HD-CAR-1 in ALL and lymphoma [29, 56] and points to the potential contribution of the third-generation design of HD-CAR-1 to its low toxicity. Despite extensive pretreatment with stem cell-toxic regimens such as fludarabine and cyclophosphamide (FC) and bendamustine and a HCT history in many patients, also ICAHT was extremely modest, again in line with previously published experience with HD-CAR-1 [29, 57].
CART products of HD-CAR-1 responders contained significantly more CD4 + T cells compared to non-responders. This observation is in keeping with Melenhorst et al. [58], who demonstrated that a CD4+ population dominated long-lasting CD19-directed CARTs in two CLL patients who showed a sustained CR for more than ten years. Furthermore, immunophenotyping of HD-CAR-1 CART products disclosed a strong enrichment of EM-like CD8 + T cells with high expression of CD39 and/or CD197 in non-responders compared to responders. This confirms the results of HD-CAR-1 in ALL patients, where a low CD39-expression on effector T cells within the CART product was associated with a higher response rate [29]. This clinical confirmation strengthens the role of CD39 within CART products as a marker for T-cell exhaustion [59, 60] and possible predictor of response in CART patients.
While the small sample size is an obvious limitation of this study, a major strength is its prospective design, demonstrating an extremely favorable safety profile and high response rate of the HD-CAR-1 approach tested here in heavily pretreated/double-refractory patients with CLL and, thus, potentially broadening patient eligibility for CART therapy in CLL.
In conclusion, treatment of heavily pretreated patients with high-risk CLL having failed multiple pathway inhibitors with the third-generation CART HD-CAR-1 is feasible and associated with only very modest CART-specific toxicity. The preliminary efficacy signals obtained suggest that HD-CAR-1 can induce prolonged complete responses in otherwise refractory patients and warrant further exploration of this approach.
Data availability
Original data are available with Patrick.Derigs@med.uni-heidelberg.de upon request.
References
Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–42.
Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398:491–502.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
Neelapu SS, Dickinson M, Munoz J, Ulrickson ML, Thieblemont C, Oluwole OO, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. 2022;28:735–42.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–54.
Fowler NH, Dickinson M, Dreyling M, Martinez-Lopez J, Kolstad A, Butler J, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28:325–32.
Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399:2294–308.
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52.
Vitale C, Griggio V, Perutelli F, Coscia M. CAR-modified cellular therapies in chronic lymphocytic leukemia: is the uphill road getting less steep? Hemasphere. 2023;7:e988.
Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23:91–103.
Neelapu SS, Chavez JC, Sehgal AR, Epperla N, Ulrickson M, Bachy E, et al. Three-year follow-up analysis of axicabtagene ciloleucel in relapsed/refractory indolent non-Hodgkin lymphoma (ZUMA-5). Blood. 2024;143:496–506.
Dreyling M, Fowler NH, Dickinson M, Martinez-Lopez J, Kolstad A, Butler J, et al. Durable response after tisagenlecleucel in adults with relapsed/refractory follicular lymphoma: ELARA trial update. Blood. 2024;143:1713–25.
Riches JC, Davies JK, McClanahan F, Fatah R, Iqbal S, Agrawal S, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–21.
Riches JC, Ramsay AG, Gribben JG. Immune dysfunction in chronic lymphocytic leukemia: the role for immunotherapy. Curr Pharm Des. 2012;18:3389–98.
Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118:2427–37.
Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126:573–81.
Griggio V, Perutelli F, Salvetti C, Boccellato E, Boccadoro M, Vitale C, et al. Immune dysfunctions and immune-based therapeutic interventions in chronic lymphocytic leukemia. Front Immunol. 2020;11:594556.
Hanna BS, Roessner PM, Yazdanparast H, Colomer D, Campo E, Kugler S, et al. Control of chronic lymphocytic leukemia development by clonally-expanded CD8(+) T-cells that undergo functional exhaustion in secondary lymphoid tissues. Leukemia. 2019;33:625–37.
Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24:563–71.
Li W, Guo L, Rathi P, Marinova E, Gao X, Wu MF. Redirecting T cells to glypican-3 with 4–1BB zeta chimeric antigen receptors results in Th1 polarization and potent antitumor activity. Hum Gene Ther. 2017;28:437–48.
Tammana S, Huang X, Wong M, Milone MC, Ma L, Levine BL. 4–1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against B-cell malignancies. Hum Gene Ther. 2010;21:75–86.
Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4–1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18:413–20.
Park S, Shevlin E, Vedvyas Y, Zaman M, Hsu YS. Micromolar affinity CAR T cells to ICAM-1 achieves rapid tumor elimination while avoiding systemic toxicity. Sci Rep. 2017;7:14366.
Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106:3360–5.
Enblad G, Karlsson H, Gammelgård G, Wenthe J, Lövgren T, Amini RM. A phase I/IIa trial using CD19-targeted third-generation CAR T cells for lymphoma and leukemia. Clin Cancer Res. 2018;24:6185–94.
Schubert ML, Schmitt A, Sellner L, Neuber B, Kunz J, Wuchter P, et al. Treatment of patients with relapsed or refractory CD19+ lymphoid disease with T lymphocytes transduced by RV-SFG.CD19.CD28.4-1BBzeta retroviral vector: a unicentre phase I/II clinical trial protocol. BMJ Open. 2019;9:e026644.
Wang L, Gong W, Wang S, Neuber B, Sellner L, Schubert ML, et al. Improvement of in vitro potency assays by a resting step for clinical-grade chimeric antigen receptor engineered T cells. Cytotherapy. 2019;21:566–78.
Schubert ML, Schmitt A, Huckelhoven-Krauss A, Neuber B, Kunz A, Waldhoff P, et al. Treatment of adult ALL patients with third-generation CD19-directed CAR T cells: results of a pivotal trial. J Hematol Oncol. 2023;16:79.
Rejeski K, Subklewe M, Aljurf M, Bachy E, Balduzzi A, Barba P, et al. Immune effector cell-associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood. 2023;142:865–77.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25:625–38.
Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32:34–48.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745–60.
Rawstron AC, Bottcher S, Letestu R, Villamor N, Fazi C, Kartsios H, et al. Improving efficiency and sensitivity: European Research Initiative in CLL (ERIC) update on the international harmonised approach for flow cytometric residual disease monitoring in CLL. Leukemia. 2013;27:142–9.
Kunz A, Gern U, Schmitt A, Neuber B, Wang L, Hückelhoven-Krauss A, et al. Optimized assessment of qPCR-based vector copy numbers as a safety parameter for GMP-grade CAR T cells and monitoring of frequency in patients. Mol Ther Methods Clin Dev. 2020;17:448–54.
McInnes L, Healy J UMAP: Uniform manifold approximation and projection for dimension reduction. ArXiv. 2018;abs/1802.03426.
Venables WN, Ripley BD Modern Applied Statistics with S. 4th ed: Springer New York, NY; 2002. XII, 498 p.
Hao Y, Stuart T, Kowalski MH, Choudhary S, Hoffman P, Hartman A, et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat Biotechnol. 2024;42:293–304.
Kokuina E, Breff-Fonseca MC, Villegas-Valverde CA, Mora-Díaz I. Normal values of T, B and NK lymphocyte subpopulations in peripheral blood of healthy Cuban adults. MEDICC Rev. 2019;21:16–21.
Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96:1679–705.
Thangavadivel S, Woyach JA. Genomics of resistance to targeted therapies. Hematol Oncol Clin North Am. 2021;35:715–24.
Thijssen R, Tian L, Anderson MA, Flensburg C, Jarratt A, Garnham AL, et al. Single-cell multiomics reveal the scale of multilayered adaptations enabling CLL relapse during venetoclax therapy. Blood. 2022;140:2127–41.
Wang E, Mi X, Thompson MC, Montoya S, Notti RQ, Afaghani J, et al. Mechanisms of resistance to noncovalent Bruton’s tyrosine kinase inhibitors. N Engl J Med. 2022;386:735–43.
Dreger P, Ghia P, Schetelig J, van Gelder M, Kimby E, Michallet M, et al. High-risk chronic lymphocytic leukemia in the era of pathway inhibitors: integrating molecular and cellular therapies. Blood. 2018;132:892–902.
Gauthier J, Hirayama AV, Purushe J, Hay KA, Lymp J, Li DH, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135:1650–60.
Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S, Chen X, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified t cells after failure of ibrutinib. J Clin Oncol. 2017;35:3010–20.
Kochenderfer JN, Dudley ME, Feldman SA. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20.
Kochenderfer JN, Dudley ME, Kassim SH. Chemotherapy- refractory diffuse large B-cell lymphoma and indolent B-cell malignan- cies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9.
Porter DL, Hwang WT, Frey NV. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139.
Frey NV, Gill S, Hexner EO. Long-term outcomes from a ran- domized dose optimization study of chimeric antigen receptor mod- ified T cells in relapsed chronic lymphocytic leukemia. J Clin Oncol. 2020;38:2862–71.
Davids MS, Kenderian SS, Flinn IW, Hill BT, Maris M, Ghia P, et al. ZUMA-8: a phase 1 study of KTE-X19, an Anti-CD19 Chimeric Antigen Receptor (CAR) t-cell therapy, in patients with relapsed/refractory chronic lymphocytic leukemia. Blood. 2022;140:7454–6.
Siddiqi T, Maloney DG, Kenderian SS, Brander DM, Dorritie K, Soumerai J, et al. Lisocabtagene maraleucel in chronic lymphocytic leukaemia and small lymphocytic lymphoma (TRANSCEND CLL 004): a multicentre, open-label, single-arm, phase 1-2 study. Lancet. 2023;402:641–54.
Greil R, Obrtlíková P, Smolej L, Kozák T, Steurer M, Andel J, et al. Rituximab maintenance versus observation alone in patients with chronic lymphocytic leukaemia who respond to first-line or second-line rituximab-containing chemoimmunotherapy: final results of the AGMT CLL-8a Mabtenance randomised trial. Lancet Haematol. 2016;3:e317–29.
Mhibik M, Gaglione EM, Eik D, Herrick J, Le J, Ahn IE, et al. Cytotoxicity of the CD3×CD20 bispecific antibody epcoritamab in CLL is increased by concurrent BTK or BCL-2 targeting. Blood Adv. 2023;7:4089–101.
Frey NV, Gill S, Hexner EO, Schuster S, Nasta S, Loren A, et al. Long-term outcomes from a randomized dose optimization study of chimeric antigen receptor modified t cells in relapsed chronic lymphocytic leukemia. J Clin Oncol. 2020;38:2862–71.
Schubert M-L, Schmitt A, Neuber B, Hückelhoven-Krauss A, Kunz A, Wang L, et al. Third-Generation Chimeric Antigen Receptor (CAR) T Cells in Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia (ALL) and Non-Hodgkin Lymphoma (NHL) - Results from the Heidelberg Trial 1 (HD-CAR-1 trial). Blood. 2021;138:1734.
Schubert ML, Dietrich S, Stilgenbauer S, Schmitt A, Pavel P, Kunz A. Feasibility and safety of CD19 chimeric antigen receptor T cell treatment for B cell lymphoma relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2020;26:1575–80.
Melenhorst JJ, Chen GM, Wang M, Porter DL, Chen C, Collins MA, et al. Decade-long leukaemia remissions with persistence of CD4. Nature. 2022;602:503–9.
Roselli E, Boucher JC, Li G, Kotani H, Spitler K, Reid K, et al. 4-1BB and optimized CD28 co-stimulation enhances function of human mono-specific and bi-specific third-generation CAR T cells. J Immunother Cancer. 2021;9:e003354.
Myers RM, Taraseviciute A, Steinberg SM, Lamble AJ, Sheppard J, Yates B, et al. Blinatumomab nonresponse and high-disease burden are associated with inferior outcomes after CD19-CAR for B-ALL. J Clin Oncol. 2022;40:932–44.
Acknowledgements
We thank the patients and their families.
Funding
This work was supported by the National Center for Tumor Diseases (NCT), by the German Cancer Research Center (DKFZ), by the Jochen Siebeneicher Foundation and by a personal donation from Dr. h.c. Karl Schlecht. PDe is supported by the Clinician Scientist Fellowship Program of the DKFZ (supported by the Dieter Morszeck Foundation). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MS is the principle investigator (PI) and PDr the deputy PI of the HD‐CAR‐1 trial. PDr, MS, PDe, MLS, AS, CMT, FK, MB, HB, GK, AL and MR provided patient care. MS, AS, BN, AH‐K and BM manufactured and performed quality control of CAR T-cell products. PDe, PDr, MS and MLS collected and analyzed clinical data. PDe, PDr and MS performed statistical analyses of clinical data. SY, SH and CR designed experiments on spectral flow cytometry, analyzed flow data, performed statistical analyses, generated Fig. 4 and wrote respective parts on flow cytometry of the manuscript. PDe, PDr and MS wrote the manuscript. All authors revised the manuscript and approved the final version of the manuscript. All authors are accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
AS: Travel grants from Hexal and Jazz Pharmaceuticals. Research grant from Therakos/Mallinckrodt. Consultancy BMS, Janssen‐Cilag. Co‐founder and part‐time employee of TolerogenixX LtD. of TolerogenixX Ltd. CMT: research support from Bayer AG. Advisory board member Pfizer, Janssen‐Cilag GmbH. Grants and/or provision of investigational medicinal products from Pfizer, Daiichi Sankyo, BiolineRx. MB: consulting fees from Amgen, research funding from Amgen, honoraria/ travel grants from Jazz, Celgene, Novartis, Pfizer and Amgen, advisory board member for Incyte, Amgen and AstraZeneca. MLS: consultancy for Kite/Gilead, Takeda. MS: research grants from Apogenix, Hexal and Novartis. Travel grants from Hexal and Kite. Financial support for educational activities and conferences from bluebird bio, Kite and Novartis. Advisory board member of MSD. (Co‐)PI of clinical trials of MSD, GSK, Kite and BMS. Co‐Founder and shareholder of TolerogenixX Ltd. PDe: honorarium from MSD. PDr: consultancy AbbVie, AstraZeneca, Gilead, Janssen, Novartis, Riemser, Roche; speakers bureau AbbVie, Gilead, Novartis, Riemser, Roche; research support from Neovii and Riemser. None of the mentioned sources supported the work described within this manuscript. ADH, AHK, AL, BM, BN, CR, FK, GK, HB, MR, SH, SY: none.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. The HD-CAR-1 trial was approved by the institutional review board as well as by the German federal regulatory authority for immunotherapy (Paul-Ehrlich-Institut, Langen, Germany; Eudra CT 2016-004808-60). Written informed consent was obtained from all participants. The trial was conducted in compliance with the principles of the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Derigs, P., Schubert, ML., Dreger, P. et al. Third-generation anti-CD19 CAR T cells for relapsed/refractory chronic lymphocytic leukemia: a phase 1/2 study. Leukemia (2024). https://doi.org/10.1038/s41375-024-02392-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41375-024-02392-7