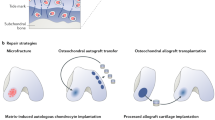
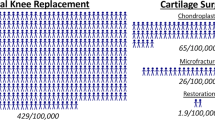
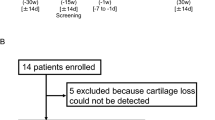
Abstract
Lesions in articular cartilage can result in significant musculoskeletal morbidity and display unique biomechanical characteristics that make repair difficult, at best. Several surgical procedures have been devised in an attempt to relieve pain, restore function, and delay or stop the progression of cartilaginous lesions. Advanced MRI and ultrasonography protocols are currently used in the evaluation of tissue repair and to improve diagnostic capability. Other nonoperative modalities, such as injection of intra-articular hyaluronic acid or supplementary oral glucosamine and chondroitin sulfate, have shown potential efficacy as anti-inflammatory and symptom-modifying agents. The emerging field of tissue engineering, involving the use of a biocompatible, structurally and mechanically stable scaffold, has shown promising early results in cartilage tissue repair. Scaffolds incorporating specific cell sources and bioactive molecules have been the focus in this new exciting field. Further work is required to better understand the behavior of chondrocytes and the variables that influence their ability to heal articular lesions. The future of cartilage repair will probably involve a combination of treatments in an attempt to achieve a regenerative tissue that is both biomechanically stable and, ideally, identical to the surrounding native tissues.
Key Points
-
Cartilage injuries remain a major cause of morbidity in both young and elderly patient populations
-
Advanced imaging with MRI and ultrasonography has resulted in improved sensitivity and specificity in the diagnosis of cartilage injuries
-
Surgical procedures have had some success in alleviating symptoms and improving function; however, the regenerative tissue has not been found to resemble the surrounding native tissue
-
Intra-articular injections of hyaluronic acid, oral glucosamine and oral chondroitin sulfate seem to confer a therapeutic effect via anti-inflammatory-mediated mechanisms
-
Tissue engineering involving scaffolds, bioactive molecules and specific cell lines has shown promising results in the treatment of cartilage defects
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Evans, P. J., Miniaci, A. & Hurtig, M. B. Manual punch versus power harvesting of osteochondral grafts. Arthroscopy 20, 306–310 (2004).
Mithoefer, K. et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J. Bone Joint Surg. Am. 87, 1911–1920 (2005).
Hangody, L., Feczko, P., Bartha, L., Bodo, G. & Kish, G. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin. Orthop. Relat. Res. 391 (Suppl.), S328–S336 (2001).
Brittberg, M. et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 331, 889–895 (1994).
Knutsen, G. et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J. Bone Joint Surg. Am. 89, 2105–2112 (2007).
Bentley, G. et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J. Bone Joint Surg. Br. 85, 223–230 (2003).
Saris, D. B. et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am. J. Sports Med. 36, 235–246 (2008).
Knutsen, G. et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J. Bone Joint Surg. Am. 86-A, 455–464 (2004).
Kiviranta, P. et al. Comparison of novel clinically applicable methodology for sensitive diagnostics of cartilage degeneration. Eur. Cell. Mater. 13, 46–55 (2007).
Brown, W. E., Potter, H. G., Marx, R. G., Wickiewicz, T. L. & Warren, R. F. Magnetic resonance imaging appearance of cartilage repair in the knee. Clin. Orthop. Relat. Res. 422, 214–223 (2004).
Saarakkala, S. et al. Ultrasonic quantitation of superficial degradation of articular cartilage. Ultrasound Med. Biol. 30, 783–792 (2004).
Charni-Ben Tabassi, N. & Garnero, P. Monitoring cartilage turnover. Curr. Rheumatol. Rep. 9, 16–24 (2007).
Garnero, P. Use of biochemical markers to study and follow patients with osteoarthritis. Curr. Rheumatol. Rep. 8, 37–44 (2006).
Wakitani, S. et al. Serum keratan sulfate is a promising marker of early articular cartilage breakdown. Rheumatology (Oxford) 46, 1652–1656 (2007).
Zhen, E. Y. et al. Characterization of metalloprotease cleavage products of human articular cartilage. Arthritis Rheum. 58, 2420–2431 (2008).
Tehranzadeh, J., Booya, F. & Root, J. Cartilage metabolism in osteoarthritis and the influence of viscosupplementation and steroid: a review. Acta Radiol. 46, 288–296 (2005).
Altman, R. D. Status of hyaluronan supplementation therapy in osteoarthritis. Curr. Rheumatol. Rep. 5, 7–14 (2003).
Snibbe, J. C. & Gambardella, R. A. Treatment options for osteoarthritis. Orthopedics 28, s215–s220 (2005).
Juni, P. et al. Efficacy and safety of intraarticular hylan or hyaluronic acids for osteoarthritis of the knee: a randomized controlled trial. Arthritis Rheum. 56, 3610–3619 (2007).
Raman, R. et al. Efficacy of Hylan G-F 20 and sodium hyaluronate in the treatment of osteoarthritis of the knee—a prospective randomized clinical trial. Knee 15, 318–324 (2008).
Lai, H. Y. et al. Intra-articular hyaluronic acid for treatment of osteoarthritis: a nationwide study among the older population of Taiwan. BMC Health Serv. Res. 8, 24 (2008).
Goggs, R. et al. Nutraceutical therapies for degenerative joint diseases: a critical review. Crit. Rev. Food Sci. Nutr. 45, 145–164 (2005).
Basalo, I. M. et al. Chondroitin sulfate reduces the friction coefficient of articular cartilage. J. Biomech. 40, 1847–1854 (2007).
Kahan, A., Uebelhart, D., De Vathaire, F., Delmas, P. D. & Reginster, J. Y. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 60, 524–533 (2009).
Gouze, J. N. et al. Exogenous glucosamine globally protects chondrocytes from the arthritogenic effects of IL-1β. Arthritis Res. Ther. 8, R173 (2006).
Clegg, D. O. et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N. Engl. J. Med. 354, 795–808 (2006).
Hughes, L. C., Archer, C. W. & ap Gwynn, I. The ultrastructure of mouse articular cartilage: collagen orientation and implications for tissue functionality. A polarised light and scanning electron microscope study and review. Eur. Cell. Mater. 9, 68–84 (2005).
Tuli, R., Li, W. J. & Tuan, R. S. Current state of cartilage tissue engineering. Arthritis Res. Ther. 5, 235–238 (2003).
Kessler, M. W. & Grande, D. A. Tissue engineering and cartilage. Organogenesis 4, 28–32 (2008).
Kujawa, M. J. & Caplan, A. I. Hyaluronic acid bonded to cell-culture surfaces stimulates chondrogenesis in stage 24 limb mesenchyme cell cultures. Dev. Biol. 114, 504–518 (1986).
Solchaga, L. A. et al. Hyaluronan-based polymers in the treatment of osteochondral defects. J. Orthop. Res. 18, 773–780 (2000).
Hollander, A. P. et al. Maturation of tissue engineered cartilage implanted in injured and osteoarthritic human knees. Tissue Eng. 12, 1787–1798 (2006).
Marcacci, M. et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin. Orthop. Relat. Res. 435, 96–105 (2005).
Nehrer, S. et al. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur. J. Radiol. 57, 3–8 (2006).
Brun, P. et al. Chondrocyte aggregation and reorganization into three-dimensional scaffolds. J. Biomed. Mater. Res. 46, 337–346 (1999).
Campoccia, D. et al. Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials 19, 2101–2127 (1998).
Liao, E., Yaszemski, M., Krebsbach, P. & Hollister, S. Tissue-engineered cartilage constructs using composite hyaluronic acid/collagen I hydrogels and designed poly(propylene fumarate) scaffolds. Tissue Eng. 13, 537–550 (2007).
Masuda, K., Sah, R. L., Hejna, M. J. & Thonar, E. J. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: the alginate-recovered-chondrocyte (ARC) method. J. Orthop. Res. 21, 139–148 (2003).
Mauck, R. L. et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 122, 252–260 (2000).
Sharma, B. & Elisseeff, J. H. Engineering structurally organized cartilage and bone tissues. Ann. Biomed. Eng. 32, 148–159 (2004).
Mauck, R. L., Nicoll, S. B., Seyhan, S. L., Ateshian, G. A. & Hung, C. T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 9, 597–611 (2003).
Huang, A. H., Yeger-McKeever, M., Stein, A. & Mauck, R. L. Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels. Osteoarthritis Cartilage 16, 1074–1082 (2008).
Moutos, F. T., Freed, L. E. & Guilak, F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 6, 162–167 (2007).
Price, R. L., Waid, M. C., Haberstroh, K. M. & Webster, T. J. Selective bone cell adhesion on formulations containing carbon nanofibers. Biomaterials 24, 1877–1887 (2003).
Chahine, N. O., Collette, N. M., Bahadori, S. & Loots, G. G. Biocompatibility of carbon nanotubes for chondrocyte growth. Transactions of the Orthopedic Research Society, San Francisco, CA, Paper #576 (2008).
Nettles, D. L., Vail, T. P., Morgan, M. T., Grinstaff, M. W. & Setton, L. A. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann. Biomed. Eng. 32, 391–397 (2004).
Solchaga, L. A. et al. A rapid seeding technique for the assembly of large cell/scaffold composite constructs. Tissue Eng. 12, 1851–1863 (2006).
Li, W. J., Jiang, Y. J. & Tuan, R. S. Cell-nanofiber-based cartilage tissue engineering using improved cell seeding, growth factor, and bioreactor technologies. Tissue Eng. Part A 14, 639–648 (2008).
Baker, B. M. et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 29, 2348–2358 (2008).
Steinwachs, M. New technique for cell-seeded collagen-matrix-supported autologous chondrocyte transplantation. Arthroscopy 25, 208–211 (2009).
Kon, E. et al. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am. J. Sports Med. 37, 33–41 (2009).
Bartlett, W. et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J. Bone Joint Surg. Br. 87, 640–645 (2005).
Mironov, V., Boland, T., Trusk, T., Forgacs, G. & Markwald, R. R. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 21, 157–61 (2003).
L'Heureux, N. et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med. 12, 361–365 (2006).
L'Heureux, N., McAllister, T. N. & de la Fuente, L. M. Tissue-engineered blood vessel for adult arterial revascularization. N. Engl. J. Med. 357, 1451–1453 (2007).
Mrugala, D. et al. Gene expression profile of multipotent mesenchymal stromal cells: identification of pathways common to TGFβ3/BMP2-induced chondrogenesis. Cloning Stem Cells 11, 61–76 (2009).
Chuma, H., Mizuta, H., Kudo, S., Takagi, K. & Hiraki, Y. One day exposure to FGF-2 was sufficient for the regenerative repair of full-thickness defects of articular cartilage in rabbits. Osteoarthritis Cartilage 12, 834–842 (2004).
Shi, S., Mercer, S., Eckert, G. J. & Trippel, S. B. Growth factor regulation of growth factors in articular chondrocytes. J. Biol. Chem. 284, 6697–6704 (2009).
Elisseeff, J., McIntosh, W., Fu, K., Blunk, B. T. & Langer, R. Controlled-release of IGF-I and TGF-β1 in a photopolymerizing hydrogel for cartilage tissue engineering. J. Orthop. Res. 19, 1098–1104 (2001).
Fan, H. et al. Porous gelatin-chondroitin-hyaluronate tri-copolymer scaffold containing microspheres loaded with TGF-β1 induces differentiation of mesenchymal stem cells in vivo for enhancing cartilage repair. J. Biomed. Mater. Res. A 77, 785–794 (2006).
Martinek, V., Ueblacker, P. & Imhoff, A. B. Current concepts of gene therapy and cartilage repair. J. Bone Joint Surg. Br. 85, 782–788 (2003).
Grande, D. A., Mason, J., Light, E. & Dines, D. Stem cells as platforms for delivery of genes to enhance cartilage repair. J. Bone Joint Surg. Am. 85-A (Suppl. 2), 111–116 (2003).
Lima, E. G. et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-β3. Osteoarthritis Cartilage 15, 1025–1033 (2007).
Wu, W. et al. Platelet-rich plasma—a promising cell carrier for micro-invasive articular cartilage repair. Med. Hypotheses 72, 455–457 (2009).
Koelling, S. et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 4, 324–335 (2009).
Erickson, G. R. et al. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 290, 763–769 (2002).
Rosenbaum, A. J., Grande, D. A. & Dines, J. S. The use of mesenchymal stem cells in tissue engineering: a global assessment. Organogenesis 4, 23–27 (2008).
Hwang, N. S. & Elisseeff, J. Application of stem cells for articular cartilage regeneration. J. Knee Surg. 22, 60–71 (2009).
Kim, H. T., Zaffagnini, S., Mizuno, S., Abelow, S. & Safran, M. R. A peek into the possible future of management of articular cartilage injuries: gene therapy and scaffolds for cartilage repair. J. Orthop. Sports Phys. Ther. 36, 765–773 (2006).
Palmer, G. et al. Development of gene-based therapies for cartilage repair. Crit. Rev. Eukaryot. Gene Expr. 12, 259–273 (2002).
Trippel, S. B., Ghivizzani, S. C. & Nixon, A. J. Gene-based approaches for the repair of articular cartilage. Gene Ther. 11, 351–359 (2004).
Gafni, Y. et al. Stem cells as vehicles for orthopedic gene therapy. Gene Ther. 11, 417–426 (2004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
N.A. Sgaglione has acted as a consultant for Arthrocare, BioSyntech, ConMed Linvatec, Smith and Nephew Endoscopy and TiGenix. D.A. Grande has acted as a consultant for Arthrocare and TiGenix. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Daher, R., Chahine, N., Greenberg, A. et al. New methods to diagnose and treat cartilage degeneration. Nat Rev Rheumatol 5, 599–607 (2009). https://doi.org/10.1038/nrrheum.2009.204
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2009.204
This article is cited by
-
Current research on pharmacologic and regenerative therapies for osteoarthritis
Bone Research (2016)
-
Heading Toward a Modern Imaging Approach in Juvenile Idiopathic Arthritis
Current Rheumatology Reports (2014)
-
Regenerative medicine in rheumatic disease—progress in tissue engineering
Nature Reviews Rheumatology (2012)
-
Surgical treatment for early osteoarthritis. Part I: cartilage repair procedures
Knee Surgery, Sports Traumatology, Arthroscopy (2012)
-
Digitally tunable physicochemical coding of material composition and topography in continuous microfibres
Nature Materials (2011)