Key Points
-
Colon cancer can be prevented through chemoprevention
-
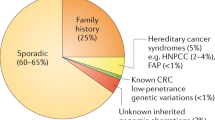
The highest return from chemopreventive strategies is in patients with a hereditary predisposition for developing colorectal cancer
-
Clinical trials have yielded conflicting results and currently no chemopreventive agent is approved in hereditary colorectal cancer syndromes
-
Chemoprevention should ultimately be aimed at delaying colectomy, reducing endoscopies and polypectomies, and preventing cancer development
-
Future trials must be carefully designed and conducted to be successful, with an effort to avoid wasting resources and time
Abstract
Colorectal cancer (CRC) is potentially preventable. Chemoprevention, a focus of research for the past three decades, aims to prevent or delay the onset of cancer through the regression or prevention of colonic adenomas. Ideal pharmacological agents for chemoprevention should be cheap and nontoxic. Although data indicate that aspirin can reduce the risk of CRC in the general population, the highest return from chemopreventive strategies would be expected in patients with the highest risk of developing the disease, particularly those with a defined hereditary predisposition. Despite compelling data showing that a large number of chemopreventive agents show promise in preclinical CRC models, clinical studies have yielded conflicting results. This Review provides a historical and methodological perspective of chemoprevention in familial adenomatous polyposis and Lynch syndrome, and summarizes the current status of CRC chemoprevention in humans. Our goal is to critically focus on important issues of trial design, with particular attention on the choice of appropriate trial end points, how such end points should be measured, and which patients are the ideal candidates to be included in a chemopreventive trial.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lewis, D. R. et al. Early estimates of SEER cancer incidence for 2012: approaches, opportunities, and cautions for obtaining preliminary estimates of cancer incidence. Cancer 121, 2053–2062 (2015).
Song, M., Garrett, W. S. & Chan, A. T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 148, 1244–1260.e16 (2015).
Wu, X., Patterson, S. & Hawk, E. Chemoprevention — history and general principles. Best Pract. Res. Clin. Gastroenterol. 25, 445–459 (2011).
Rothwell, P. M. et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 376, 1741–1750 (2010).
Jasperson, K. W., Tuohy, T. M., Neklason, D. W. & Burt, R. W. Hereditary and familial colon cancer. Gastroenterology 138, 2044–2058 (2010).
Hawk, E., Lubet, R. & Limburg, P. Chemoprevention in hereditary colorectal cancer syndromes. Cancer 86, 2551–2563 (1999).
Stoffel, E. M. & Boland, C. R. Genetics and genetic testing in hereditary colorectal cancer. Gastroenterology 149, 1191–1203.e2 (2015).
Grady, W. M. & Carethers, J. M. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology 135, 1079–1099 (2008).
Bodmer, W. F. et al. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature 328, 614–616 (1987).
Groden, J. et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66, 589–600 (1991).
Kinzler, K. W. et al. Identification of FAP locus genes from chromosome 5q21. Science 253, 661–665 (1991).
Nishisho, I. et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 253, 665–669 (1991).
Petersen, G. M., Slack, J. & Nakamura, Y. Screening guidelines and premorbid diagnosis of familial adenomatous polyposis using linkage. Gastroenterology 100, 1658–1664 (1991).
Burt, R. W. et al. Genetic testing and phenotype in a large kindred with attenuated familial adenomatous polyposis. Gastroenterology 127, 444–451 (2004).
Carethers, J. M. & Stoffel, E. M. Lynch syndrome and Lynch syndrome mimics: the growing complex landscape of hereditary colon cancer. World J. Gastroenterol. 21, 9253–9261 (2015).
Dowty, J. G. et al. Cancer risks for MLH1 and MSH2 mutation carriers. Hum. Mutat. 34, 490–497 (2013).
Edelstein, D. L. et al. Rapid development of colorectal neoplasia in patients with Lynch syndrome. Clin. Gastroenterol. Hepatol. 9, 340–343 (2011).
Haanstra, J. F. et al. Quality colonoscopy and risk of interval cancer in Lynch syndrome. Int. J. Colorectal Dis. 28, 1643–1649 (2013).
Cohen, S. et al. Polyposis syndromes in children and adolescents: a case series data analysis. Eur. J. Gastroenterol. Hepatol. 26, 972–977 (2014).
Vasen, H. F., Tomlinson, I. & Castells, A. Clinical management of hereditary colorectal cancer syndromes. Nat. Rev. Gastroenterol. Hepatol. 12, 88–97 (2015).
Committee, A. S.o. P. et al. Complications of colonoscopy. Gastrointest. Endosc. 74, 745–752 (2011).
Johnson, M. D. et al. Outcome based on management for duodenal adenomas: sporadic versus familial disease. J. Gastrointest. Surg. 14, 229–235 (2010).
Rajaratnam, S. G., Eglinton, T. W., Hider, P. & Fearnhead, N. S. Impact of ileal pouch-anal anastomosis on female fertility: meta-analysis and systematic review. Int. J. Colorectal Dis. 26, 1365–1374 (2011).
van Balkom, K. A., Beld, M. P., Visschers, R. G., van Gemert, W. G. & Breukink, S. O. Long-term results after restorative proctocolectomy with ileal pouch-anal anastomosis at a young age. Dis. Colon Rectum 55, 939–947 (2012).
Van Duijvendijk, P. et al. Quality of life after total colectomy with ileorectal anastomosis or proctocolectomy and ileal pouch-anal anastomosis for familial adenomatous polyposis. Br. J. Surg. 87, 590–596 (2000).
Spigelman, A. D., Williams, C. B., Talbot, I. C., Domizio, P. & Phillips, R. K. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet 2, 783–785 (1989).
Gouma, D. J. et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann. Surg. 232, 786–795 (2000).
Kotwall, C. A., Maxwell, J. G., Brinker, C. C., Koch, G. G. & Covington, D. L. National estimates of mortality rates for radical pancreaticoduodenectomy in 25,000 patients. Ann. Surg. Oncol. 9, 847–854 (2002).
Schmeler, K. M. et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N. Engl. J. Med. 354, 261–269 (2006).
Jenkins, M. A. et al. Short-term risk of colorectal cancer in individuals with lynch syndrome: a meta-analysis. J. Clin. Oncol. 33, 326–331 (2015).
Bussey, H. J. et al. A randomized trial of ascorbic acid in polyposis coli. Cancer 50, 1434–1439 (1982).
Waddell, W. R. & Loughry, R. W. Sulindac for polyposis of the colon. J. Surg. Oncol. 24, 83–87 (1983).
DeCosse, J. J., Miller, H. H. & Lesser, M. L. Effect of wheat fiber and vitamins C and E on rectal polyps in patients with familial adenomatous polyposis. J. Natl Cancer Inst. 81, 1290–1297 (1989).
Labayle, D. et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology 101, 635–639 (1991).
Giardiello, F. M. et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N. Engl. J. Med. 328, 1313–1316 (1993).
Steinbach, G. et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 342, 1946–1952 (2000).
van Stolk, R. et al. Phase I trial of exisulind (sulindac sulfone, FGN-1) as a chemopreventive agent in patients with familial adenomatous polyposis. Clin. Cancer Res. 6, 78–89 (2000).
Giardiello, F. M. et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N. Engl. J. Med. 346, 1054–1059 (2002).
Phillips, R. K. et al. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut 50, 857–860 (2002).
Higuchi, T. et al. A randomized, double-blind, placebo-controlled trial of the effects of rofecoxib, a selective cyclooxygenase-2 inhibitor, on rectal polyps in familial adenomatous polyposis patients. Clin. Cancer Res. 9, 4756–4760 (2003).
Cruz-Correa, M. et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 4, 1035–1038 (2006).
Lynch, P. M. et al. The safety and efficacy of celecoxib in children with familial adenomatous polyposis. Am. J. Gastroenterol. 105, 1437–1443 (2010).
West, N. J. et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut 59, 918–925 (2010).
Burn, J. et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev. Res. (Phila.) 4, 655–665 (2011).
Ishikawa, H. et al. Preventive effects of low-dose aspirin on colorectal adenoma growth in patients with familial adenomatous polyposis: double-blind, randomized clinical trial. Cancer Med. 2, 50–56 (2013).
Lynch, P. M. et al. An international randomised trial of celecoxib versus celecoxib plus difluoromethylornithine in patients with familial adenomatous polyposis. Gut 65, 286–295 (2016).
Kim, B. & Giardiello, F. M. Chemoprevention in familial adenomatous polyposis. Best Pract. Res. Clin. Gastroenterol. 25, 607–622 (2011).
Niv, Y. & Fraser, G. M. Adenocarcinoma in the rectal segment in familial polyposis coli is not prevented by sulindac therapy. Gastroenterology 107, 854–857 (1994).
Thorson, A. G., Lynch, H. T. & Smyrk, T. C. Rectal cancer in FAP patient after sulindac. Lancet 343, 180 (1994).
Nugent, K. P., Farmer, K. C., Spigelman, A. D., Williams, C. B. & Phillips, R. K. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br. J. Surg. 80, 1618–1619 (1993).
Spagnesi, M. T. et al. Rectal proliferation and polyp occurrence in patients with familial adenomatous polyposis after sulindac treatment. Gastroenterology 106, 362–366 (1994).
Oshima, M. et al. Suppression of intestinal polyposis in ApcΔ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87, 803–809 (1996).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Arber, N. et al. Celecoxib for the prevention of colorectal adenomatous polyps. N. Engl. J. Med. 355, 885–895 (2006).
Bertagnolli, M. M. et al. Celecoxib for the prevention of sporadic colorectal adenomas. N. Engl. J. Med. 355, 873–884 (2006).
Hallak, A. et al. Rofecoxib reduces polyp recurrence in familial polyposis. Dig. Dis. Sci. 48, 1998–2002 (2003).
Meyskens, F. L. Jr et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev. Res. (Phila.) 1, 32–38 (2008).
Lao, C. D. et al. Irreversible ototoxicity associated with difluoromethylornithine. Cancer Epidemiol. Biomarkers Prev. 13, 1250–1252 (2004).
US National Library of Medicine. ClinicalTrials.gov[online], (2016).
Samadi, A. K. et al. A multi-targeted approach to suppress tumor-promoting inflammation. Semin. Cancer Biol. 35, S151–S184 (2015).
US National Library of Medicine. ClinicalTrials.gov[online], (2016).
Fini, L. et al. Highly purified eicosapentaenoic acid as free fatty acids strongly suppresses polyps in ApcMin/+ mice. Clin. Cancer Res. 16, 5703–5711 (2010).
Kortum, B. et al. Mesalazine and thymoquinone attenuate intestinal tumour development in Msh2loxP/loxP Villin–Cre mice. Gut 64, 1905–1912 (2015).
McIlhatton, M. A. et al. Aspirin and low-dose nitric oxide-donating aspirin increase life span in a Lynch syndrome mouse model. Cancer Prev. Res. (Phila.) 4, 684–693 (2011).
Itano, O. et al. Sulindac effects on inflammation and tumorigenesis in the intestine of mice with Apc and Mlh1 mutations. Carcinogenesis 30, 1923–1926 (2009).
Sansom, O. J., Stark, L. A., Dunlop, M. G. & Clarke, A. R. Suppression of intestinal and mammary neoplasia by lifetime administration of aspirin in ApcMin/+ and ApcMin/+, Msh2−/− mice. Cancer Res. 61, 7060–7064 (2001).
Burn, J. et al. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N. Engl. J. Med. 359, 2567–2578 (2008).
Burn, J. et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 378, 2081–2087 (2011).
ISRCTN Registry. Finding the best dose of aspirin to prevent Lynch Syndrome cancers [online], (2015).
Heo, I. & Clevers, H. Expanding intestinal stem cells in culture. Cell Res. 25, 995–996 (2015).
Amos-Landgraf, J. M. et al. A target-selected Apc-mutant rat kindred enhances the modeling of familial human colon cancer. Proc. Natl Acad. Sci. USA 104, 4036–4041 (2007).
Femia, A. P., Becherucci, C., Crucitta, S. & Caderni, G. Apc-driven colon carcinogenesis in pirc rat is strongly reduced by polyethylene glycol. Int. J. Cancer 137, 2270–2273 (2015).
Taketo, M. M. & Edelmann, W. Mouse models of colon cancer. Gastroenterology 136, 780–798 (2009).
Kucherlapati, M. H. et al. An Msh2 conditional knockout mouse for studying intestinal cancer and testing anticancer agents. Gastroenterology 138, 993–1002.e1 (2010).
Colussi, D., Brandi, G., Bazzoli, F. & Ricciardiello, L. Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int. J. Mol. Sci. 14, 16365–16385 (2013).
Samadder, N. J. et al. Effect of sulindac and erlotinib vs placebo on duodenal neoplasia in familial adenomatous polyposis: a randomized clinical trial. JAMA 315, 1266–1275 (2016).
Zhang, L. et al. Chemoprevention of colorectal cancer by targeting APC-deficient cells for apoptosis. Nature 464, 1058–1061 (2010).
Baron, J. A. et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N. Engl. J. Med. 373, 1519–1530 (2015).
Lynch, P. M. et al. Global quantitative assessment of the colorectal polyp burden in familial adenomatous polyposis by using a web-based tool. Gastrointest. Endosc. 77, 455–463 (2013).
European Medicine Agency. Assessment report for celecoxib for the reduction of the number of adenomatous intestinal polyps in familial adenomatous polyposis, as an adjunct to surgery and further endoscopic surveillance [online], (2011).
Lynch, P. M. et al. A proposed staging system and stage-specific interventions for familial adenomatous polyposis. Gastrointest. Endosc. http://dx.doi.org/10.1016/j.gie.2015.12.029 (2016).
Klein, W. A., Miller, H. H., Anderson, M. & DeCosse, J. J. The use of indomethacin, sulindac, and tamoxifen for the treatment of desmoid tumors associated with familial polyposis. Cancer 60, 2863–2868 (1987).
Waddell, W. R. & Kirsch, W. M. Testolactone, sulindac, warfarin, and vitamin K1 for unresectable desmoid tumors. Am. J. Surg. 161, 416–421 (1991).
Moreira, L. et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA 308, 1555–1565 (2012).
Acknowledgements
L.R. is supported by the Italian Association for Cancer Research (AIRC) IG Investigator Grant N. 14281 and the European Community's Seventh Framework Program FP7/2007–2013 under grant agreement 311876, Pathway-27.
Author information
Authors and Affiliations
Contributions
L.R. researched the data for the article and drafted the manuscript. D.J.A. and P.M.L. edited and reviewed the manuscript and provided a critical contribution to discussions of the content.
Corresponding author
Ethics declarations
Competing interests
L.R. has received an unrestricted research grant from SLA Pharma UK. D.J.A. serves on the Scientific Advisory Boards for EXACT Sciences and Cancer Prevention Pharmaceuticals. P.M.L. declares no competing interests.
Supplementary information
Supplementary information S1 (table)
Selected clinical randomized, placebo-controlled trials in FAP and Lynch syndrome, and limits in their end point evaluation (PDF 183 kb)
Rights and permissions
About this article
Cite this article
Ricciardiello, L., Ahnen, D. & Lynch, P. Chemoprevention of hereditary colon cancers: time for new strategies. Nat Rev Gastroenterol Hepatol 13, 352–361 (2016). https://doi.org/10.1038/nrgastro.2016.56
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.56
This article is cited by
-
Primary and Secondary Prevention Interventions to Reduce Risk Factors Associated with Colorectal Cancer in High-Risk Groups: a Systematic Literature Review
Journal of Cancer Education (2023)
-
The preimplantation genetic testing for monogenic disorders strategy for blocking the transmission of hereditary cancers through haplotype linkage analysis by karyomapping
Journal of Assisted Reproduction and Genetics (2023)
-
The CHAMP-study: the CHemopreventive effect of lithium in familial AdenoMatous Polyposis; study protocol of a phase II trial
BMC Gastroenterology (2022)
-
Intestinal microbiota profiles in a genetic model of colon tumorigenesis correlates with colon cancer biomarkers
Scientific Reports (2022)
-
A risk-stratified approach to colorectal cancer prevention and diagnosis
Nature Reviews Gastroenterology & Hepatology (2020)