Abstract
Intensive research in the field of cancer biology has revealed unique methods of communication between cells through extracellular vesicles called exosomes. Exosomes are released from a broad spectrum of cell types and serve as functional mediators under physiological or pathological conditions. Hence, blocking the release of exosome bio carriers may prove useful for slowing the progression of certain types of cancers. Therefore, efforts are being made to develop exosome inhibitors to be used both as research tools and as therapies in clinical trials. Thus, studies on exosomes may lead to a breakthrough in cancer research, for which new clinical targets for different types of cancers are urgently needed. In this review, we briefly outline exosome inhibitors and discuss their modes of action and potential for use as therapeutic tools for cancer.
Similar content being viewed by others
Introduction
Exosomes are small nanosized extracellular vesicles (EVs) generated by the fusion of multivesicular bodies (MVBs) with the plasma membrane in eukaryotic cells and released under physiological and pathological conditions1,2,3,4,5,6. Exosomes secreted from cells were initially thought to be cellular waste resulting from cell damage or byproducts of cell homeostatic processes that exerted no significant impact on cell communication7,8,9,10. However, recent reports have shown that these proteins transport important signaling molecules involved in cell communication, migration, and numerous physiological processes11,12,13.
In cancer progression, tumor-derived exosomes have been closely associated with promoted tumor growth, increased abnormal angiogenesis, attenuated apoptosis, and even immune escape in proximal or distant microenvironments. During cancer metastasis, exosomes have been implicated in increasing metastatic cell migration and vascular invasion from the premetastatic niche14,15. Recently, chemoresistance, a state in which cancer cells resist chemotherapy drugs, has become a major challenge in cancer treatment and is known to be acquired through functional exosomes secreted from neighboring cancer cells16,17,18.
Moreover, a recent study reported that exosomes containing immune checkpoint proteins, such as PD-L1, on their surface are secreted, contributing to immunosuppression19,20. Exosomal PD-L1 binds to PD-1 on T cells, similar to cellular PD-L1, to inhibit the T-cell activity or attenuate the efficacy of PD-1/PD-L1 blockade therapy by binding to anti-PD-L121,22. In addition, exosomes can contribute to immune evasion by transferring PD-L1 to distant cancer cells. PD-L1-enriched exosomes derived from breast cancer cells with high PD-L1 levels have been shown to increase PD-L1 levels by transferring PD-L1 to breast cancer cells with low PD-L1 expression23. Furthermore, the transfer of PD-L1 to macrophages by exosomal PD-L1 induces macrophage-mediated inhibition of CD8+ T-cell activity24. In addition to PD-L1, exosomes have been reported to transfer tumor-derived antigens that contribute to lymph node remodeling and immunosuppression through exosome uptake by lymphatic endothelial cells25. Therefore, the effect of exosomes on antitumor immunity is important, and the regulation of exosomes in immunotherapy is a new therapeutic target.
To date, many studies have been published on bona fide exosomes under diverse physiological conditions, and many exosome inhibitors that disrupt different stages of biogenesis and release from cells have been discovered3,6. However, only a few reviews have been published to date, highlighting the significant roles of exosome inhibitors and their classification. In this review, the characteristics and physical properties of major drug inhibitors targeting specific mechanisms in the biogenesis and release of exosomes are discussed (Fig. 1). Furthermore, the use of exosome inhibitors in the treatment of various types of cancers exhibits great potential for developing effective therapeutic strategies in the future.
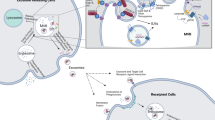
Exosomes are generated from endosomes, which are formed by the inward budding of early multivesicular body (MVB) membranes. These cells undergo a series of changes into late MVBs, which can be directed for lysosomal degradation or fusion with the plasma membrane, which leads to the release of exosomes or microvesicles. This image shows potential inhibitors that attenuate exosome biogenesis or release in the exosome manufacturing process.
Biogenesis of exosomes
Biogenesis of exosomes occurs via the fusion of late endosomes or MVBs with the plasma membrane. Early endosomes mature into late endosomes (MVBs), and in the process, intraluminal vesicles (ILVs) are formed by the invagination of the endosomal membrane. These MVBs may either subsequently fuse with lysosomes, leading to ILV destruction, or fuse with the cell membrane, releasing ILVs into the extracellular space. These secreted ILVs are known as exosomes26. MVB biogenesis is realized by the endosomal sorting complexes required for transport (ESCRT) machinery via two distinct mechanisms, the ESCRT-dependent or ESCRT-independent pathways27,28,29,30,31.
The ESCRT-dependent pathway requires ESCRT protein complexes with associated proteins to facilitate the generation of ILVs32,33,34. The ESCRT protein complex comprises multimolecular machinery consisting of four main complexes, ESCRT-0, -I, -II, and -III, and some additional associated proteins, namely, ALIX, VPS4, and ATPase. ESCRT-0 and -I interact with ubiquitylated transmembrane cargos, form microdomains in the endosomal membrane, and recruit ESCRT-III. ESCRT-III contains either ESCRT-II or ALIX. The polymerization of these components causes membrane neck constriction and cleavage to form ILVs. VPS4 and ATPase disassemble ESCRT, allowing it to be recycled. Thus, the ESCRT pathway can be recruited via other exosome biogenesis mechanisms.
On the other hand, the ESCRT-independent pathway involves lipid rafts inside endosomal membranes35,36,37,38,39. These lipid rafts contain high levels of cholesterol and sphingolipids, primarily sphingomyelin, which are produced by the neutral sphingomyelinase (nSMase) family. The enzyme nSMase converts sphingomyelin to ceramide, a cone-shaped rigid lipid. Ceramide is associated with the formation of microdomains that drive ILV formation from MVBs. The transport of MVBs to the cell membrane is dependent on interactions with actin and the microtubules of the cytoskeleton. Here, we discuss the key drugs that have been evaluated to date for blocking exosome biogenesis (Table 1).
Exosome biogenesis inhibitors
Pantethine
Pantethine is a pantothenic acid (vitamin B5) derivative used as an intermediate in the production of coenzyme A and plays a role in cholesterol metabolism40. Cholesterol is important for maintaining the fluidity of the cell membrane during membrane lipid bilayer reorganization. Therefore, pantethine can be used to disrupt the shedding of microvesicles (MVs). Previously, it had been shown to block the translocation of phosphatidylserine to the outer membrane leaflet, a fundamental process for MV production. A study by Roseblade et al. on breast cancer showed that pantethine reduced overall MV shedding by 24% in MCF-7 breast cancer cells41. Kavian et al. examined the role of MVs in systemic sclerosis (SSc), where endothelial cells (ECs) and fibroblasts are damaged and studied the effect of pantethine in preclinical in vivo SSc mouse models. The number of MVs released from ECs was decreased in a concentration-dependent manner, indicating that the addition of high concentrations of pantethine (50 μM and 100 μM) inhibited MV release. Moreover, pantethine substantially reduced the quantity of circulating EC-released MVs in these mice. In addition, oral administration of 150 mg/kg pantethine attenuated fibrosis and vascular alterations42.
GW4869
GW4869 is a cell-permeable, selective compound that acts as a potent noncompetitive inhibitor of the membrane enzyme nSMase43. nSMase is a ubiquitous enzyme that generates bioactive lipid ceramides through hydrolysis of the membrane lipid sphingomyelin. GW4869 is the most widely used pharmacological agent to block exosome generation and reduce exosome release. The cone-shaped structure of ceramide released via nSMase activity is important for creating the large lipid raft domains involved in exosome shedding during the formation of spontaneous membrane curvature36. As a result, inhibition of nSMase reduces the number of exosomes released. Therefore, nSMase is a potential therapeutic target for the inhibition of exosome release. Many efforts have been made to develop nSMase inhibitors as exosome-inhibiting agents because exosomes have been implicated in tumor progression. Matsumoto et al. reported that GW4869 significantly decreased the growth of B16BL6 melanoma cells compared to untreated cells, indicating that inhibition of exosome secretion by GW4869 suppressed the autocrine-regulated proliferation of murine melanoma cells. The same effect was observed in mice injected subcutaneously with B16BL6 cells. In this study, B16BL6-derived exosomes promoted tumor growth in mice treated with PBS, whereas GW4869 treatment significantly reduced tumor growth and increased murine survival36. Panigrahi et al. reported that exosomes were secreted at higher levels in prostate cancer (PC) cells (LNCaP, 22Rv1, and PC3) under hypoxic conditions than under normoxic conditions, indicating their roles in a survival mechanism to remove metabolic waste, including lactic acid encapsulated in exosomes. Taken together, these studies showed that the inhibition of exosome secretion with GW4869 reduced cell viability, supporting the supposition suggestion of the fundamental role that exosomes play in the survival of these cancer cells44.
Imipramine
Imipramine, a tricyclic antidepressant also known as a cyclic antidepressant, was introduced in the late 1950s. It inhibits the activity of acid sphingomyelinase (aSMase), which catalyzes the hydrolysis of sphingomyelin to ceramide, a process involved in membrane fluidity, exosome release, and MV generation45. Upon uptake into cells (especially inside endosomes and lysosomes), basic imipramine becomes protonated. In this form, it stimulates the proteolytic degradation of aSMase, which is then detached from the membrane owing to the loss of its negative charge. Thus, imipramine prevents the translocation of aSMase, thereby inhibiting MV and exosome secretion46. Kosgodage et al. demonstrated that 25 μM imipramine induced a 77% reduction in total EV release (both exosomes and MVs) from PC3 cells, although the separate effect of imipramine on exosomes and MVs was not tested. Imipramine can also be used to inhibit micropinocytosis47. However, further in-depth studies are required to investigate the role of imipramine at subtoxic concentrations and to characterize these vesicles extensively.
Spiroepoxide
Spiroepoxide, a natural product, is a potent and selective irreversible inhibitor of nSMase48. This compound is known to block exosome release. Similar to GW4869, spiroepoxide at a 5 μM concentration has been shown to inhibit exosome release by 20%; however, the structure of this compound is entirely different from that of GW4869. Spiroepoxide has only one benzene ring and one ternary ring, whereas GW4869 has three benzene rings and two diazole groups.
DPTIP (2,6-dimethoxy-4–(5-phenyl-4-thiophen-2-yl-1H-imidazole-2-yl)-phenol)
DPTIP is the most potent neutral sphingomyelinase 2 (nSMase 2) inhibitor, and the first inhibitor described with nanomolar potency (Rojas et al. 2018). The IC50 of DPTIP has been reported to be 30 nM, which indicates that it is more potent than the prototype inhibitor GW4869 (1 μM). Figuera-Losada et al. utilized DPTIP as a brain penetrant inhibitor to treat brain injury, and its hydroxyl group was shown to inhibit exosome release. Moreover, DPTIP has been shown to inhibit exosome release in a dose-dependent manner, decreasing exosome release by 50% in astrocytes at a high concentration of 30 μM49. Therefore, the inhibitory effect of DPTIP on exosome release strengthens the argument for its use as a potential agent for the synergistic treatment of cancer in the future.
Simvastatin
Simvastatin, an HMG-CoA reductase inhibitor, prevents cholesterol synthesis and is often used to decrease elevated lipid levels. Since cholesterol is an integral constituent of endosomal membranes, simvastatin has been shown to significantly reduce exosome secretion in both epithelial cells and monocytes50. However, the effect of simvastatin in cancer cell lines has not been tested. However, simvastatin has been shown to inhibit endocytosis in a dose-dependent manner and decrease intracellular proteins associated with exosomes, such as ALIX, CD63, and CD81.
Glibenclamide (Glyburide)
Glibenclamide, known as glyburide, belongs to the sulfonylureas family and inhibits MV release through an ATP-binding cassette (ABC) transporter. It has also been used as an antidiabetic drug that inhibits the SUR receptor (a member of the ABC family), which is involved in insulin release via the regulation of potassium channels. Glibenclamide has been shown to interact with other proteins of the ABC family, which has been implicated by the recycling of cholesterol and phospholipids mediated by circulating apolipoprotein A1 (apoA-I) during their maturation. Since cholesterol plays a fundamental role in MV and exosome production, this compound can be used as a potential inhibitor of exosome release51. Henricksson et al. suggested that glibenclamide also has an anticoagulant effect on monocytes in vitro, indicating a reduction in MV release52. In contrast, Kosgodage et al. reported that glibenclamide did not affect MV release from prostate cancer (PC3) and breast cancer (MCF7) cells47.
Indomethacin
Indomethacin, a member of the nonsteroidal anti-inflammatory drug (NSAID) family, is used to decrease prostaglandin production during inflammation. It has been shown to nonselectively inhibit cyclooxygenase I and II, as well as downregulate the transcription of the ABCA3 transporter, which is involved in lipid transport. Moreover, inhibition of ABCA3 has been shown to attenuate exosome release, as lipids are important for exosome biogenesis53. Koch et al. demonstrated that doxorubicin, a cytotoxic drug, was encapsulated in released exosomes. Treatment with 10 μM indomethacin inhibited the release of drug-containing exosomes, and this inhibition likely increased the cytotoxic effect on cancer cells due to their accumulation inside the cells54.
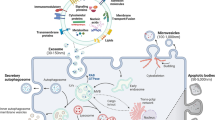
Exosome release
Exosomes are known to be involved in diverse pathophysiological conditions related to disease development and progression3,4,55 and are released via various mechanisms involving protein kinases, calcium channels, Rab signaling, and ESCRT-dependent pathways. These pathways have been identified as targets for exosome release inhibition and may not be directly related to lipid-related pathways or cytoskeletal organization; hence, they are classified separately56,57,58,59. Many pharmacological agents that inhibit exosome release have been investigated both as research tools and as potential therapeutic agents. Here, we discuss the key drugs that have been evaluated to date for blocking exosome release (Table 2)60,61,62,63.
Exosome release inhibitors
Calpeptin
Calpeptin is an inhibitor of a cysteine protease, calpain. Calpain is a heterodimer enzyme consisting of a large catalytic subunit (80 kDa) and a regulatory subunit (28 kDa) harboring a calcium-binding site. Calpeptin is a calcium-dependent neutral, cytosolic cysteine protease that is dysregulated in cancer cells and involved in various processes, including apoptosis, cell proliferation, migration, tumor invasion, and cancer progression. The calpain proenzyme is activated by calcium binding, which induces a conformational change, leading to self-cleavage and activation of the proenzyme. Active calpain has been found to regulate the activity of numerous protein substrates, including G-proteins and cytoskeletal proteins, thereby suggesting its role in promoting MV shedding through the regulation of cytoskeleton remodeling62. However, this regulatory activity of calpain is inhibited by calpeptin, which leads to a reduction in the release of MV from cells.
Calpeptin is a reversible semisynthetic peptidomimetic aldehyde inhibitor developed via the modification of a Streptomyces peptide aldehyde64. It has been extensively used as a microvesiculation inhibitor. Atanassoff et al. investigated the effects of calpeptin on MV release using streptolysin-O (known to damage cell membranes) in HEK293 cells and observed that calpeptin treatment led to streptolysin-O accumulation inside these cells and increased the rate of cell lysis65. Studying PC3 cells, Jorfi et al. reported that the inhibition of MV release by calpeptin resulted in the accumulation of the cytotoxic drugs docetaxel and methotrexate inside cells, which significantly decreased the cell proliferation and increased the cell death rates. Moreover, a subsequent preclinical experiment with an in vivo mouse model showed results similar to those obtained with the in vitro model, indicating that the combined treatment of docetaxel and calpeptin significantly attenuated tumor mass compared to the effect of treatment with docetaxel alone66. According to studies reported herein, the efficacy of exosome release inhibition by calpeptin is clear in experimental in vitro or in vivo mouse models. However, the selective effect of calpeptin on exosome release needs to be further evaluated on the basis of its concentration.
Bisindolylmaleimide I
Bisindolylmaleimide I is a highly selective protein kinase C inhibitor that is known to be a cell-permeable and reversible competitive inhibitor. This inhibitor can attenuate the activity of different isoforms of protein kinase C (PKC), that is, α, βI, βII, γ, δ, and ε, in diverse cells. MV release is dependent on calcium and the externalization of lipids, both of which involve PKC activation by diacylglycerol. Stratton et al. demonstrated that MV release was inhibited by bisindolylmaleimide I, which blocked MV shedding in PC cells63.
Y-27632
Rho-associated protein kinase (ROCK) is a serine-threonine kinase in the PKA/PKG/PKC family and is expressed in two isoforms: ROCK1 and ROCK2. ROCK controls the shape and movement of cells by regulating the cytoskeleton. Reorganization of the cytoskeleton by actin filaments is important for MV shedding. Y27632 is a cell-permeable, highly potent, competitive inhibitor of both ROCK1 and ROCK2, and it competes with ATP by interacting with the catalytic sites of these two kinases67. Li et al. investigated the role of ROCK in MV formation in diverse cancer cell lines, including triple-negative breast cancer (MDA-MB-231) and ovarian cancer (HeLa) cells, which express high levels of these kinases, and a substantial decrease in MV release was observed upon treatment of these cells with RhoA siRNA or the ROCK inhibitor Y-2763258. Sapet et al. also reported that MV release was inhibited by a ROCK inhibitor in a microvascular endothelial cell line (the HMEC-1 cell line) activated by thrombin, which was used to stimulate an increase in MV shedding by these cells67.
U0126
U0126 is a highly selective noncompetitive inhibitor of MEK1 and MEK2, two protein kinases in the mitogen-activated protein kinase kinase (MAPKK) family. U0126 has been found to functionally antagonize AP-1 transcriptional activity via noncompetitive inhibition of the dual specificity kinase MEK, with an IC50 of 72 nM for MEK1 and 58 nM for MEK2. ERK, an upstream kinase of MEK, stimulates MV biogenesis, and its inhibition has been associated with MV shedding reduction. Mingzhen et al. demonstrated that MAPK activation by tobacco smoke extract increased the release of procoagulant MV from THP-1 monocytic cell lines and primary human monocyte-derived macrophages. Subsequently, it was observed that inhibition of MEK by U0126 reduced procoagulant MV release from these stimulated monocyte and macrophage cell lines60.
Manumycin A
Ras is a small GTPase that contributes to the regulation of diverse proteins and many different cellular processes, including cytoskeletal rearrangement, cell adhesion, migration, proliferation, differentiation, apoptosis, and exosome release. In cancer cells, Ras activation is involved in the invasiveness, metastasis, and suppression of apoptosis. Ras consists of two domains: the G domain (binds to GTP) and the C-terminal domain (membrane-targeting domain). The C-terminal domain is important for membrane recruitment because it is modified by a lipid chain that affects membrane trafficking. Lipid modification involves farnesylation mediated by farnesyltransferase (FTase). When this modification is inhibited by a farnesyltransferase inhibitor (FTI), Ras activity is inhibited. Thus, the Ras-induced downstream signaling cascade is blocked in cells.
One of the most commonly used FTIs is manumycin A, a cell-permeable, selective, and potent inhibitor of Ras FTase. Thus, since one of the cellular functions of Ras is exosome release, manumycin A has been investigated to ascertain its inhibitory effect on exosome function, including exosome biogenesis release. Datta et al. investigated the effects of manumycin A on ESCRT-dependent exosome biogenesis in PC cells. Manumycin A administered at a 250 nM concentration, induced a reduction in exosome production by 50–60% in these cancer cells. Moreover, treatment with manumycin A and GW4869 (nSMase inhibitor) in combination further reduced exosome production in these cancer cells compared with either treatment alone, indicating that other proteins may be involved in additional pathways of exosome biogenesis68. Zhou et al. examined the role of exosomes in the wound-healing process of BUMPT cells. Exosome secretion in these cells was induced by scratching a cell monolayer in a wound-healing assay. The wound area increased upon treatment with manumycin A or GW4869, indicating that exosomes facilitate the wound healing process in BUMPT cells69. However, the limited number of studies on manumycin A reported to date is relevant to the conclusion that it inhibits exosome release. Future studies are necessary to determine the noncytotoxic concentration of this drug that is sufficient for inhibiting exosome release.
Dimethyl amiloride
Dimethyl amiloride (DMA) is a derivative of amiloride used to treat high blood pressure. Intracellular calcium levels induce exosome release, and calcium homeostasis is maintained by calcium channels. Therefore, because DMA impairs the function of calcium channels, it has been proposed as a prospective exosome blocker70. Chalmin et al. reported that DMA inhibited the release of exosomes in various cell lines (CT26 colon carcinoma cells, EL4lymphoma cells, and H23lung adenocarcinoma cells) and different mouse models (C57BL/6, BALB/c, and nude mice)71.
Tipifarnib
Tipifarnib is a potent FTI that interferes with cell growth and apoptosis. It was one of four compounds identified from 1280 pharmacologically active compounds by72. It inhibits the biogenesis and secretion of exosomes to slow cancer progression. It was observed that tipifarnib at concentrations ranging from 0.25 to 1 μM significantly decreased the number of released exosomes from aggressive prostate cancer (PC) CD63-GFP-expressing C4-2B cells, substantiating its inhibitory effect on exosome release. Moreover, tipifarnib decreased the levels of Rab27A, Alix, and nSMase2 in cancer cells but not in normal cells. This result is crucial for its clinical use as an exosome inhibitor56,61,73.
Ketoconazole
Sella et al. reported ketoconazole to be an exosome release inhibitor after it was identified from compounds approved by the United States FDA for treating PC patients74. Ketoconazole (5 μM) decreased the levels of exosomes produced by C4-2B and PC3 cells and showed a robust dose-dependent decrease in RAB27A, Alix, and nSMase2 expression both in C4-2B and PC3 cells but not in RWPE-1 cells. The efficacy of ketoconazole has been observed to be lower than that of tipifarnib. Furthermore, both compounds carry the same components, namely diazole and aromatic moieties, suggesting the importance of both of these moieties in the inhibition of exosome release.
Endothelin A receptor antagonist
Recently, endothelin A receptor (ETA) antagonists, such as sulfisoxazole (SFX) and macitentan (MAC), were discovered to be new agents for the inhibition of exosome secretion75,76. SFX is an orally administered FDA-approved drug that is not cytotoxic at effective doses. It was first identified as a competitive inhibitor of dihydropteroate synthetase, which generates dihydropteroic acid by condensing dihydropteroate diphosphate and p-aminobenzoic acid77. Im et al. showed that SFX efficiently reduced the release of small vesicles via an ESCRT-dependent pathway, as shown in in vitro and preclinical in vivo studies. SFX blocked exosome release and cancer cell activity by targeting ETA in breast cancer cells (MCF10A, MCF7, and MDA-MB-231 cells)75. Moreover, it decreased the expression of several Rab and ESCRT-related components, such as VPS4B and Alix, without affecting other vesicle-producing pathways, such as components in ESCRT-dependent pathways, or altering intracellular calcium levels.
Another ETA antagonist, MAC, has been reported to inhibit exosomal PD-L1 secretion76. MAC is an ETA/ETB dual antagonist approved by the FDA for the treatment of pulmonary arterial hypertension. Inhibition of exosomal PD-L1 secretion by MAC has been shown to reduce the number of exosomes that bind to PD-1 on T cells and to enhance antitumor immunity by inducing the reinvigoration of exhausted T cells. These results suggest that an effective drug can be developed by targeting different stages of exosome biogenesis and secretion.
Cannabidiol
Cannabidiol (CBD), a phytocannabinoid derived from Cannabis sativa currently used as an anxiolytic drug with anti-inflammatory, antineoplastic, and analgesic properties, can inhibit exosome and MV release78,79,80,81. Kosgodage et al. reported that 5 μM CBD blocked exosome release by 50% and specifically decreased exosome release in diverse cancer cells (PC3 cells; liver cancer, HEPG2 cells; breast cancer, MDA-MB231 cells). These results indicate that this compound is a potential therapeutic agent for reducing EV82,83. The mode of action of CBD involves its interference with CD63 expression, as a lower CD63 expression level was observed after CBD treatment in three cancer cell lines.
Ketotifen
Ketotifen, an antihistamine, is a store-operated calcium channel blocker that stabilizes mast cells84. Khan et al. reported that ketotifen (10 μM) decreased exosome release from various cancer cells (HeLa, MCF7, and BT549 cells) by 70%85. It has been shown to increase the sensitivity of cancer cells to doxorubicin and attenuate the progression of cancer cell survival. Moreover, it blocks calcium influx into cells, thereby inhibiting exosome release via calcium-dependent mechanisms, as calcium is required for exosome release. The mode of action of ketotifen is the blockade of calcium channels, which might be important for developing new potential strategies to inhibit exosome release86,87.
Other inhibitors
Since the mechanism of exosome release is complex, it is not surprising that the same pathways can be impaired by different drugs targeting diverse proteins involved in the same signaling cascade. Therefore, although the inhibitors mentioned above are commonly used, other inhibitors have also been investigated for their ability to inhibit exosome release. Here, we describe other exosome inhibitors that have the potential to be used in future strategies (Table 3).
Lansoprazole, omeprazole, esomeprazole, and pantoprazole
Exosome inhibition can be realized via multiple strategies that affect different mechanisms, note merely exosome biogenesis and exosome release discussed thus far in this review. Parolini et al. reported that exosome trafficking is regulated by microenvironmental pH in cancer cells88. Although exosome release is increased at a low pH, it is reduced under alkalizing conditions in tumor cells89. In cancer cells, proton transporter V-ATPase plays a key role in maintaining an alkaline intracellular pH and an acidic extracellular pH. Therefore, V-ATPase may be a potential target to block exosome release. A proton pump inhibitor (PPI) of V-ATPase is a therapeutic tool used for improving the anti-acidic properties of cancer cells because it can inhibit exosome release90,91,92,93,94. Federici et al. investigated the reduction in the number of exosomes released by a PPI in tumor cells and showed that the PPI lansoprazole caused a marked reduction in exosome release from human melanoma cells and showed the inhibition of plasmatic exosomes release by treatment with a PPI in human tumor cells in vivo95. Two previous studies also reported that pretreatment with PPIs such as omeprazole, esomeprazole, or pantoprazole induced the sensitivity of human solid tumors to cytotoxic drugs, indicating that these inhibitors (lansoprazole, omeprazole, esomeprazole, or pantoprazole) inhibited the acidification of the extracellular medium of cancer cells (melanoma, adenocarcinoma, and lymphoma cells) and can therefore be used to block exosome release93,96.
Dynasore, and methyl-β-cyclodextrin (MβCD) (an inhibitor of proteins involved in endocytosis)
Exosomes are released via endocytosis, which involves two important proteins, clathrin and caveolin. Although dynasore is a widely used clathrin-dependent endocytosis (CDE) inhibitor, it exerts many nonspecific effects97. Dynasore, a noncompetitive inhibitor, inhibits the GTPase activity of dynamin 1 and dynamin 298. Dynamin is necessary for the CDE process and regulates the assembly of actin filaments into bundles during endocytosis99. Dynasore inhibits the endocytosis of exosomes and exhibits some additional functions in exosome release from cells. However, it has not been extensively tested in cancer cells and neuronal cells since it had been reported that dynasore failed to inhibit the exocytosis of synaptic vesicles100.
Methyl-β-cyclodextrin (MβCD) inhibits caveolin-mediated endocytosis, which involves caveolin, a protein that regulates endocytosis. MβCD depletes cholesterol in the plasma membrane, disturbs lipid rafts, and subsequently diminishes the uptake of exosome-sized vesicles. This compound is not an exosome-specific inhibitor because it decreases micropinocytosis and exosome secretion from the cells. Kosgodage et al. reported that MβCD reduced exosome secretion but severely impaired cell morphology at high concentrations (5 mM), which was beyond the range used to inhibit exocytosis47. MβCD has also been used in combination with lovastatin (an inhibitor of cholesterol synthesis) to prevent cholesterol synthesis, leading to dramatically depleted levels of cholesterol in cells.
Conclusion and further perspective
Although EVs, including exosomes, are constantly released by most mammalian cells, they had largely been considered to be byproducts of membrane biosynthesis and shedding101,102. However, their importance has increased since their critical biological function in cell-to-cell communication was discovered103. Intercellular communication via exosomes is evident throughout cancer progression and pathogenesis104. During tumorigenesis, exosomes can facilitate the programming of a tumoral microenvironment that favors early tumor initiation stages, and subsequently, they can regulate the immune response to enable tumor progression and induce tumor cell survival by promoting angiogenesis, metastasis, and drug resistance, thereby maintaining tumors105,106,107. The biogenesis and release of exosomes are regulated during cancer progression, and the exosomes released from normal cells and cancer cells are quantitatively and qualitatively different108,109,110,111,112,113. In addition to their roles in cancer pathogenesis, exosomes may help in developing two cancer management fields, specifically, the fields associated with noninvasive diagnostic tools for liquid biopsy and with therapeutic tools for immunotherapy, vaccine development, and drug delivery. Although preclinical studies have shown the therapeutic potential of exosomes in cancer treatment, many clinical trials have not led to successful outcomes, which has been attributed to major hurdles in understanding exosome biogenesis/release, protein sorting, isolation of pure exosomes, large-scale exosome production, and exosome transport.
Recently, a significant contribution of exosomal PD-L1 was reported; it lowered the response to immune checkpoint blockade (ICB) therapy, such as PD-1/PD-L1 blockade therapy21,22,23. Several studies demonstrated the potential of exosomal PD-L1 inhibition in enhancing immunotherapy responsiveness. Genetic inhibition of Rab27a, an exosome secretion regulator, or nSMase, which is involved in exosome biogenesis, significantly reduced tumor progression by suppressing exosomal PD-L1 activity22,114. It has also been reported that the genetic inhibition of ETA or LSD1, which are other exosome release-related genes, increased antitumor immunity76,115. A similar phenomenon has been reported after treatment with GW4869, which inhibits exosome secretion by inhibiting the activity of nSMase and showed a synergistic effect in combination with anti-PD-L1 therapy114. The FDA-approved ETA antagonists SFX and MAC have been reported to improve the efficacy of anti-PD-L1 treatment in breast, lung, and colon cancer models76,116. These results show the potential of exosome inhibitors as effective administrated agents for ICB therapy.
Although many in vitro, preclinical in vivo, and clinical studies have indicated that many compounds can inhibit the biogenesis and release of exosomes or EVs, more extensive research with both in vitro and in vivo models is required to investigate the activities of these compounds for the development of single or combination treatment strategies. These compounds target different proteins at different stages of exosome biogenesis and release. Furthermore, the importance of exosome inhibitors being used as synergistic agents for cancer therapy will eventually increase as research on exosome biology is further explored. Therefore, novel strategies targeting exosomes for attenuating pathological communication in cancer will show significant therapeutic potential in cancer patients in the future. Moreover, it is crucial to develop pharmacological compounds that can selectively reduce exosome biogenesis/release without inducing many side effects, and the consolidated overview provided in this review of the current research being carried out in this field may be a reference for future drug development.
References
van der Pol, E., Boing, A. N., Harrison, P., Sturk, A. & Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 64, 676–705 (2012).
Harding, C. V., Heuser, J. E. & Stahl, P. D. Exosomes: looking back three decades and into the future. J. Cell Biol. 200, 367–371 (2013).
Yuana, Y., Sturk, A. & Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 27, 31–39 (2013).
Minciacchi, V. R., Freeman, M. R. & Di Vizio, D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 40, 41–51 (2015).
Schorey, J. S., Cheng, Y., Singh, P. P. & Smith, V. L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 16, 24–43 (2015).
Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Invest. 126, 1208–1215 (2016).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell. Biol. 9, 654–659 (2007).
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell. Biol. 17, 816–826 (2015).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Tkach, M. & Thery, C. Communication by extracellular vesicles: where we are and where we need to go. Cell 164, 1226–1232 (2016).
Gangoda, L., Boukouris, S., Liem, M., Kalra, H. & Mathivanan, S. Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics 15, 260–271 (2015).
Ohno, S., Drummen, G. P. & Kuroda, M. Focus on extracellular vesicles: development of extracellular vesicle-based therapeutic systems. Int. J. Mol. Sci. 17, 172 (2016).
Pitt, J. M., Kroemer, G. & Zitvogel, L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J. Clin. Invest. 126, 1139–1143 (2016).
Milane, L., Singh, A., Mattheolabakis, G., Suresh, M. & Amiji, M. M. Exosome mediated communication within the tumor microenvironment. J. Control. Release 219, 278–294 (2015).
Spugnini, E. P., Logozzi, M., Di Raimo, R., Mizzoni, D. & Fais, S. A Role of Tumor-Released Exosomes in Paracrine Dissemination and Metastasis. Int. J. Mol. Sci. 19, 3968 (2018).
Namee, N. M. & O’Driscoll, L. Extracellular vesicles and anti-cancer drug resistance. Biochim. Biophys. Acta Rev. Cancer 1870, 123–136 (2018).
Santos, J. C. et al. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci. Rep. 8, 829 (2018).
O’Neill, C. P., Gilligan, K. E. & Dwyer, R. M. Role of extracellular vesicles (EVs) in cell stress response and resistance to cancer therapy. Cancers (Basel) 11, 136 (2019).
Xie, F., Xu, M., Lu, J., Mao, L. & Wang, S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol. Cancer 18, 146 (2019).
Tang, Y. et al. The biogenesis, biology, and clinical significance of exosomal PD-L1 in cancer. Front. Immunol. 11, 604 (2020).
Chen, G. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382–386 (2018).
Poggio, M. et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 177, 414–427 e413 (2019).
Ricklefs, F. L. et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 4, eaar2766 (2018).
Chen, J. et al. GOLM1 exacerbates CD8(+) T cell suppression in hepatocellular carcinoma by promoting exosomal PD-L1 transport into tumor-associated macrophages. Signal Transduct. Target. Ther. 6, 397 (2021).
Leary, N. et al. Melanoma-derived extracellular vesicles mediate lymphatic remodelling and impair tumour immunity in draining lymph nodes. J. Extracell. Vesicles 11, e12197 (2022).
Huotari, J. & Helenius, A. Endosome maturation. EMBO J. 30, 3481–3500 (2011).
Henne, W. M., Stenmark, H. & Emr, S. D. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb. Perspect. Biol. 5, a016766 (2013).
Chiaruttini, N. et al. Relaxation of loaded ESCRT-III spiral springs drives membrane deformation. Cell 163, 866–879 (2015).
Lee, I. H., Kai, H., Carlson, L. A., Groves, J. T. & Hurley, J. H. Negative membrane curvature catalyzes nucleation of endosomal sorting complex required for transport (ESCRT)-III assembly. Proc. Natl Acad. Sci. USA 112, 15892–15897 (2015).
McCullough, J. et al. Structure and membrane remodeling activity of ESCRT-III helical polymers. Science 350, 1548–1551 (2015).
Christ, L., Raiborg, C., Wenzel, E. M., Campsteijn, C. & Stenmark, H. Cellular functions and molecular mechanisms of the ESCRT membrane-scission machinery. Trends Biochem. Sci. 42, 42–56 (2017).
Baietti, M. F. et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14, 677–685 (2012).
Friand, V., David, G. & Zimmermann, P. Syntenin and syndecan in the biogenesis of exosomes. Biol. Cell 107, 331–341 (2015).
Roucourt, B., Meeussen, S., Bao, J., Zimmermann, P. & David, G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res 25, 412–428 (2015).
Pike, L. J. Lipid rafts: bringing order to chaos. J. Lipid Res. 44, 655–667 (2003).
Trajkovic, K. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 (2008).
Lingwood, D. & Simons, K. Lipid rafts as a membrane-organizing principle. Science 327, 46–50 (2010).
Record, M., Poirot, M. & Silvente-Poirot, S. Emerging concepts on the role of exosomes in lipid metabolic diseases. Biochimie 96, 67–74 (2014).
Skotland, T., Sandvig, K. & Llorente, A. Lipids in exosomes: current knowledge and the way forward. Prog. Lipid Res. 66, 30–41 (2017).
Ranganathan, S., Jackson, R. L. & Harmony, J. A. Effect of pantethine on the biosynthesis of cholesterol in human skin fibroblasts. Atherosclerosis 44, 261–273 (1982).
Roseblade, A., Luk, F., Ung, A. & Bebawy, M. Targeting microparticle biogenesis: a novel approach to the circumvention of cancer multidrug resistance. Curr. Cancer Drug Targets 15, 205–214 (2015).
Kavian, N. et al. Pantethine prevents murine systemic sclerosis through the inhibition of microparticle shedding. Arthritis Rheumatol. 67, 1881–1890 (2015).
Shamseddine, A. A., Airola, M. V. & Hannun, Y. A. Roles and regulation of neutral sphingomyelinase-2 in cellular and pathological processes. Adv. Biol. Regul. 57, 24–41 (2015).
Panigrahi, G. K. et al. Hypoxia-induced exosome secretion promotes survival of African-American and Caucasian prostate cancer cells. Sci. Rep. 8, 3853 (2018).
Arenz, C. Small molecule inhibitors of acid sphingomyelinase. Cell. Physiol. Biochem. 26, 1–8 (2010).
Bianco, F. et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 28, 1043–1054 (2009).
Kosgodage, U. S., Trindade, R. P., Thompson, P. R., Inal, J. M. & Lange, S. Chloramidine/bisindolylmaleimide-I-mediated inhibition of exosome and microvesicle release and enhanced efficacy of cancer chemotherapy. Int. J. Mol. Sci. 18, 1007 (2017).
Li, J. et al. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat. Immunol. 14, 793–803 (2013).
Figuera-Losada, M. et al. Cambinol, a novel inhibitor of neutral sphingomyelinase 2 shows neuroprotective properties. PLoS ONE 10, e0124481 (2015).
Kulshreshtha, A. et al. Simvastatin mediates inhibition of exosome synthesis, localization and secretion via multicomponent interventions. Sci. Rep. 9, 16373 (2019).
Nieland, T. J. et al. Cross-inhibition of SR-BI- and ABCA1-mediated cholesterol transport by the small molecules BLT-4 and glyburide. J. Lipid Res. 45, 1256–1265 (2004).
Henriksson, C. E. et al. Anticoagulant effects of an antidiabetic drug on monocytes in vitro. Thromb. Res. 128, e100–e106 (2011).
Aung, T. et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc. Natl Acad. Sci. USA 108, 15336–15341 (2011).
Koch, R. et al. Nuclear trapping through inhibition of exosomal export by indomethacin increases cytostatic efficacy of doxorubicin and pixantrone. Clin. Cancer Res. 22, 395–404 (2016).
Lowry, M. C., Gallagher, W. M. & O’Driscoll, L. The role of exosomes in breast cancer. Clin. Chem. 61, 1457–1465 (2015).
Martin, L. A. et al. The farnesyltransferase inhibitor R115777 (tipifarnib) in combination with tamoxifen acts synergistically to inhibit MCF-7 breast cancer cell proliferation and cell cycle progression in vitro and in vivo. Mol. Cancer Ther. 6, 2458–2467 (2007).
McConnell, R. E. et al. The enterocyte microvillus is a vesicle-generating organelle. J. Cell Biol. 185, 1285–1298 (2009).
Li, B., Antonyak, M. A., Zhang, J. & Cerione, R. A. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31, 4740–4749 (2012).
van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018).
Li, M., Yu, D., Williams, K. J. & Liu, M. L. Tobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophages. Arterioscler. Thromb. Vasc. Biol. 30, 1818–1824 (2010).
Colombo, M., Raposo, G. & Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014).
Siklos, M., BenAissa, M. & Thatcher, G. R. Cysteine proteases as therapeutic targets: does selectivity matter? A systematic review of calpain and cathepsin inhibitors. Acta Pharm. Sin. B 5, 506–519 (2015).
Stratton, D., Moore, C., Zheng, L., Lange, S. & Inal, J. Prostate cancer cells stimulated by calcium-mediated activation of protein kinase C undergo a refractory period before re-releasing calcium-bearing microvesicles. Biochem. Biophys. Res. Commun. 460, 511–517 (2015).
Leloup, L. & Wells, A. Calpains as potential anti-cancer targets. Expert Opin. Ther. Targets 15, 309–323 (2011).
Atanassoff, A. P. et al. Microvesicle shedding and lysosomal repair fulfill divergent cellular needs during the repair of streptolysin O-induced plasmalemmal damage. PLoS ONE 9, e89743 (2014).
Jorfi, S. et al. Inhibition of microvesiculation sensitizes prostate cancer cells to chemotherapy and reduces docetaxel dose required to limit tumor growth in vivo. Sci. Rep. 5, 13006 (2015).
Sapet, C. et al. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood 108, 1868–1876 (2006).
Datta, A. et al. Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells. Cancer Lett. 408, 73–81 (2017).
Zhou, X. et al. Exosome production and its regulation of EGFR during wound healing in renal tubular cells. Am. J. Physiol. Ren. Physiol. 312, F963–F970 (2017).
Savina, A., Furlan, M., Vidal, M. & Colombo, M. I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 278, 20083–20090 (2003).
Chalmin, F. et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Invest. 120, 457–471 (2010).
Datta, A. et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: a drug repurposing strategy for advanced cancer. Sci. Rep. 8, 8161 (2018).
Haluska, P., Dy, G. K. & Adjei, A. A. Farnesyl transferase inhibitors as anticancer agents. Eur. J. Cancer 38, 1685–1700 (2002).
Sella, A. et al. Phase II study of ketoconazole combined with weekly doxorubicin in patients with androgen-independent prostate cancer. J. Clin. Oncol. 12, 683–688 (1994).
Im, E. J. et al. Sulfisoxazole inhibits the secretion of small extracellular vesicles by targeting the endothelin receptor A. Nat. Commun. 10, 1387 (2019).
Lee, C. H. et al. Macitentan improves antitumor immune responses by inhibiting the secretion of tumor-derived extracellular vesicle PD-L1. Theranostics 12, 1971–1987 (2022).
Hong, Y. L., Hossler, P. A., Calhoun, D. H. & Meshnick, S. R. Inhibition of recombinant Pneumocystis carinii dihydropteroate synthetase by sulfa drugs. Antimicrob. Agents Chemother. 39, 1756–1763 (1995).
Mechoulam, R., Parker, L. A. & Gallily, R. Cannabidiol: an overview of some pharmacological aspects. J. Clin. Pharm. 42, 11S–19S (2002).
Martin-Moreno, A. M. et al. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer’s disease. Mol. Pharmacol. 79, 964–973 (2011).
Blessing, E. M., Steenkamp, M. M., Manzanares, J. & Marmar, C. R. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics 12, 825–836 (2015).
Pisanti, S. et al. Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol. Ther. 175, 133–150 (2017).
Kosgodage, U. S. et al. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front. Pharmacol. 9, 889 (2018).
Kosgodage, U. S. et al. Cannabidiol affects extracellular vesicle release, miR21 and miR126, and reduces prohibitin protein in glioblastoma multiforme cells. Transl. Oncol. 12, 513–522 (2019).
Franzius, D., Hoth, M. & Penner, R. Non-specific effects of calcium entry antagonists in mast cells. Pflug. Arch. 428, 433–438 (1994).
Khan, F. M. et al. Inhibition of exosome release by ketotifen enhances sensitivity of cancer cells to doxorubicin. Cancer Biol. Ther. 19, 25–33 (2018).
Soboloff, J., Zhang, Y., Minden, M. & Berger, S. A. Sensitivity of myeloid leukemia cells to calcium influx blockade: application to bone marrow purging. Exp. Hematol. 30, 1219–1226 (2002).
Kim, H. J., Park, M. K., Kim, S. Y. & Lee, C. H. Novel suppressive effects of ketotifen on migration and invasion of MDA-MB-231 and HT-1080 cancer cells. Biomol. Ther. 22, 540–546 (2014).
Parolini, I. et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 284, 34211–34222 (2009).
Logozzi, M. et al. Microenvironmental pH and exosome levels interplay in human cancer cell lines of different histotypes. Cancers 10, 370 (2018).
De Milito, A. & Fais, S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 1, 779–786 (2005).
Fais, S. Evidence-based support for the use of proton pump inhibitors in cancer therapy. J. Transl. Med. 13, 368 (2015).
Fais, S., De Milito, A., You, H. & Qin, W. Targeting vacuolar H+-ATPases as a new strategy against cancer. Cancer Res. 67, 10627–10630 (2007).
Taylor, S. et al. Microenvironment acidity as a major determinant of tumor chemoresistance: Proton pump inhibitors (PPIs) as a novel therapeutic approach. Drug Resist. Updat. 23, 69–78 (2015).
Canitano, A., Iessi, E., Spugnini, E. P., Federici, C. & Fais, S. Proton pump inhibitors induce a caspase-independent antitumor effect against human multiple myeloma. Cancer Lett. 376, 278–283 (2016).
Federici, C. et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS ONE 9, e88193 (2014).
Luciani, F. et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J. Natl Cancer Inst. 96, 1702–1713 (2004).
Dutta, D. & Donaldson, J. G. Search for inhibitors of endocytosis: intended specificity and unintended consequences. Cell. Logist. 2, 203–208 (2012).
Kirchhausen, T., Macia, E. & Pelish, H. E. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol. 438, 77–93 (2008).
Gu, C. et al. Direct dynamin-actin interactions regulate the actin cytoskeleton. EMBO J. 29, 3593–3606 (2010).
Newton, A. J., Kirchhausen, T. & Murthy, V. N. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc. Natl Acad. Sci. USA 103, 17955–17960 (2006).
Delmas, C. et al. The farnesyltransferase inhibitor R115777 reduces hypoxia and matrix metalloproteinase 2 expression in human glioma xenograft. Clin. Cancer Res. 9, 6062–6068 (2003).
Iraci, N., Leonardi, T., Gessler, F., Vega, B. & Pluchino, S. Focus on extracellular vesicles: physiological role and signalling properties of extracellular membrane vesicles. Int. J. Mol. Sci. 17, 171 (2016).
Zhang, H. G. & Grizzle, W. E. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am. J. Pathol. 184, 28–41 (2014).
Wang, M. et al. High throughput cell-based assay for identification of glycolate oxidase inhibitors as a potential treatment for primary hyperoxaluria type 1. Sci. Rep. 6, 34060 (2016).
De Toro, J., Herschlik, L., Waldner, C. & Mongini, C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front. Immunol. 6, 203 (2015).
Salem, K. Z. et al. Exosomes in tumor angiogenesis. Methods Mol. Biol. 1464, 25–34 (2016).
Lobb, R. J., Lima, L. G. & Moller, A. Exosomes: key mediators of metastasis and pre-metastatic niche formation. Semin. Cell Dev. Biol. 67, 3–10 (2017).
Yu, J. L. et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood 105, 1734–1741 (2005).
Di Vizio, D. et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 181, 1573–1584 (2012).
Chin, A. R. & Wang, S. E. Cancer-derived extracellular vesicles: the ‘soil conditioner’ in breast cancer metastasis? Cancer Metastasis Rev. 35, 669–676 (2016).
Isola, A. L., Eddy, K. & Chen, S. Biology, therapy and implications of tumor exosomes in the progression of melanoma. Cancers 8, 110 (2016).
Allenson, K. et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. 28, 741–747 (2017).
Nuzhat, Z. et al. Tumour-derived exosomes as a signature of pancreatic cancer—liquid biopsies as indicators of tumour progression. Oncotarget 8, 17279–17291 (2017).
Yang, Y. et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 28, 862–864 (2018).
Shen, D. D. et al. LSD1 deletion decreases exosomal PD-L1 and restores T-cell response in gastric cancer. Mol. Cancer 21, 75 (2022).
Shin, J. M. et al. Sulfisoxazole elicits robust antitumour immune response along with immune checkpoint therapy by inhibiting exosomal PD-L1. Adv. Sci. 9, e2103245 (2021).
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant (NRF-2021R1A2C2004705, NRF-2021R1A5A2021614) funded by the Korean government, Ministry of Science and ICT (MSIT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, J.H., Lee, CH. & Baek, MC. Dissecting exosome inhibitors: therapeutic insights into small-molecule chemicals against cancer. Exp Mol Med 54, 1833–1843 (2022). https://doi.org/10.1038/s12276-022-00898-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-022-00898-7
This article is cited by
-
Tumorigenic and tumoricidal properties of exosomes in cancers; a forward look
Cell Communication and Signaling (2024)
-
Impairing Gasdermin D-mediated pyroptosis is protective against retinal degeneration
Journal of Neuroinflammation (2023)
-
The effects of intracellular and exosomal ncRNAs on cancer progression
Cancer Gene Therapy (2023)