Abstract
Syndromic monogenic obesity is a rare and severe early-onset form of obesity. It is characterized by intellectual disability, congenital malformations, and/or dysmorphic facies. The diagnosis of patients is challenging due to the genetic heterogenicity of this condition. However, the use of microarray technology in combination with public databases has been successful on genotype–phenotype correlations, especially for body mass index (BMI) alteration. In this study, the relationship between copy number variations (CNVs) detected by microarray mapping on 16p region and BMI alterations in syndromic patients were assessed. In order to achieve this goal, 680 unrelated Spanish children with intellectual disability were included. 16p region was characterized by using microarray platforms. All detected variants were classified as: (I) one previously non-described 10-Mb duplication in 16p13.2p12.3 region considered causal of intellectual disability and severe overweight, and (II) eleven 16p11.2 CNVs of low prevalence but with recurrence in syndromic patients with severe BMI alteration (nine proximal and two distal). Proximal 16p11.2 CNVs have a dose-dependent effect: underweight in carriers of duplication and obesity in carriers of deletion. KCTD13 was identified as a possible candidate gene for BMI alteration on proximal syndromes, whereas SH2B1 gene was identified as candidate for distal syndromes. The results shown in this paper suggest that syndromic patients could constitute a reliable model to evaluate hypothalamic satiety and obesity disorders as well as generate a wide expectation for primary prevention of comorbidities. Furthermore, 16p13.2p12.3 showed to be an important region on the regulation of body fatness.
Similar content being viewed by others
Introduction
Monogenetic forms of human obesity are described as rare and severe early-onset obesity forms associated with several endocrine disorders [1]. These forms of obesity are mainly due to single mutations located in genes linked to the leptin–melanocortin pathway, resulting in an imbalance between food intake and energy expenditure [2]. Monogenic obesity syndromes correspond to severe overweight as a predominant sign associated with additional phenotypes, including intellectual disability, congenital malformations, and/or dysmorphic facies [3]. Around 30 characterized obesity syndromes are described in the literature so far [4]. Moreover, several cases of syndromic patients with a very high BMI, mainly occurred due to chromosomal abnormalities and/or highly penetrant genetic variants in genes related to the leptin–melanocortin pathway [5]. Patients affected by syndromic obesity suffer, on the one hand, an evident intellectual disability associated with severe overweight and, on the other hand, they frequently manifest a mild neurodevelopment retardation characterized by a decrease of their language skills, and other cognitive, motor, and behavioral alterations [6].
Several authors proposed that syndromic individuals with early-onset obesity, who maintain overweight throughout their life, could be a good model to study hypothalamic control of satiety regulated by the leptin–melanocontin pathway [7, 8]. This hypothesis outcome from the fact that the non-controlled food intake presented by these patients is due to the abnormalities in brain structure and functionality [9]. Therefore, syndromic patients could be the key to discover new candidate genes involved in the uncontrolled food intake [9, 10]. The physiological alteration of this pathway triggers an increase in body weight, mainly by an excessive deposition of adipose tissue [11]. The dysfunction of the leptin–melanocortin pathway is partially measurable and comparable, allowing the demonstration of specific genotype–phenotype correlations in groups of syndromic obese patients [12, 13].
Furthermore, in the last years, the consideration of underweight in syndromic patients has been added to the evaluation of overweight and obesity, which could be crucial to the development of prevention programs as well as improving the design of research projects with more efficacy and efficiency [14]. Therefore, the description of the genetic causes and the risk prediction for anthropometric parameters is much more complex and has to consider the molecular basis of common obesity [7].
Copy number variations (CNVs) have been proposed as modifier variants of body mass index (BMI), depending on underlying genetic mechanisms [15]. For this reason, the identification of CNVs could explain part of the susceptibility risk to develop an obese phenotype [10, 16,17,18,19,20,21]. This approach has been addressed in several case–control studies using single nucleotide polymorphisms (SNPs) variations [22,23,24]. Several genes and SNPs have been identified on chromosome 16 associated with an obese phenotype [22,23,24]. Thus, chromosome 16 could be considered as a genomic hotspot for syndromic [13, 25, 26], monogenic [27], and polygenic [22,23,24] forms of obesity.
In the present study, the characterization and analysis of CNVs located in the chromosome 16p region was targeted in patients with intellectual disability. Hence, the aim was to identify and validate new genes which are possibly associated with increasing BMI in these patients.
Materials and methods
Patients
This study has been performed using a cohort of 680 unrelated children patients (37.5% were girls). The inclusion criteria used for the participant’s study were: age under 18-years-old, presence of abnormal facies/congenital malformations and intellectual disability or autism spectrum disorders. The presence of X-Fragile or Prader–Willi/Angelman Syndromes was an exclusion criteria. All included children were none carriers of any of these two syndromes after following the Spanish national algorithm for non-affiliated phenotypes proposed by Cigudosa and colleagues [28].
The cohort has a Spanish descent, located in different geographical regions of Spain: 160 from Valencia (East coast Spain), 30 from Extremadura (Western Spain), and 490 from Galicia (Northwestern Spain). All these patients were phenotypically characterized by the homogeneous criteria established in the Human Phenotype Ontology (HPO) [29]. Besides, some anthropometric parameters were estimated in all patients such as weight (kg), height (cm), and standard deviation (SD) BMI, classifying each child as: underweight for SD < −1, normal weight for SD ≥ −1 and SD < 1, overweight for SD ≥ 1 and SD < 2, and obese for SD ≥ 2, following the guidelines for the Spanish population proposed by the Fundación Faustino Orbezogo [30]. In the present sample, there were patients with all body weight phenotypes. This homogeneous unbiased weight or intellectual disability cohort contains all BMI phenotypes, allowing to perform a genotype–phenotype correlation considering weight and BMI as continuous variables. Normal weight patients in the present cohort allow to discard putative regions involved in BMI modulation.
The study protocol was approved by the ethical Committee of Ethics and Clinical Research from the Consorcio Hospital General Universitario de Valencia and was conducted in accordance with the Declaration of Helsinki and its subsequent revisions. Prior to study inclusion, all children’s parents or legal guardian gave consent for the molecular cytogenetic investigation and signed written informed consent forms saved in medical records.
Chromosomal microarray (CMA) and CNV analysis
A peripheral blood sample was collected from each child. Genomic DNA was isolated using Chemagic DNA Blood100 kit (PerkinElmer Chemagen Technologies GmbH, Germany) and QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany), following the manufacturer’s recommendations.
The Genome-Wide Human CytoScan HD Array (Thermo Fisher Scientific, CA, USA) was used in patients referred to the General University Hospital Consortium of Valencia and Maternal Infant Hospital of Badajoz, to analyze genomic alterations following the manufacturer’s recommendations. The arrays were scanned using an Affymetrix GeneChip® Scanner 3000 7G. Scanned files were generated using Affymetrix GeneChip® Command Console Software, version 4.3.2. To analyze CNVs.cel files were imported into Thermo Fischer® Chromosome Analysis Suite v3.3 (ChAS) (Thermo Fisher Scientific, CA, USA) to generate copy number variants from raw intensity. Chromosome 16 was filtered by the query segments tool and the selected CNVs in the 16p region had at least 25 probes and a size ≥ 100 kpb.
Comparative Genomic Hybridization array (aCGH) for patients referred to the Hospital Álvaro Cunqueiro located in Vigo was performed using the Agilent SurePrint G3 ISCA V2 CGH 8 × 60 K microarray. Bioinformatic interpretation was done by using Agilent Cytogenomics 4 and Cartagenia Bench Lab CNV software (Agilent Technologies, CA, USA). CNVs in the 16p region had at least 5 probes and a mean size ≥ 32 kpb.
All observed CNVs were compared with those cataloged in two public databases; Database Genomic Variants (DGV; http://dgv.tcag.ca/), and International Standards for Cytogenomic Arrays (ISCA; http://dbsearch.clinicalgenome.org/search/). Each CNVs was classified into five categories: (1) benign variations; (2) likely benign; (3) uncertain clinical significance; (4) likely pathogenic, and (5) pathogenic variations, according to ACMG guidelines. Common polymorphic/benign CNVs were defined as those overlapped completely with CNVs reported in at least one study cataloged in the DGV with more than 100 cases studied and a frequency higher than 1%, or at least two studies cataloged in the DGV. Unique pathogenic CNVs were defined as those that showed no or incomplete overlap with CNVs in the DGV database and/or overlap with pathogenic CNVs reported in ISCA database. Gene annotation was based on the ISCN 2016 human reference genome sequence GRCh37 (hg19). Unique CNVs were assessed by searching in DECIPHER (https://decipher.sanger.ac.uk/) and PubMed (https://www.ncbi.nlm.nih.gov/pubmed) databases. Unique CNVs were also classified as causal of pathology or syndrome when this variant was previously non-reported, and pathogenic recurrent CNVs when this variant has been previously reported associated with a syndromic-specific phenotype. Familiar segregation of CNVs was performed using microarrays technology when DNA was available.
To identify and postulate genes involved in BMI modulation encompassed in CNVs, gene information was obtained from OMIM (https://www.omim.org/), Ensembl (http://www.ensembl.org), and NCBI (https://www.ncbi.nlm.nih.gov/gene/) databases. The affected gene was also studied in case it could have a described animal model to determine a possible related phenotype with patients carrying the CNVs.
Representation of the CNVs overlapping
All CNVs detected in this study were visually represented using PhenoGram Plot tool (freely available on http://visualization.ritchielab.org/phenograms/plot). In all identified CNVs, a breakpoint was defined to represent them in scale. The same approach was applied for the included genes (using the first and last nucleotide).
Results
As a first step in this study, data from the microarray had to meet the Quality Control (QC) metrics for Thermo Fisher array (including SNP-QC ≥ 15, and Median of the Absolute values of all Pairwise Differences (MAPD) ≤ 0.25 to be included in the cohort) and Agilent array (including DSLR < 0.20). All of the 680 children passed the quality control and were analyzed by using the following strategy: (I) phenotype classification of patients into normal weight, underweight, overweight or obesity categories, apart from the intellectual disability; (II) detection and classification of all CNVs located in chromosome 16; (III) after variants filtration we investigate possible candidate genes associated with body weigh modulation and the development of intellectual disability.
Phenotype classification of the cohort
The 680 children included in the cohort were from different Spanish locations (Valencia, Badajoz and Vigo) and all of them presented an age < 18 years, being 10.9 (±4.78) years the average age of the sample. The percentage of girls in the cohort was 37.5% (n = 255), being both sexes represented without any bias. All of the patients manifest intellectual disability and, at least, one of the next features: abnormal facies, congenital malformations and/or BMI alteration, highlighting the severe obesity or underweight phenotype. Following the SD parameters for BMI classification, we observed that 16.8% of the study sample presented an obese phenotype, 16.1% showed overweight, 8.4% manifested underweight, and 58.7% presented a normal weight. All phenotypical information and features of each patient was referred using the HPO criteria for the exhaustive phenotyping. All the information was exposed in Table 1.
Detected 16p CNVs involved in BMI alteration
Causal CNV of syndromic obesity: 16p13.2p12.3 region
A 10-Mb duplication localized in the 16p13.2p12.3 region (arr[GRCh37] 16p13.2p12.3(8815381_18893088)×3 dn) was identified in a 5-year-old girl from Valencia region. This unique and causal duplication included 69 genes, whereas 46 with an OMIM entry (Supplementary information, Table S1).
This patient was born from an ovule donation process, confirming a de novo chromosomal alteration due to the normal karyotype observed in her father. She was remitted to Neuropediatrics consultation from primary attention because of the manifestation of a mature retardation and episodes of absences. The Neuropediatrics evaluation, following the HPO catalog, described dysmorphic facies including wide forehead (HP:0000337), abnormality of the posterior hairline (HP:0030141), anteverted ears (HP:0040080), poor fine motor coordination (HP:0007010) with poor digital opposition, dysdiadochokinesia (HP:0002075), moderate expressive language delay (HP:0011345), mild intellectual disability (HP:0001256) and childhood-onset truncal obesity (HP:0008915).
Therefore, due to the size and the number of duplicated genes (Supplementary information, Table S1) in the 16p13.2p12.3 region, the alteration was considered causal and responsible of the developed phenotype of this patient, highlighting the manifestation of early-onset obesity, suggesting that one or several genes located on this region could explain this feature.
Recurrence of CNVs with an early and maintained extreme BMI: 16p11.2 region
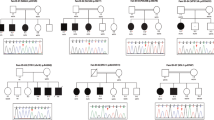
Thereafter the analysis of the observed variants in this cohort and their comparison with the previously reported CNVs described in the general population as well as in patients with similar phenotypes, we detected 11 CNVs in this total cohort with a recurrence on the 16p11.2 region, representing 1.6% of the cohort. Distal and proximal CNVs were reported (Table 2). To correlate CNVs with their potential role in weight modulation, we identified the phenotype presented by carriers with the SD BMI (Table 2). For the 9 proximal variants (4 duplications and 5 deletions), we observed a dose-dependent association between the type of the variant (deletion or duplication) and BMI (obesity and underweight, respectively). Only 2 deletions were found with distal localization and the carriers presented an obese phenotype. These evidences suggest that one or several genes located in the 16p11.2 region (Tables 3 and 4) could be responsible for the modulation of BMI in patients with intellectual disability (Fig. 1).
Overlapping of proximal and distal 16p11.2 CNVs detected in the cohort of Spanish children using PhenoGram Plot tool. a Two distal deletions (in red) in the 16p11.2 region associated with obesity were detected in the cohort (patients #10 and #11; see Table 2 for the breakpoints). They were represented in scale with the proximal CNVs. Nine deleted OMIM genes (represented in black) were encompassed. The SH2B1 gene, the responsible of syndromic obesity in these patients, was represented with a red box. Further information about the deleted genes is available in Table 4. b Nine CNVs in the 16p11.2 proximal region associated with a modulation of BMI were detected in the cohort. Duplications (in blue) and deletions (in red) detected in the 16p11.2 region in patients #1 to #9 were represented in scale with distal deletions (see Table 2 for the breakpoints). Complete overlapping is observed. 21 encompassed OMIM genes (represented in black) in proximal CNVs were shown in scale as well. The altered gene-dose in these patients was manifested in the development of opposite phenotypes: obesity for deletions and underweight for duplications. The KCTD13 gene, postulated as responsible of the BMI alteration in these patients, was represented with a red box. Further information about the encompassed genes is available in Table 3
In order to identify new candidate genes involved in body weight modulation in syndromic patients, only genes with an OMIM entry and common in all proximal CNVs were investigated for this region [SPN (#182160), QPRT (#606248), KIF22 (#603213), MAZ (#600999), PRRT2 (#614386), PAGR1 (#612033), MVP (#605088), CDIPT (#605893), KCTD13 (#608947), TAOK2 (#613199), HIRIP3 (#603365), DOC2A (#604567), FAM57B (#615175), ALDOA (#103850), PPP4C (#602035), TBX6 (#602427), YPEL3 (#609724), MAPK3 (#601795)] (Table 3). The same approach was performed for distal CNVs, considering common OMIM genes for this region [ATXN2L (#607931), TUFM (#602389), SH2B1 (#608937), ATP2A1 (#108730), RABEP2 (#611869), CD19 (#107265), NFATC2IP (#614525), SPNS1 (#612583), LAT (#602354)] (Table 4).
Evaluation of candidate genes located in 16p11.2, with respect to body weight modulation in syndromic patients
Correlation of affected genes and phenotypic characterization was investigated considering the encompassed genes in CNVs located in the 16p11.2 region, their function and their expression pattern. From all detected genes with a different copy number state detected by the whole-genome array, only genes with an OMIM entry were analyzed.
All common OMIM genes of the proximal CNVs (duplications and deletions) were studied. After analyzing gene function, expression patterns and reported animal models, we highlighted that KCTD13 gene (OMIM #608947) could be responsible of BMI alteration phenotype. Following the same strategy for distal deletions, it highlighted the SH2B1 gene (OMIM #608937), which takes part of the hypothalamic satiety signaling pathway.
Discussion
Pathologic alteration of BMI, both severe underweight and overweight, is a common and a remarkable feature in patients that suffer intellectual disability [31]. This alteration was a clearly observed trait in the present cohort, being BMI alteration presented in 41.3% of the studied patients (Table 1). The appearance and existence throughout the life of these patients affects and conditions their prognostics and life quality, being the body weight alteration treatment costly and ineffective for health care units [32, 33]. The etiology of intellectual disability associated and non-associated with BMI alteration is widely heterogeneous, from punctual mutations in specific genes to high chromosomal abnormalities [7]. The same occurs for their clinical manifestation, showing a reliable variability between patients with the same pathology, resulting in a complex diagnostic.
One of these etiologies is the large-scale of chromosomal alterations or rearrangements, such as deletions or duplications, which used to be unique genetic causes of specific signs and symptoms of each patients, highlighting intellectual disability and BMI alteration [16,17,18, 20, 21, 26]. This is the case of the patient that was born from an ovule donation process, who carried a 10-Mb duplication in the 16p13.2p12.3 region. Reported duplication was responsible of the developed phenotype characterized by intellectual disability and obesity. This type of etiology makes to study the patients as unique cases of intellectual disability, with or without excess of body fat. In this reported patient and according to the literature [34], the development of early-onset obesity makes patent to consider her syndrome as a case of a non-canonical unique obesity syndrome. However, the duplication of the patient had a partial overlapping with the previously reported [34]. In the studied cohort several variants overlapping the 10-Mb duplication of the patient were detected with a high recurrence. However, these variants were considered polymorphic. This observation suggested that some of the involved genes could be responsible of BMI variation in general population but not causing other phenotypic traits. More genetic, molecular and functional studies are required to evaluate the gene or set of genes that could be responsible of the development of obesity.
CNVs could be benign and common in the general population as well as CNVs of low prevalence associated with a syndrome or pathology with a clear phenotypic trait. This is the case showed with the 11 variants localized in the 16p11.2 region (Table 2), representing the 1.6% of the cohort. They corroborate the recurrence of deletions and duplications in proximal (9 CNVs) and distal (2 CNVs) location of this chromosomal region [25, 26, 35, 36]. The study of common genes in proximal CNVs allowed us to propose KCTD13 gene as a candidate gene involved in the development of an altered BMI associated with intellectual disability. This was verified through previous associations of this gene with an obese phenotype when it is deleted [37]. Although it has been reported that this gene presents a ubiquitous expression in all tissues, being predominant in brain and testis, KCTD13gene has been postulated as a major driver of mirrored neuroanatomical phenotypes of 16p11.2 CNVs, highlighting autism [37]. In zebrafish and mouse models, this gene is underlying the same neurologic effects reported in patients with these cytogenetic alterations, deletions and duplications [37]. Moreover, KCTD13 gene has a dose-dependent effect for specific phenotypic features, such as the cephalic perimeter: microcephaly for duplications and macrocephalia for deletions [37]. Although, underweight has been reported in several studies [38,39,40], here, the same dose-dependent effect caused by KCTD13 gene regarding weight is assumed: underweight for duplications and overweight for deletions, possibly due to its participation in the hypothalamic satiety control. Any information was previously reported about which gene is the responsible of this dose-dependent effect for weight phenotype in patients [38,39,40] and animal models [37]. However, further functional studies were needed to corroborate this hypothesis involving KCTD13 gene.
Recently, variants in KCTD15 gene, a component of the same family of KCTD13 gene [41], have been reported in the literature associated to the development of obesity [42]. The analysis of KCTD15 gene ruled out a difference in the distribution of its genetic variants, between obese and normal weight individuals; this was ruled out in both univariate and multivariate analyzes [24]. In spite of this, other research regarding KCTD15 gene variants concluded that the presence of specific variants in this gene supposed an increased risk to the development of bulimia nervosa [43]. That was analyzed in different series belonging to the same descent than case–control research for obesity [24]. So, the association to binge eating disorders and/or distinct anthropometric and psychological parameters for KCTD15 gene variants was evidenced [43]. Besides, a recent study pointed KCTD15 gene as a key regulator gene involved in early neural crest development [44]. Nevertheless, further studies are needed to address a putative relationship of both KCTD13 and KCTD15 genes of this family in the development of BMI alterations and in the neural crest formation. Moreover, a clear relationship between KCTD15 and overweight is needed to establish it as an obesity-related gene.
The same approximation for the KCTD13 gene was performed in the distal deletions, pointing the previously postulated SH2B1 gene as responsible of the phenotype, which participates in the leptin–melanocortin pathway [45]. Therefore, the 2 reported deletions in the cohort corroborate previous associations that correlated the genetic alteration of the SH2B1 gene with the developed obese phenotype manifested with intellectual disability [27]. Therefore, the patients who carried these CNVs would be cases of monogenic syndromic obesity caused by the haploinsufficiency of this gene and detected by whole-genome array [27, 46]. For that reason, an evaluation of the family and a variant segregation would be needed. A knock-out mouse model for this gene developed leptin resistance, hyperlipidemia, hyperphagia, hyperglycemia, insulin resistance, glucose intolerance and obesity [45]. Consequently, SH2B1 gene is postulated as principal responsible of the obesity and intellectual disability development in these patients [27, 45, 46].
On the basis of the foregoing, Doche et al. [27] expose that deletions in the 16p11.2 region could constitute a new specific obesity syndrome to diagnose patients that carry genetic alteration in this location due to the high correlation between genetic and phenotypic data. Some posterior studies confirmed this point of view, considering it as one of the principal regions of syndromic obesity [47,48,49,50], recognized in the recent reviews as 16p11.2 microdeletion syndrome of obesity [4].
Moreover, other loci with a recurrence of variants associated to general population is the centromeric region of the 16p11.2 variants exposed in the Table 2, suggesting a putative role in common obesity. Case–control studies were needed to evaluate the effect of these regions in the development of overweight to establish if they are new regions for the susceptibility to obesity.
Following the strategy proposed in this article, other chromosomal regions could be evaluated to establish recurrent CNVs in patients with intellectual disability and severe BMI alteration, postulating new candidate genes and loci to participate in the development of syndromic obesity or severe modulation of BMI (obesity and underweight) in syndromic patients as occurs in the 16p11.2 proximal region [35, 36].
Conclusions
Applying whole-genome microarray technology in a high Iberian cohort of syndromic patients has allowed reporting and characterizing CNVs associated with BMI alteration, especially related with obesity. One patient has been diagnosed with a new non-canonical obesity syndrome characterized by a 10-Mb 16p13.2p12.3 causal duplication. Further CNVs analyses in this cohort have permitted to establish and corroborate a recurrence of 16p11.2 CNVs associated with BMI alteration in 11 syndromic patients. The analyses have identified the KCTD13 gene as a main BMI modulator for the 9 proximal CNVs (obesity in carriers of deletions and underweight in carriers of duplications) and SH2B1 gene for 2 distal CNVs with a putative role in hypothalamic alteration of satiety. CNVs detected in the 16p11.2 region can explain the developed phenotype of intellectual disability and/or autism with severe BMI alteration of 1.6% of the cohort, showing the high prevalence of this etiology. Therefore, patients that carried distal deletions should be considered cases of monogenic syndromic obesity diagnosed by microarray. In conclusion, this study corroborates and exposes several 16p loci involved in the development of syndromic phenotypes with a BMI alteration and report new cases. The diagnostic of this type of alterations in syndromic patients and the involved genes could help to implement prevention strategies in Endocrinology and Neuropediatric Services to improve their quality of life and retard the appearance of comorbidities associated with an obese phenotype.
References
Singh RK, Kumar P, Mahalingam K. Molecular genetics of human obesity: a comprehensive review. CR Biol. 2017;340:87–108.
Albuquerque D, Stice E, Rodríguez-López R, Manco L, Nóbrega C. Current review of genetics of human obesity: from molecular mechanisms to an evolutionary perspective. Mol Genet Genom. 2015;290:1191–221.
Geets E, Meuwissen MEC, Van Hul W. Clinical, molecular genetics and therapeutic aspects of syndromic obesity. Clin Genet. 2018. https://doi.org/10.1111/cge.13367.
Kaur Y, de Souza RJ, Gibson WT, Meyre D. A systematic review of genetic syndromes with obesity. Obes Rev. 2017;18:603–34.
Huvenne H, Dubern B, Clément K, Poitou C. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obes Facts. 2016;9:158–73.
Pratt HD, Greydanus DE. Intellectual disability (mental retardation) in children and adolescents. Prim Care. 2007;34:375–86.
Delrue MA, Michaud JL. Fat chance: genetic syndromes with obesity. Clin Genet. 2004;66:83–93.
Abdalla MMI. Central and peripheral control of food intake. Endocr Regul. 2017;51:52–70.
Chung WK. An overview of mongenic and syndromic obesities in humans. Pediatr Blood Cancer. 2012;58:122–8.
Vuillaume ML, Naudion S, Banneau G, Diene G, Cartault A, Cailley D, et al. New candidate loci identified by array-CGH in a cohort of 100 children presenting with syndromic obesity. Am J Med Genet Part A. 2014;164:1965–75.
Campbell LV. Genetics of obesity. Aust Fam Physician. 2017;46:456–9.
D’Angelo CS, Koiffmann CP. Copy number variants in obesity-related syndromes: review and perspectives on novel molecular approaches. J Obes. 2012. https://doi.org/10.1155/2012/845480.
Gill R, Chen Q, D’Angelo D, Chung WK. Eating in the absence of hunger but not loss of control behaviors are associated with 16p11.2 deletions. Obesity. 2014;22:2625–31.
Phillips KL, Schieve LA, Visser S, Boulet S, Sharma AJ, Kogan MD, et al. Prevalence and impact of unhealthy weight in a national sample of US adolescents with autism and other learning and behavioral disabilities. Matern Child Health J. 2014;18:1964–75.
Serra-Juhé C, Martos-Moreno G, Bou de Pieri F, Flores R, González JR, Rodríguez-Santiago B, et al. Novel genes involved in severe early-onset obesity revealed by rare copy number and sequence variants. PLoS Genet. 2017. https://doi.org/10.1371/journal.pgen.1006657.
D’Angelo CS, Kohl I, Varela MC, de Castro CIE, Kim CA, Bertola DR, et al. Obesity with associated developmental delay and/or learning disability in patients exhibiting additional features: report of novel pathogenic copy number variants. Am J Med Genet Part A. 2013;161:479–86.
J. Dasouki M,L, Youngs E, Hovanes K. Structural chromosome abnormalities associated with obesity: report of four new subjects and review of literature. Curr Genom. 2011;12:190–203.
Lespinasse J, Bugge M, Réthoré MO, North MO, Lundsteen C, Kirchhoff M. De novo complex chromosomal rearrangements (CCR) involving chromosome 1, 5, and 6 resulting in microdeletion for 6q14 in a female carrier with psychotic disorder. Am J Med Genet. 2004;128 A:199–203.
Menten B, Buysse K, Vandesompele J, De Smet E, De Paepe A, Speleman F, et al. Identification of an unbalanced X-autosome translocation by array CGH in a boy with a syndromic form of chondrodysplasia punctata brachytelephalangic type. Eur J Med Genet. 2005;48:301–9.
Probst FJ, Roeder ER, Enciso VB, Ou Z, Cooper ML, Eng P, et al. Chromosomal microarray analysis (CMA) detects a large X chromosome deletion including FMR1, FMR2, and IDS in a female patient with mental retardation. Am J Med Genet Part A. 2007;143:1358–65.
Zung A, Rienstein S, Rosensaft J, Aviram-Goldring A, Zadik Z. Proximal 19q trisomy: a new syndrome of morbid obesity and mental retardation. Horm Res. 2007;67:105–10.
González JR, González-Carpio M, Hernández-Sáez R, Serrano Vargas V, Hidalgo GT, Rubio-Rodrigo M, et al. FTO risk haplotype among early onset and severe obesity cases in a population of Western Spain. Obesity. 2012;20:909–15.
Albuquerque D, González LM, Gimeno-Ferrer F, Bruna M, Sánchez C, Benito GM, et al. Association study of six single nucleotide polymorphisms with obesity in two independent Iberian samples. Meta Gene. 2018;17:17–22.
González JR, Estévez MN, Giralt PS, Cáceres a, Pérez LML, González-Carpio M, et al. Genetic risk profiles for a childhood with severe overweight. Pediatr Obes. 2014;9:272–80.
Bachmann-Gagescu R, Mefford HC, Cowan C, Glew GM, Hing AV, Wallace S, et al. Recurrent 200-kb deletions of 16p11.2 that include the SH2B1 gene are associated with developmental delay and obesity. Genet Med. 2010;12:641–7.
Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463:666–70.
Doche ME, Bochukova EG, Su HW, Pearce LR, Keogh JM, Henning E, et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J Clin Invest. 2012;122:4732–6.
Cigudosa JC, Lapunzina P, Coords. Consenso para la Implementación de los Arrays [CGH y SNP-arrays] en la Genética Clínica. Madrid, Spain: Instituto Roche; 2012.
Köhler S, Vasilevsky NA, Engelstad M, Foster E, McMurry J, Aymé S, et al. The human phenotype ontology in 2017. Nucleic Acids Res. 2017;45:865–76.
Sobradillo B, Aguirre A, Uresti U, Bilbao A, Fernández-Ramos C, Lizarraga A, et al. Curvas y tablas de crecimiento. Bilbao, Spain: Estudios longitudinal y transversal. Fundación Faustino Orbegozo; 2004.
Bhaumik S, Watson JM, Thorp CF, Tyrer F, Mcgrother CW. Body mass index in adults with intellectual disability: Distribution, associations and service implications: a population-based prevalence study. J Intellect Disabil Res. 2008;52:287–98.
Au N. The health care cost implications of overweight and obesity during childhood. Health Serv Res. 2012;47:655–76.
Li H, Fujiura G, Magaña S, Parish S. Health care expenditures of overweight and obese U.S. adults with intellectual and developmental disabilities. Res Dev Disabil. 2018;75:1–10.
Bădescu GM, Fîlfan M, Sandu RE, Surugiu R, Ciobanu O, Popa-Wagner A. Molecular mechanisms underlying neurodevelopmental disorders, ADHD and autism. Rom J Morphol Embryol. 2016;57:361–6.
Battaglia A, Novelli A, Bernardini L, Igliozzi R, Parrini B. Further characterization of the new microdeletion syndrome of 16p11.2-p12.2. Am J Med Genet Part A. 2009;149:1200–4.
Walters RG, Jacquemont S, Valsesia A, De Smith AJ, Martinet D, Andersson J, et al. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature. 2010;463:671–5.
Golzio C, Willer J, Talkowski ME, Oh EC, Taniguchi Y, Jacquemont S, et al. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature. 2012;485:363–7.
McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, Yoon S, et al. Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet. 2009;41:1223–7.
Shinawi M, Liu P, Kang SHL, Shen J, Belmont JW, Scott DA, et al. Recurrent reciprocal 16p11.2 rearrangements associated with global developmental delay, behavioural problems, dysmorphism, epilepsy, and abnormal head size. J Med Genet. 2010;47:332–41.
Jacquemont S, Reymond A, Zufferey F, Harewood L, Walters RG, Kutalik Z, et al. Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11.2 locus. Nature. 2011;478:97–102.
Liu Z, Xiang Y, Sun G. The KCTD family of proteins: structure, function, disease relevance. Cell Biosci. 2013. https://doi.org/10.1186/2045-3701-3-45.
Lv D, Zhang DD, Wang H, Zhang Y, Liang L, Fu JF, et al. Genetic variations in SEC16B, MC4R, MAP2K5 and KCTD15 were associated with childhood obesity and interacted with dietary behaviors in Chinese school-age population. Gene. 2015;560:149–55.
Gamero-Villarroel C, González LM, Rodríguez-López R, Albuquerque D, Carrillo JA, García-Herráiz A, et al. Influence of TFAP2B and KCTD15 genetic variability on personality dimensions in anorexia and bulimia nervosa. Brain Behav. 2017. https://doi.org/10.1002/brb3.784.
Wong TCB, Rebbert M, Wang C, Chen X, Heffer A, Zarelli VE, et al. Genes regulated by potassium channel tetramerization domain containing 15 (Kctd15) in the developing neural crest. Int J Dev Biol. 2016;60:159–66.
Ren D, Zhou Y, Morris D, Li M, Li Z, Rui L. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest. 2007;117:397–406.
Chen Z, Morris DL, Jiang L, Liu Y, Rui L. SH2B1 in β-cells promotes insulin expression and glucose metabolism in mice. Mol Endocrinol. 2014;28:696–705.
Barber JCK, Hall V, Maloney VK, Huang S, Roberts AM, Brady AF, et al. 16p11.2-p12.2 duplication syndrome: a genomic condition differentiated from euchromatic variation of 16p11.2. Eur J Hum Genet. 2013;21:182–9.
Qureshi AY, Mueller S, Snyder AZ, Mukherjee P, Berman JI, Roberts TPL, et al. Opposing brain differences in 16p11.2 deletion and duplication carriers. J Neurosci. 2014;34:11199–211.
Tabet AC, Pilorge M, Delorme R, Amsellem F, Pinard JM, Leboyer M, et al. Autism multiplex family with 16p11.2p12.2 microduplication syndrome in monozygotic twins and distal 16p11.2 deletion in their brother. Eur J Hum Genet. 2012;20:540–6.
Zufferey F, Sherr EH, Beckmann ND, Hanson E, Maillard AM, Hippolyte L, et al. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. J Med Genet. 2012;49:660–8.
Acknowledgements
We are very grateful to all patients involved in this study.
Funding
David Albuquerque is recipient of a postdoctoral grant from Fundação para a Ciência e a Tecnologia (SFRH/BPD/109043/2015). This project was supported by the Carlos III Health Institute of the Spanish Ministry of Economy and Competitiveness (17PI1551).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gimeno-Ferrer, F., Albuquerque, D., Guzmán Luján, C. et al. The effect of copy number variations in chromosome 16p on body weight in patients with intellectual disability. J Hum Genet 64, 221–231 (2019). https://doi.org/10.1038/s10038-018-0545-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-018-0545-5
This article is cited by
-
A Comprehensive Review of Syndromic Forms of Obesity: Genetic Etiology, Clinical Features and Molecular Diagnosis
Current Obesity Reports (2024)