Abstract
Humans are not (and have never been) alone. From the moment we are born, millions of micro-organisms populate our bodies and coexist with us rather peacefully for the rest of our lives. This microbiome represents the totality of micro-organisms (and their genomes) that we necessarily acquire from the environment. Micro-organisms living in or on us have evolved to extract the energy they require to survive, and in exchange they support the physiological, metabolic and immune capacities that have contributed to our evolutionary success. Although currently categorized as an autoimmune disorder and regarded as a complex genetic disease, the ultimate cause of rheumatoid arthritis (RA) remains elusive. It seems that interplay between predisposing genetic factors and environmental triggers is required for disease manifestation. New insights from DNA sequence-based analyses of gut microbial communities and a renewed interest in mucosal immunology suggest that the microbiome represents an important environmental factor that can influence autoimmune disease manifestation. This Review summarizes the historical clues that suggest a possible role for the microbiota in the pathogenesis of RA, and will focus on new technologies that might provide scientific evidence to support this hypothesis.
Key Points
-
In rheumatoid arthritis (RA)—a complex, polygenic, autoimmune disorder with a major impact on individuals and society—genes have a role, but environmental factors are required for disease manifestation
-
Multiple lines of epidemiological and clinical investigation have implicated several micro-organisms in RA pathogenesis; however, causation could not be established
-
The microbiome is defined as the totality of micro-organisms and their genes inhabiting a unique environment; the human microbiome outnumbers human genes by several orders of magnitude
-
Understanding of the role of micro-organisms in modulating health and disease has been greatly advanced by culture-independent DNA sequencing technologies and novel insights into mucosal immunology
-
Germ-free and gnotobiotic experiments have provided a deeper understanding of host–microbial interactions and have shown that gut bacteria can induce autoimmunity in genetically predisposed animal models
-
Studies are underway to assess the role of the microbiome in human RA and related diseases in the hope that disease mechanisms will be elucidated and therapeutic targets identified
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Savage, D. C. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 31, 107–133 (1977).
Backhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A. & Gordon, J. I. Host–bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005).
Lederberg, J. Infectious history. Science 288, 287–293 (2000).
Lederberg, J. & McCray, A. T. 'Ome sweet 'omics—A genealogical treasury of words. Scientist 15, 8–9 (2001).
Turnbaugh, P. J. et al. The human microbiome project. Nature 449, 804–810 (2007).
Chervonsky, A. V. Influence of microbial environment on autoimmunity. Nat. Immunol. 11, 28–35 (2010).
Klareskog, L., Catrina, A. I. & Paget, S. Rheumatoid arthritis. Lancet 373, 659–672 (2009).
MacGregor, A. J. et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 43, 30–37 (2000).
Stahl, E. A. et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat. Genet. 42, 508–514 (2010).
Aho, K., Koskenvuo, M., Tuominen, J. & Kaprio, J. Occurrence of rheumatoid arthritis in a nationwide series of twins. J. Rheumatol. 13, 899–902 (1986).
Silman, A. J. et al. Twin concordance rates for rheumatoid arthritis: results from a nationwide study. Br. J. Rheumatol. 32, 903–907 (1993).
Svendsen, A. J., Holm, N. V., Kyvik, K., Petersen, P. H. & Junker, P. Relative importance of genetic effects in rheumatoid arthritis: historical cohort study of Danish nationwide twin population. BMJ 324, 264–266 (2002).
Tobón, G. J., Youinou, P. & Saraux, A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J. Autoimmun. 35, 10–14 (2010).
Short, C. L. The antiquity of rheumatoid arthritis. Arthritis Rheum. 17, 193–205 (1974).
Ruffer, M. A. & Rietti, A. On osseous lesions in ancient Egyptians. J. Pathol. Bacteriol. 16, 439–465 (1912).
Bourke, J. B. A review of the paleopathology of arthritic diseases in Diseases in Antiquity (eds Brothwell, D. & Sandison, A. T.) 352–369 (Thomas, Springfield, IL, USA, 1967).
Zorab, P. A. Historical and prehistorical background of ankylosing spondylitis. Proc. R. Soc. Med. 54, 415–420 (1961).
Wells, C. Joint pathology in ancient Anglo-Saxons. J. Bone Joint. Surg. 44B, 948–949, (1962).
Appelboom, T. Hypothesis: Rubens—one of the first victims of an epidemic of rheumatoid arthritis that started in the 16th–17th century? Rheumatology (Oxford) 44, 681–683 (2005).
Rothschild, B. M., Turner, K. R. & DeLuca, M. A. Symmetrical erosive peripheral polyarthritis in the Late Archaic Period of Alabama. Science 241, 1498–1501 (1988).
Rothschild, B. M., Woods, R. J., Rothschild, C. & Sebes, J. I. Geographic distribution of rheumatoid arthritis in ancient North America: implications for pathogenesis. Semin. Arthritis Rheum. 22, 181–187 (1992).
Ferucci, E. D., Templin, D. W. & Lanier, A. P. Rheumatoid arthritis in American Indians and Alaska Natives: a review of the literature. Semin. Arthritis Rheum. 34, 662–667 (2005).
Zeng, Q. Y. et al. Rheumatic diseases in China. Arthritis Res. Ther. 10, R17 (2008).
McGill, P. E. & Oyoo, G. O. Rheumatic disorders in Sub-saharan Africa. East Afr. Med. J. 79, 214–216 (2002).
Neovius, M., Simard, J. F. & Askling, J. Nationwide prevalence of rheumatoid arthritis and penetration of disease-modifying drugs in Sweden. Ann. Rheum. Dis. 70, 624–629 (2011).
Myasoedova, E., Crowson, C. S., Kremers, H. M., Therneau, T. M. & Gabriel, S. E. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007 Arthritis Rheum. 62, 1576–1582 (2010).
Warden, C. C. The toxemic factor in rheumatoid arthritis. Cal. State J. Med. 7, 299–301 (1909).
Eerola, E. et al. Intestinal flora in early rheumatoid arthritis. Br. J. Rheumatol. 33, 1030–1038 (1994).
Hunter, W. Oral sepsis as a cause of disease. Br. Med. J. 2, 215–216 (1900).
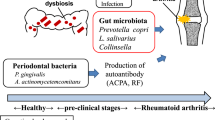
Mikuls, T. R. et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int. Immunopharmacol. 9, 38–42, (2009).
Hitchon, C. A. et al. Antibodies to Porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J. Rheumatol. 37, 1105–1112 (2010).
Loyola-Rodriguez, J. P., Martinez-Martinez, R. E., Abud-Mendoza, C., Patino-Marin, N. & Seymour, G. J. Rheumatoid arthritis and the role of oral bacteria. J. Oral Microbiol. http://dx.doi.org/10.3402/jom.v2i0.5784 (2010).
Lundberg, K., Wegner, N., Yucel-Lindberg, T. & Venables, P. J. Periodontitis in RA—the citrullinated enolase connection. Nat. Rev. Rheumatol. 6, 727–730 (2010).
Koch, R. An address on bacteriological research. Br. Med. J. 2, 380–383 (1890).
Eckburg, P. B. et al. Diversity of the human intestinal microbial flora. Science 308, 1635–1638 (2005).
Weisburg, W. G., Barns, S. M., Pelletier, D. A. & Lane, D. J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173, 697–703 (1991).
Hugenholtz, P., Goebel, B. M. & Pace, N. R. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180, 4765–4774 (1998).
Huse, S. M. et al. Exploring microbial diversity and taxonomy using SSU rRNA hypervariable tag sequencing. PLoS Genet. 4, e1000255 (2008).
Zhao, L. Genomics: The tale of our other genome. Nature 465, 879–880 (2010).
Nelson, K. E. et al. A catalog of reference genomes from the human microbiome. Science 328, 994–999 (2010).
Peterson, J. et al. The NIH Human Microbiome Project. Genome Res. 19, 2317–2323 (2009).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
Koenig, J. E. et al. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4578–4585 (2011).
Palmer, C., Bik, E. M., DiGiulio, D. B., Relman, D. A. & Brown, P. O. Development of the human infant intestinal microbiota. PLoS Biol. 5, e177 (2007).
Ley, R. E., Lozupone, C. A., Hamady, M., Knight, R. & Gordon, J. I. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 6, 776–788 (2008).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature 473, 174–180 (2011).
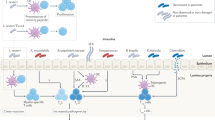
Round, J. L. & Mazmanian, S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323 (2009).
Johansson, M. E. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069, (2008).
Meyer-Hoffert, U. et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut 57, 764–771 (2008).
Macpherson, A. J. & Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303, 1662–1665 (2004).
Hooper, L. V. & Macpherson, A. J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 10, 159–169 (2010).
Kelsall, B. Recent progress in understanding the phenotype and function of intestinal dendritic cells and macrophages. Mucosal. Immunol. 1, 460–469, (2008).
Cerf-Bensussan, N. & Gaboriau-Routhiau, V. The immune system and the gut microbiota: friends or foes? Nat. Rev. Immunol. 10, 735–744 (2010).
Round, J. L. et al. The Toll-Like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977 (2011).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Frank, D. N. et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl Acad. Sci. USA 104, 13780–13785 (2007).
Xavier, R. J. & Podolsky, D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
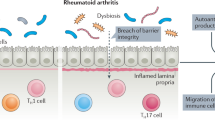
Abdollahi-Roodsaz, S. et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118, 205–216 (2008).
Wu, H. J. et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827 (2010).
Ochoa-Reparaz, J., Mielcarz, D. W., Begum-Haque, S. & Kasper, L. H. Gut, bugs, and brain: role of commensal bacteria in the control of central nervous system disease. Ann. Neurol. 69, 240–247 (2011).
Kochetkova, I., Trunkle, T., Callis, G. & Pascual, D. W. Vaccination without autoantigen protects against collagen II-induced arthritis via immune deviation and regulatory T cells. J. Immunol. 181, 2741–2752 (2008).
Kochetkova, I., Golden, S., Holderness, K., Callis, G. & Pascual, D. W. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J. Immunol. 184, 7144–7153 (2010).
Hyrich, K. L. & Inman, R. D. Infectious agents in chronic rheumatic diseases. Curr. Opin. Rheumatol. 13, 300–304 (2001).
Kohashi, O. et al. Susceptibility to adjuvant-induced arthritis among germfree, specific-pathogen-free, and conventional rats. Infect. Immun. 26, 791–794 (1979).
Bjork, J., Kleinau, S., Midtvedt, T., Klareskog, L. & Smedegard, G. Role of the bowel flora for development of immunity to hsp 65 and arthritis in three experimental models. Scand. J. Immunol. 40, 648–652 (1994).
Kohashi, O., Kohashi, Y., Takahashi, T., Ozawa, A. & Shigematsu, N. Reverse effect of gram-positive bacteria vs. gram-negative bacteria on adjuvant-induced arthritis in germfree rats. Microbiol. Immunol. 29, 487–497 (1985).
Kohashi, O., Kohashi, Y., Takahashi, T., Ozawa, A. & Shigematsu, N. Suppressive effect of Escherichia coli on adjuvant-induced arthritis in germ-free rats. Arthritis Rheum. 29, 547–553 (1986).
Rath, H. C. et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J. Clin. Invest. 98, 945–953 (1996).
Sinkorova, Z., Capkova, J., Niederlova, J., Stepankova, R. & Sinkora, J. Commensal intestinal bacterial strains trigger ankylosing enthesopathy of the ankle in inbred B10.BR (H-2(k)) male mice. Hum. Immunol. 69, 845–850 (2008).
Taurog, J. D. et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 180, 2359–2364 (1994).
van den Broek, M. F., van Bruggen, M. C., Koopman, J. P., Hazenberg, M. P. & van den Berg, W. B. Gut flora induces and maintains resistance against streptococcal cell wall-induced arthritis in F344 rats. Clin. Exp. Immunol. 88, 313–317 (1992).
Yoshitomi, H. et al. A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 201, 949–960 (2005).
Rodriguez-Reyna, T. S., Martinez-Reyes, C. & Yamamoto-Furusho, J. K. Rheumatic manifestations of inflammatory bowel disease. World J. Gastroenterol. 15, 5517–5524 (2009).
Carter, J. D. & Hudson, A. P. Reactive arthritis: clinical aspects and medical management. Rheum. Dis. Clin. North Am. 35, 21–44 (2009).
Ross, C. B., Scott, H. W. & Pincus, T. Jejunoileal bypass arthritis. Baillieres Clin. Rheumatol. 3, 339–355 (1989).
Moos, V. & Schneider, T. Changing paradigms in Whipple's disease and infection with Tropheryma whipplei. Eur. J. Clin. Microbiol. Infect. Dis. http://dx.doi.org/10.1007/s10096-011-1209-y.
Svartz, N. The primary cause of rheumatoid arthritis is an infection—the infectious agent exists in milk. Acta Med. Scand. 192, 231–239 (1972).
Svartz, N. The treatment of rheumatic polyarthritis with acid azo compounds. Rheumatism 4, 180–185 (1948).
Hannonen, P., Mottonen, T., Hakola, M. & Oka, M. Sulfasalazine in early rheumatoid arthritis. A 48-week double-blind, prospective, placebo-controlled study. Arthritis Rheum. 36, 1501–1509 (1993).
O'Dell, J. R. et al. Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N. Engl. J. Med. 334, 1287–1291 (1996).
Saag, K. G. et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 59, 762–784 (2008).
Moreland, L. W. et al. TEAR: Treatment of Early Aggressive RA; a randomized, double-blind, 2-year trial comparing immediate triple DMARD versus MTX plus etanercept to step-up from initial MTX monotherapy. Arthritis Rheum. 60 (Suppl. 10), 1895 (2009).
Tilley, B. C. et al. Minocycline in rheumatoid arthritis. A 48-week, double-blind, placebo-controlled trial. MIRA Trial Group. Ann. Intern. Med. 122, 81–89 (1995).
O'Dell, J. R. et al. Treatment of early seropositive rheumatoid arthritis: doxycycline plus methotrexate versus methotrexate alone. Arthritis Rheum. 54, 621–627 (2006).
Zanin-Zhorov, A. et al. Protein kinase C-θ mediates negative feedback on regulatory T cell function. Science 328, 372–376 (2010).
Hot, A. & Miossec, P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann. Rheum. Dis. 70, 727–732 (2011).
Colin, E. M. et al. 1, 25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 62, 132–142 (2010).
Scher, J. U. et al. Characteristic oral and intestinal microbiota in rheumatoid arthritis (RA): a trigger for autoimmunity? Arthritis Rheum. 62 (suppl. 10) doi:10.1002/art.29156 (2010).
Acknowledgements
The writing of this manuscript has been supported in part by Grant No. RC2 AR05898 to S. B. Abramson from the US NIH through the American Recovery and Reinvestment Act (ARRA) of 2009, and by KL2 Program in Translational Research to J. U. Scher, Grant No. 1 UL1 RR029893 from the National Center for Research Resources, NIH. The authors thank Ms. Ann Rupel for assistance in preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J. U. Scher and S. B. Abramson contributed equally to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Scher, J., Abramson, S. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol 7, 569–578 (2011). https://doi.org/10.1038/nrrheum.2011.121
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2011.121