Abstract
Women with asymptomatic Neisseria gonorrhoeae infection are at risk of developing pelvic inflammatory disease (PID) if the bacteria ascend from the endocervix into the uterus and oviducts. Factors that affect disease severity, ranging from mild discomfort to severe inflammation, pain, and infertility, remain elusive. Herein we perform direct transcervical inoculation of N. gonorrhoeae into the uterus of mice to establish an infection that leads to PID. Profoundly different disease outcomes were apparent at different stages of the reproductive cycle. Mice that were infected during the diestrus stage of the reproductive cycle displayed extensive gonococcal penetration into the submucosa, severe inflammation, and clinical signs reflecting discomfort. Meanwhile, infection during the intervening estrus stage showed only modest effects. Furthermore, a gonococcal-specific humoral response was only elicited following the penetrative upper genital tract (UGT) infection during diestrus but not estrus. Strikingly, the potential for antibodies to contribute to protection during re-infection also depends upon the reproductive stage, as antigonococcal antibodies within the genital tract were markedly higher when mice were in diestrus. Combined, this work establishes a robust new model reflecting gonococcal PID in humans and reveals how the reproductive cycle determines the pathogenic outcome of gonococcal infections of the UGT.
Similar content being viewed by others
INTRODUCTION
The female reproductive tract consists of continuous, yet functionally, structurally, and immunologically distinct mucosal compartments.1, 2, 3, 4 The ability to harbor a dense microbiome in the lower portion (vagina) while at the same time maintain relatively sparse microbial populations within the upper portion (uterus and fallopian tubes) insinuate distinct immunological outcomes in response to microbial pathogens accessing different regions along the genital tract. The sexually transmitted bacterium Neisseria gonorrhoeae (also referred to as the gonococcus, Ngo) is a human-restricted pathogen that typically establishes an infection within the cervix, which links the vagina to the uterus. Infections that remain localized here are generally asymptomatic; however, in 10–25% of untreated cases5 the gonococci ascend into the upper reproductive tract to cause endometritis, salpingitis, tubo-ovarian abscess, and peritonitis, any combination of which falls under the clinical diagnosis of pelvic inflammatory disease (PID). In severe cases, inflammation-induced tissue damage can lead to persistent pain, ectopic pregnancies, and infertility.5, 6
Host factors that contribute to the development of pathology during ascending infections remain elusive. The female reproductive cycle may affect gonococcal progression into the upper genital tract (UGT) owing to physical changes within the cervix apparent at certain stages. For instance, thinning of cervical mucus during ovulation and/or retrograde blood flow during menstruation may provide a means for gonococci to access the UGT. The latter is supported by the observation that patients often present with abrupt and intense PID symptoms within the first 10 days after the onset of menses.7 Importantly, cyclic fluctuations in ovarian hormones during the menstrual cycle cause remarkable restructuring of both the endometrium and resident immune cell populations1, 8, 9, 10 and impact Ngo survival within primary epithelial cells,11 which can impact susceptibility to infection and severity of disease.
Although ethical considerations preclude experimental infections in female volunteers, a mouse cervico-vaginal infection model12 has provided useful insights into gonococcal pathogenesis and has been used as a platform for evaluating vaccine and therapeutic candidates (reviewed in Liu et al.13 and Zhu et al.14). This well-established model involves the administration of antibiotics to suppress the vaginal microbiome and β-estradiol to prolong an estrus-like stage and avoid the natural cycle–dependent influx of neutrophils into the vaginal lumen. Although this facilitates gonococcal persistence in the lower genital tract (LGT) and can be considered to reflect an uncomplicated infection scenario, bacterial migration into the UGT only occasionally occurs in mice when using this approach.12, 15, 16 Direct instillation of gonococci into the uterus had previously been accomplished to compare the relative susceptibility of mouse lines to either genital or disseminated infection, but uterine pathology was not investigated.17
In this study, we utilize a transcervical delivery method to deposit Ngo directly into the uterine horn, thereby simulating an UGT infection, which leads to PID. Distinct pathological outcomes were apparent in the upper vs. lower portions of the genital tract, with inflammatory cytokine and immune cell responses markedly higher within the uterus. We reveal striking differences in disease outcome of UGT infection during different stages of the murine estrous cycle, with significant impacts on gonococcal tissue penetration, the acute inflammatory response, and the development of humoral immunity. Finally, we illustrate stage-dependent differences in genital availability of gonococcal-specific antibodies upon systemic immunization and assess their effect on disease outcome.
RESULTS
Gonococcal localization within the UGT depends on the stage of the estrous cycle
As vaginal instillation only sporadically allows bacteria to ascend into the upper reproductive tract, we adapted a previously described method to bypass the vagina and cervix so as to reliably inoculate the uterine horns.18 Validation of appropriate inoculum delivery using vaginal vs. transcervical methods was performed using a dye. The transcervical method allowed infection of the uterine horn(s) and uterine corpus (together referred to as the uterus or UGT), as well as the cervix and vagina (referred to as the LGT). Presence of the dye was not apparent in the oviducts (Figure 1a). For gonococcal infections, mice used in this study were either naturally at estrus (when ovulation occurs) or diestrus so as to consider the effect of structural and functional differences occurring between these stages. As the duration of the murine reproductive cycle is only 4 days, with mice remaining at either estrus or diestrus for 1–2 days, for some experiments β-estradiol or DepoProvera were administered to induce and prolong these two stages, respectively (hormone treatment denoted by H).
Differences in gonococcal localization upon transcervical instillation of Neisseria gonorrhoeae (Ngo) into the uterus of female mice. (a) Visualization of inoculums delivered vaginally vs. transcervically using India Ink immediately after delivery. (b) Recovery of Ngo from the upper and lower genital tracts of mice infected either vaginally or transcervically with 108 mix of clinical gonococcal isolates. Mice were either naturally cycling at estrus and diestrus stages for the duration of the infection, or estrusH and diestrusH were induced by administration of β-estradiol and DepoProvera, respectively. Bacterial loads quantified by quantitative PCR (QPCR) amplification of gonococcal DNA 24 h after infection. (c) Comparison of gross anatomy of the genital tract 24 h after transcervically administering phosphate-buffered saline (PBS) and 108 clinical Ngo. Region of interest (dashed line) in the middle panel is magnified in the bottom panel. Black arrows indicate nodes of inflammation. (d) Bacterial localization in infected estrusH and diestrusH tissues at 4 and 24 h after infection. Ngo is stained with an antigonococcal antibody (red) while nuclei are counterstained with DAPI (4,6-diamidino-2-phenylindole; blue). Region of interest (white box) in the top panel is magnified in the middle panel. White dotted line traces the outline of the epithelia, and asterisks denote the lumen. White arrows point at Ngo. Images were representative of at least three animals per time point. (e) Groups of ⩾5 mice were transcervically infected with 107 clinical Ngo isolates during diestrusH, and viable bacterial count was obtained from homogenized upper genital tract and lower genital tract tissues for 3 days. Bars represent mean±s.e.m. CFU, colony-forming unit.
Consistent with past studies, vaginal infection with Ngo allowed persistent detection of gonococcal DNA from the LGT of mice during estrusH, but not diestrusH;19 Ngo were not detected in the UGT of any vaginally infected mice regardless of the stage. Intriguingly, transcervically delivered Ngo were detected within the UGT during both stages, in hormone-induced or naturally cycling mice, after 24 h (Figure 1b). Notably, Ngo was detected in the LGT of both estrus/estrusH and diestrus/diestrusH mice that had been infected transcervically, suggesting that the bacteria persistently descend downwards once uterine infection is established. Signs of inflammation were apparent in transcervically infected mice even at the gross anatomical level by 24 h, suggestive of a robust host inflammatory response. In particular, swollen nodules were noticeable in those infected during diestrusH (black arrows, Figure 1c), indicative of an exaggerated immune response. Reflecting this rapid host response, and likely also the lack of human-specific factors that Ngo utilize for nutrition and immune evasion in wild-type mice,20, 21, 22 infection did not persist, and the viable bacteria could only be cultured until day 3 (Figure 1e). Mice displayed overt signs of distress, such as disheveled coats and lethargy, upon infection with a high bacterial dose of 108 Ngo during diestrus. Neither swollen nodules nor distress were apparent in mice infected during estrus, indicating that this was not an inevitable consequence of infection via the transcervical route.
To further characterize gonococcal localization, we performed immunofluorescence staining at 4 and 24 h postinfection using an antigonococcal antibody (Figure 1d). In mice infected during estrusH, Ngo stayed within the uterine and vaginal lumens, and tissue penetration was rarely observed at 4 h. By 24 h, few Ngo remained in the uterus of estrusH-stage mice (white arrow), but they were detected in the vaginal lumen, indicating that bacteria did not associate with the epithelia and so tended to be washed down. In stark contrast, tissue sections from diestrusH mice displayed several infected foci along the length of the uterine horns, where Ngo had already penetrated into the subepithelial spaces in large numbers within 4 h. Although Ngo appears to take a paracellular route, it is unclear whether infection had initially led to the breakdown of cellular junctions to allow further entry or whether the gonococci exploited gaps present transiently as a result of normal tissue remodeling. However, as there was no evidence of substantial disruption of the columnar epithelia outside of these infected foci or in uninfected mice during diestrus, we hypothesize that the former might be a more probable cause. If this is the case, the Ngo must associate with some unknown murine protein or factor on the mucosal surface to initiate infection. By 24 h, Ngo appear to have been cleared from the subepithelial tissues and were now found within the uterine lumen associated with host cells, which we later identified to be neutrophils (below). Unlike in the uterus, Ngo was only detected in the vaginal lumen regardless of stage. Thus dramatically different outcomes of infection can occur depending on the stage of the estrus cycle and whether the bacteria access the LGT or UGT.
Comparing local and systemic cytokine profiles during UGT infections at distinct stages
In considering the ability of Ngo to penetrate into the uterine tissues of mice at diestrus, we considered whether the early inflammatory response would be differentially affected depending on what stage of the cycle the uterine infection occurred. To this end, we analyzed the local, as well as systemic, cytokine milieu in Ngo-infected mice during estrus vs. diestrus (Figure 2a). We utilized naturally cycling mice here to avoid possible immunomodulatory effects of using high doses of exogenous hormones.23, 24 The data revealed both tissue- and stage-dependent trends.
Local and systemic cytokine induction during gonococcal infections. Naturally cycling mice at estrus or diestrus were infected transcervically with 107 MS11 Neisseria gonorrhoeae (Ngo) for 6 and 18 h. Levels of cytokines in the upper genital tract (UGT) and lower genital tract (LGT) homogenates and sera samples were detected by LUMINEX muliplex assays. (a) Heat map showing fold change normalized to uninfected mice (UN) phosphate-buffered saline (PBS) control. (b) Bar graphs showing the levels of select cytokines in the uterus illustrating differences between estrus and diestrus levels at 6 h and 18 h. Mean±s.e.m. plotted on graph, n=3. *P<0.05, ***P<0.001, or ****P<0.0001 as determined by two-way analysis of variance using the GraphPad PRISM 5.0 software. GM-CSF, granulocyte-macrophages colony-stimulating factor; IL, interleukin; IFN, interferon; IP-10, interferon gamma inducible protein 10; KC, keratinocyte chemoattractant; MCP, monocyte chemotactic protein; MIG, monokine induced by gamma-interferon; MIP, macrophage-inflammatory protein; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
In the UGT, several pro-inflammatory cytokines and chemokines, including interleukin (IL)-1α, IL-1β, macrophage-inflammatory protein-1α, monocyte chemotactic protein-1, monokine induced by gamma-interferon, interferon gamma inducible protein 10, and IL-12, were about fivefold higher by as early as 6 h in both estrus- and diestrus-infected mice. However, by 18 h, while most of these levels had returned to baseline in the estrus cohort, they were instead further elevated during diestrus (Figure 2b). Aside from the cytokines induced during both stages, certain cytokines were differentially induced; for example, vascular endothelial growth factor was detected only during estrus, while tumor necrosis factor-α, interferon-γ, keratinocyte chemoattractant (a functional homologue of human IL-8), granulocyte-macrophages colony-stimulating factor, IL-17, IL-13, and IL-10 were only evident during diestrus. Overall, the data revealed a markedly broader and more intense cytokine induction in diestrus mice, presumably reflecting gonococcal presence within subepithelial spaces, which allows exposure to resident and recruited inflammatory cells during this stage.
To investigate whether endotoxin detection in the UGT could be responsible for cytokine production, we inoculated purified lipopolysaccharide, as a prototypical Toll-like receptor 4 ligand, at a concentration estimated to correspond to a dose of 108 bacteria.25 Curiously, lipopolysaccharide alone elicited modest (relative to Ngo) macrophage-inflammatory protein-1α and IL-1β but no keratinocyte chemoattractant or IL-6, indicating that the response to Ngo did not simply reflect a response to this potent inflammatory mediator (see Supplementary Figure S2 online), suggesting that additional factors also contribute to this effect. In LGT tissues, relatively little cytokine induction was evident during estrus, with no noticeable response at 6 h and then a relatively modest response only emerging after 18 h (Figure 2a). This is in harmony with past studies using the vaginal infection model, where an inflammatory response takes a few days to develop.26 Compared with the more pronounced pro-inflammatory cytokine production observed at 6 h in the UGT of the same mice, the lack of stimulation in the LGT, together with the induction of regulatory IL-10, suggests differences in microbial tolerance along the genital tract during estrus. However, during diestrus, a sharp increase in LGT cytokines was noted at 6 h and was mostly sustained over 18 h, indicating that the LGT is capable of responding to infection at this stage.
Looking systemically, a similar trend was evident when we measured serum cytokine responses, with a very rapid and robust induction being apparent only during diestrus. This response did not simply reflect release from the mucosal tissues as, for example, IL-6 was apparent in serum at 6 h during both estrus and diestrus phases yet was not detected in either upper or lower genital tissues (Figure 2a). Taken together, distinct cytokine responses are induced in different parts of the genital tract, and each is significantly affected by the stage of the reproductive cycle.
Neutrophil recruitment during UGT infections and its role in gonococcal clearance
Given that a purulent exudate comprised almost exclusively of neutrophils is characteristic of symptomatic gonorrhea, and that we observed both a significant increase in pro-inflammatory cytokines and uterine abscesses upon infection of diestrus mice, we measured the accumulation of neutrophil-derived myeloperoxidase (MPO) in the UGT. Signs of elevated MPO were evident in the UGT upon administration of as little as 102 Ngo, leading to a fivefold increase using a minimum dose of 106 for estrusH and 104 in diestrusH mice (Figure 3ai). MPO accumulation was not apparent when Ngo inoculation was localized to the vagina, even with the highest dose of 108 Ngo. For each dose over 102, twofold to threefold higher levels of MPO were detected during diestrusH (Figure 3aii), paralleling the increased levels of pro-inflammatory cytokine apparent in mice infected at diestrus and likely resulting from increased Ngo tissue penetration relative to estrus.
Recruitment of neutrophils into the infected tissues and their role in gonococcal clearance. (ai) Myeloperoxidase (MPO) enzyme-linked immunosorbent assays (ELISAs) performed on uterine homogenates as a read out for neutrophil infiltration 24 h after infection with clinical isolates. EstrusH or diestrusH mice received phosphate-buffered saline (PBS) or the indicated dose of Neisseria gonorrhoeae (Ngo) either vaginally or transcervically. Cohort sizes are indicated on graphs. Mean±s.e.m. is plotted for each group. *P<0.05, **P<0.01 in one-way analysis of variance comparing each column to PBS control using Bonferroni’s multiple comparison test. (aii) Comparison of MPO levels in the uteri of transcervically infected mice at estrusH or diestrusH using the same data sets as in ai. (b) Mice, synchronized at estrusH or diestrusH by hormone administration, were inoculated transcervically with either PBS or increasing doses of clinical Ngo isolates. Immunohistological staining was performed on uterine and vaginal sections 24 h after infection using anti-Gr-1 antibody to visualize neutrophils (brown). Nuclei are counterstained purple. Images were taken under × 20 magnification. Effect of neutrophil depletion on mice synchronized at (c) estrusH and (d) diestrusH. Mice were either injected with PBS (+PMN) or PMN depleted (−PMN) using anti-Gr-1 antibody clone RB6-8C5. Neutrophil depletion was confirmed by MPO ELISA on homogenized uterine tissue on the left panel of c and d. Representative data from one of two independent experiments has been graphed. Mean±s.e.m. is plotted. **P<0.01 or ***P<0.001, respectively, as determined by Mann–Whitney test on infected samples (uninfected groups were excluded from statistical analysis owing to group sizes). Effect of neutrophil depletion of gonococcal load is plotted on the right panel of c and d. Mice were infected transcervically with 107 clinical Ngo and bacterial load 24 h after infection was determined by quantitative PCR (QPCR). Horizontal bars represent mean±s.e.m. *P<0.1 by Mann–Whitney test. All statistical analyses were performed using the GraphPad PRISM 5.0 software. NS, not significant. PMN, polymorphonuclear neutrophils.
To validate that changes in MPO levels reflected an accumulation of neutrophils, and to localize neutrophils within the infected tissues, we looked for neutrophils within upper and lower genital tissues using immunohistochemistry (Figure 3b). A dose-dependent increase in the density of neutrophils was visually evident within the uterine stroma during both estrusH and diestrusH stages at 24 h postinfection. However, while the neutrophils accumulated into a dense band directly below the columnar epithelia during estrusH, they instead passed into the uterine lumen to form dramatic pus-like exudates in mice infected during diestrusH. Although there also tended to be more neutrophils within the vaginal lumen during diestrusH relative to estrusH mice (in addition to the baseline stage–dependent changes evident in uninfected mice), this difference was insignificant relative to the number of neutrophils apparent in the purulent discharge seen during uterine infection.
To understand the relationship between bacterial load and neutrophil levels within the UGT after infection, we measured MPO levels at 6, 24, 48, and 72 h postinfection (see Supplementary Figure S1). MPO levels closely resembled the kinetics of gonococcal clearance. The positive correlation between the two led us to further investigate the importance of neutrophils during bacterial clearance. To this end, we adopted a systemic depletion approach by administering the Gr1-specific antibody clone RB6-8C5, which depletes neutrophils and some monocytes, prior to infection. MPO levels were significantly reduced in the RB6-8C5-treated mice (Figure 3c,d; left panels). Intriguingly, while neutrophil depletion in the tissues was more complete during estrusH, it had little effect on gonococcal clearance from the UGT during this stage (Figure 3c; right panel), suggesting that the increased mucous secretions that coat the uterine lining during estrus may be the primary defense against the infection (see Supplementary Figure S3). Despite almost complete clearance of neutrophils from the blood of RB6-8C5-treated mice, there was still an elevation in MPO within the uterine tissues of mice infected at diestrus, consistent with the persistence of some tissue-resident Gr1-resistant but MPO-expressing phagocytes.27 Regardless, this level of neutrophil depletion led to an ∼10-fold increase in Ngo burden in the uterus of diestrusH mice, highlighting that the influx of neutrophils is important in mediating gonococcal clearance at this stage (Figure 3d). Based upon our observations regarding the cellular and cytokine response to Ngo uterine infection, we have developed a schematic to depict the Ngo-induced inflammation apparent during UGT infection (Figure 4).
Schematic showing the inflammatory response in the upper and lower genital tracts during different stages of the estrous cycle upon infection with Neisseria gonorrhoeae (Ngo). Early pathological outcome upon gonococcal infection is summarized diagrammatically. The color of the stroma indicates the level of tissue inflammation, where beige is not inflamed and red denotes the presence of inflammatory cytokines. During upper genital tract infections occurring at estrus, Ngo localizes within the uterine lumen with little tissue penetration and mild levels of inflammation. During diestrus, Ngo penetrate into the stroma in large numbers, eliciting a potent inflammatory response that is characterized by neutrophil recruitment. In the lower genital tract, Ngo is found mainly within the vaginal lumen at both estrus and diestrus but is only able to persist during estrus, presumably owing to the lack of inflammation at this stage.
Ngo-specific immunoglobulin G (IgG) is induced upon UGT infection during diestrus but not during estrus
The lack of a humoral response during uncomplicated gonococcal infection is widely accepted.16, 28 However, bactericidal antibodies have been detected in convalescent phase sera of 70% women suffering from severe PID, suggesting that under certain pathological conditions an antibody response can be induced.29 Considering the differences in uterine tissue penetration observed during estrus and diestrus, we contemplated whether the humoral response might be determined by the stage of the reproductive cycle at which the bacteria access the UGT. Therefore, we assessed whether the adaptive response differed in mice that were infected within the UGT or LGT during estrus or diestrus phases of their natural cycle (Figure 5a).
Immunoglobulin G (IgG) response during upper genital tract gonococcal infections. (a) Schematic representation of the experimental setup. The estrous cycle was monitored for 5 days. Mice that entered the first day of estrus or second day of diestrus received 107 clinical Neisseria gonorrhoeae (Ngo) vaginally, transcervically, or intranasally. Serum and vaginal washes were sampled for 21 days, after which all the groups received DepoProvera and were subsequently infected with 107 bacteria. (b) Induction of Ngo-specific IgG in sera (top panel) and vaginal washes (bottom panel) as determined by whole-bacterial enzyme-linked immunosorbent assays for 21days after 1o infection. Mean±s.e.m. plotted on graph. ****P<0.0001 as determined by two-way analysis of variance using Bonferroni post-hoc method comparing means of each treatment to the Vaginal (estrus) group for that particular time point. Group sizes are indicated in the legend. All statistical analyses were performed using the GraphPad PRISM 5.0 software.
Sera and vaginal washes collected over a period of 21 days were tested for the presence of Ngo-specific antibodies using whole-bacterial enzyme-linked immunosorbent assays (Figure 5b). Consistent with previous studies,16 Ngo inoculated intravaginally during estrus or diestrus did not trigger IgG production. Intranasal inoculation, which we used as a positive control owing to its ability to act as an immune-inductive site (reviewed in Kiyono and Fukuyama30), did lead to an IgG response irrespective of the stage of the estrous cycle. Notably, Ngo-specific IgG was detected in the cohort that was transcervically infected at diestrus, with titers that exceeded with that obtained in intranasally infected mice. This response failed to develop in mice transcervically infected at estrus, demonstrating that the UGT is not always an effective immune-inductive site. An independent experiment using transcervically infected estrusH and diestrusH mice also led to antibody production only upon infection during diestrusH (data not shown), signifying that this outcome is reproducible in hormone-treated animals as well. Together, these results are consistent with a humoral response being elicited upon Ngo penetration into the uterine submucosal tissues of diestrus mice.
Implication of Ngo-specific antibodies during recurrent UGT infections
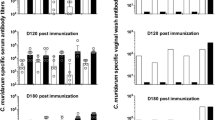
Next we considered whether Ngo-specific antibodies induced by infection conferred protection against subsequent infections. For re-infection studies, we utilized mice that were previously infected during their natural cycles, and three groups were established: mice that had been either vaginally or transcervically infected during estrus (both of which fail to trigger antibodies); mice that had been transcervically infected during diestrus (which produce antibodies); and mice that were intranasally infected (as a control that produces antibodies). One month later, DepoProvera was administered to induce diestrusH, and mice were subsequently reinfected using a 107 dose of the same Ngo strain used during primary infection (Figure 5a). Prior to secondary infection, sera and vaginal washes were tested to confirm the presence of antibodies. Ngo-specific IgG was detected in serum and vaginal washes of diestrus uterine- and nasal-infected, but not estrus-infected, mice (Figure 6ai, aii). Local IgG titers positively correlated with amount present in sera regardless of the route of immunization (Figure 6aiii). IgA was detected in the sera of nasally infected mice (Figure 6bi) and vaginal washes of a subset of mice in all three groups at very low levels (Figure 6bii); however, there was no correlation between serum and vaginal Ngo-specific IgA titers (Figure 6bii). Curiously, mice transcervically infected during diestrus tended to have the highest genital Ngo-specific IgG but the lowest genital IgA response (Figure 6a,b), although the latter difference was not statistically significant.
Effect of previous gonococcal exposure during recurrent upper genital tract infection. Detection of Neisseria gonorrhoeae (Ngo)-specific (a) immunoglobulin G (IgG) and (b) IgA from previously infected mice prior to secondary challenge (collected on day 33, according to Figure 5a). All mice received DepoProvera treatment to induce diestrusH and were grouped according to stage and method of primary challenge. Estrus T/V, transcervically or vaginally infected during estrus; Diestrus T, transcervically infected during diestrus; Both IN, intranasally infected during estrus or diestrus. Antibody levels in (i) sera and (ii) vaginal washes are shown. Mean±s.e.m. *P<0.05, **P<0.01, and ***P<0.0001, respectively, using one-way analysis of variance and Bonferroni’s post-hoc test comparing each group to all other groups. (iii) Scatter plots showing correlation of Ngo-specific Ig in sera to levels found in vaginal washes. Pearson’s r correlation values are shown on the graphs. (c) Quantitative PCR (QPCR) quantification of Ngo load in the uterus 24 h after 2o transcervical challenge with 107 of the same strains of Ngo that was used for 1o infection. Mean±s.e.m. is plotted, and the percentage of infected for each group is shown at the bottom. (d) Cytokine levels in homogenized uteri 24 h after 2o infection. Control group contains a mix of previously infected mice that received phosphate-buffered saline (PBS) during 2o challenge. Mean±s.e.m. is plotted. All statistical analyses were performed using the GraphPad PRISM 5.0 software. IL, interleukin; KC, keratinocyte chemoattractant; MCP, monocyte chemotactic protein; MIP, macrophage-inflammatory protein.
Cytokine and MPO levels were elevated in all re-infected animals at 24 h postinfection, indicative of an innate inflammatory response; however, there was no difference in their levels among the three immunized groups (Figure 6d). Despite this, a difference in bacterial burdens attained in the UGT was apparent. For the group that was initially infected during estrus (and did not raise IgG), Ngo was detected in the UGT of two out of the three mice that were previously infected vaginally and of two out of the six mice that was previously infected transcervically. Together, the UGT of 44% (4 out of 9) of re-infected mice in this group were positive for Ngo, which is indistinguishable from non-immunized mice of this older age (see below). In contrast, for the two groups that did raise antigonococcal antibodies from previous exposure, Ngo could be detected in the UGT of only 1 out of the 7 (14% diestrus uterine) and of 1 out of the 6 (intranasal) after the secondary challenge (Figure 6c). This may be indicative of a role for Ngo-specific IgG during bacterial clearance; however, this difference did not achieve statistical significance.
Local availability of Ngo-specific IgG fluctuates during different stages of the estrous cycle
Considering the marked difference in antibody induction in mice infected during estrus or diestrus phases, we considered whether the reproductive cycle would also affect the production of anti-Ngo antibodies and/or its delivery into the genital tract upon parenteral immunization. To address this, we injected 107 heat-inactivated Ngo intraperitoneally (Figure 7a). This led to a robust IgG production, with titers exceeding that seen with mucosal infections (Figure 7b). The stage of the cycle on the day of immunization did not affect antibody titers achieved in the blood (see Supplementary Figure S4). However, levels of Ngo-specific IgG present in vaginal lavages fluctuated depending on the stage mice were at on each sampling day, with significantly higher levels present during diestrus (Figure 7b, bottom panel). No variance was observed for systemic IgG levels (Figure 7b, top panel). This stage-specific difference in genital mucosal IgG availability was further accentuated upon treatment with hormones to elicit estrus- or diestrus-like states (Figure 7c). Notably, low titers of anti-Ngo IgA were also detected in vaginal washes at diestrusH, despite the distal (intraperitoneal) immunization route and it being not significantly elevated in sera (Figure 7d).
Local and systemic immunoglobulin (Ig) responses during parenteral immunization with heat-inactivated gonococci. (a) Schematic representation of the experimental setup. Hormone treatment for inducing estrusH or diestrusH was initiated 2 or 5 days prior to infection, respectively. (b) Neisseria gonorrhoeae (Ngo)-specific IgG, detected by whole-bacterial enzyme-linked immunosorbent assays, following parenteral immunization with 107 heat-inactivated clinical Ngo strains (hk Ngo) in sera (top panel) and vaginal washes (bottom panel). Total number of immunized mice, n=20; phosphate-buffered saline (PBS) controls, n=20. For every time point, mice are grouped according to the stage of the estrous cycle on the sampling day. Group sizes ranged from n=4 to n=16. Mean±s.e.m. plotted. *P<0.05, ***P<0.001, respectively, as calculated by two-way analysis of variance (ANOVA) with Bonferroni post-hoc test comparing each group with every other group. (c) Ngo-specific IgG and (d) IgA in sera (top panel) and vaginal washes (bottom panel) sampled on day 33 after administering β-estradiol or DepoProvera to synchronize mice to estrusH or diestrusH, respectively. Bars represent mean±s.e.m., n=7 for each group. ***P<0.001 using one-way ANOVA with Bonferroni’s multiple comparison test. Statistical analysis was performed out using the GraphPad PRISM 5.0 software. NS, not significant.
Effect of whole-bacterial immunization on uterine infection
To ascertain whether antibodies elicited by parenteral immunization could affect Ngo uterine infection, we immunized mice intraperitoneally with heat-killled Ngo and then transcervically infected them with 107 viable Ngo during estrusH or diestrusH (Figure 7a). Immunization did not affect the bacterial burden observed during estrusH (Figure 8ai); however, it did reduce the number of animals infected during diestrusH (Figure 8bi). During both stages, there tended to be a reduction in the levels of MPO and proinflammatory cytokines in the immunized mice, but this difference was not statistically significant at the experimental end point (Figure 8aii,bii). Considering that diestrus is when infection-induced inflammation is observed (see Figures 1, 2, 3, 4), we performed a focused immunization experiment where diestrusH mice were used for the challenge. Although the observation that immunized mice had reduced levels of proinflammatory cytokines was again noted, this also did not reach statistical significance (data not shown). However, taken together, our results suggest that vaccine-induced antibodies may have the potential to contribute to protection against the pathology associated with gonococcal-induced PID.
Effect of parenteral immunization during upper genital tract infections. Mice were immunized with 107 heat-inactivated clinical Neisseria gonorrhoeae (hkNgo) or phosphate-buffered saline (PBS), according to the schematic in Figure 7a. (ai) Bacterial load, (aii) myeloperoxidase (MPO), macrophage-inflammatory protein (MIP)-1α, and interleukin (IL)-β levels 24 h postinfection in the uterus of mice challenged during estrusH. (bi) Bacterial load, (bii) MPO, MIP-1α, and IL-β levels 24 h postinfection in the uterus of mice challenged during diestrusH. Mean±s.e.m. is plotted, n=⩾5 for each group. Bonferroni’s multiple comparison test was performed using the GraphPad PRISM 5.0 software. Changes were not statistically significant. QPCR, quantitative PCR.
DISCUSSION
Despite obvious differences in the gross anatomy of their reproductive tracts, functional and microscopic similarities have made it possible to model gonococcal infections in women using female mice.31 Prior to this study, mouse modeling of gonococcal infections had solely focused on simulating uncomplicated infection of the LGT of β-estradiol-treated mice. Herein we instead characterize immunological response and pathology associated with UGT gonococcal infections and show that the outcome is highly specific to the stage of the reproductive cycle at which infection occurs. It is important to bear in mind that the murine estrous cycle does vary from the human menstrual cycle, notably in terms of its shorter duration and the lack of menstruation; yet analogous changes in the uterine architecture during the respective reproductive cycles of these species make it feasible to draw parallels.32
The intense clinical manifestations of PID observed upon infecting mice at diestrus, during which significant epithelial damage allowed gonococcal entry into the stroma (schematically illustrated in Figure 4), is reminiscent of the scenario that may develop in infected humans during or shortly after the onset of menses when the uterine lining gets sloughed.33 The prolonged induction of pro-inflammatory cytokines in the uterus at this stage led to a rapid and severe neutrophilic inflammation, including a pus-like exudate that contributed to gonococcal clearance. In addition, inflammatory cytokines such as IL-1, IL-6, and tumor necrosis factor α were systemically induced, and mice displayed noticeable signs of discomfort at high doses of Ngo.34 These outcomes are also consistent with the sudden intense symptoms of PID that arise shortly after menses in humans.7
Although symptoms are most severe during diestrus, the UGT is also susceptible to bacteria spreading from the cervix around the time of ovulation owing to thinning of cervical mucus.35 The uterine microenvironment in mice during estrus is comparable to that in humans around ovulation, in that uterine glands release an abundance of mucus that lines an unbroken epithelium.33, 36 Our findings suggest that the endometrium is effectively protected from gonococcal invasion during this stage, evident from the relative absence of Ngo associated with the epithelium and, presumably owing to the absence of tissue penetration, only a transient spike in pro-inflammatory cytokines. However, when extrapolating this observation to human diseases, it is important to consider that mucosal receptors present on the human endometrial lining would allow for cellular association and transmigration.37 The global suppression of cytokine production caused by naturally elevated levels of circulating β-estradiol during estrus may be another protective factor.23 Interestingly, we observed lower levels of neutrophil influx into the uterus during the estrus phase but that gonococci can be cleared from the UGT even when neutrophils are depleted. Such clearance could be mediated by physical defenses such as complement, antimicrobial peptides, and/or the flushing action of mucus (reviewed in Linden et al. and McGuckin et al.38, 39); however, the relative contribution of these and other factors to antigonococcal defense remains to be explored. Regardless, when considered together, our findings are consistent with UGT infections having a milder outcome during this stage.
One of the most prominent observations in this study was the compartmentalized nature of the gonococcal-specific immune response along the genital tract, attributable to the differences in cellular populations and expression of pattern-recognition receptors in the UGT vs. LGT.4, 40 Compared with the infected uterus, the vagina showed very little sign of inflammation during estrus. In contrast, anti-inflammatory cytokines such as IL-4, IL-13, and IL-10 were induced instead.41 During diestrus, the difference in cytokine induction in the UGT and LGT was less pronounced (both are elevated), presumably reflecting the fact that the potent inflammatory response of uterine tissues during this stage are evident systemically. Given that mouse studies up to this point have utilized vaginal infection model of β-estradiol-treated mice to study the host immune response, it should be kept in mind that the effects seen are specific to the tissue and stage of the cycle. Importantly, the development of severe PID during diestrus presents a new paradigm by which to consider gonococcal disease. Notably, this ability to cause disease during the progesterone-dominant diestrus stage is not unique to Ngo, as progesterone also renders mice susceptible to the sexually transmitted pathogens that cause chlamydia and genital herpes, albeit for different reasons.24, 42
Studies looking at naturally infected human populations show that the humoral response generally does not develop in patients with uncomplicated gonococcal infections.28, 29, 43 This is consistent with our observation that there was no significant response to Ngo when mice were exposed to uterine infection during estrus or were vaginally infected at either stage. However, we observed that the invasive uterine infections that develop during diestrus trigger high titers of systemic and local (mucosal) Ngo-specific IgG. Although additional studies need to be conducted to determine the mechanism for induction, this is the first experimental evidence that we are aware of to show that Ngo-specific antibodies can be induced in the female UGT in a stage-restricted manner. It is enticing to consider this link between the reproductive cycle, disease, and immunity in the context of classical studies, showing that gonococcal-specific bactericidal antibodies are evident in the convalescent-phase sera of 70% of women who suffer from severe PID.29
Importantly, this cycle-dependent Ig induction is unique to the UGT as intranasal Ngo infection at either stage produces similar levels of IgG. Also notable, there is a direct correlation between serum and vaginal Ngo-specific IgG levels. This suggests that, regardless of the route of immunization, most genital IgG is derived from circulation rather than being locally produced. On the other hand, no obvious correlation was apparent when comparing serum vs. genital mucosa IgA or between IgA and bacterial persistence during secondary infection. Therefore, important in the context of infection, the presence of IgG from prior exposure better correlates with protection of mice re-infected during diestrus. This may explain the clinical findings which suggest that a history of PID can confer protection against recurrent salpingitis from the same strain.45
In consideration of the fact that systemically produced IgG may be delivered into the genital tract,44, 46 we parenterally immunized mice with heat-inactivated Ngo to assess whether this can protect against ascending infections. The availability of plasma-derived IgG in genital secretions has been previously shown to fluctuate during the estrous and menstrual cycles.47, 48, 49 We observed a similar trend, with significantly higher levels of Ngo-specific IgG being present during diestrus as compared with estrus. The neonatal FcRn receptor on genital epithelia has been implicated for the transport of IgG into the mucosal lumen;47 however, it remains unclear whether the striking difference in genital Ngo-specific IgG between mice in estrus and diestrus, with nearly 20-fold higher IgG levels in the latter, stems from differences in the regulation of FcRn and/or other cycle-dependent effects. Perhaps related to this fact, we found no difference in gonococcal recovery when these immunized mice were infected while in estrusH. For mice infected during diestrusH, the immunization may have a role in reducing the number of animals that were infected, though larger vaccine-focused studies must be performed to establish how robust and cross-protective this effect is. Notably, however, there tended to be a modest reduction in the inflammatory cytokine expression, suggesting that the potentially pathogenic inflammatory response may also be alleviated.
In summary, this study takes advantage of a robust new model of gonococcal-induced PID to reveal that the outcome of upper reproductive tract infection is determined by the stage of the reproductive cycle, both with respect to uterine inflammation and the adaptive immune response to infection. Given that Ngo utilizes various factors of human origin for survival, mucosal colonization, and immune evasion during infection in humans,20, 21, 22 it is not surprising that infection did not result in long-term colonization in wild-type animals. Another limitation that should be considered is the lack of gonococcal progression into the oviducts and signs of salpingitis in mice, which is a common outcome of ascendant infections in women. Whether this stemmed from anatomical differences, in that the murine oviduct is tightly coiled,31 or the lack of colonization factors remains unclear. An important avenue for future research will be to utilize transgenic mice to dissect the contribution of host-restricted factors during bacterial progression, persistence, and disease, with the exciting prospect that this would lead to a more “natural” infection scenario and permit chronic infection of the UGT. Notable in the ongoing search for a gonococcal-specific vaccine, a robust immune response has the potential to suppress both bacterial burden and the inflammatory response. Further efforts to understand molecular aspects of the gonococcal tissue invasion apparent during diestrus may yield insights as to how to obstruct this pathogenic process. At the same time, efforts to increase the efficacy of Ngo-specific antibody responses, either by vaccine-induced targeting of the response toward essential antigens or by increasing the delivery of protective antibodies throughout the cycle, is an essential next step in our battle against the emerging global health problems associated with antibiotic-resistant N. gonorrhoeae.
METHODS
Additional methods are provided in Supplementary Materials.
Mouse strains. Six-week-old female FVB wild-type mice were from Charles River (Quebec, Canada) and acclimatized for 1–2 weeks prior to experimentation.
Mouse infections. An overnight lawn of gonococci was harvested into 1 ml of phosphate-buffered saline (PBS) supplemented to obtain 0.9 mM CaCl2 and 0.5 mM MgCl2 (PBS++, Life Technologies), and OD550 was measured to calculate the number of bacteria. Appropriate dilutions were made in PBS++ to obtain the desired concentration. Naturally cycling or hormone-treated mice at estrus or diestrus stages were anesthetized via Isofluorane inhalation. A modified version of the previously described transcervical protocol was used in this study.18 Briefly, mice were placed in a prone position with the lower back tilted at a 45-degree angle. A blunted 25-gauge needle was inserted through the vagina so as to bypass the cervix and deposit a total of 20 μl suspension of the indicated amount of gonococci into the uterine horn(s). For vaginal and intranasal infections, a 10-μl micropipette tip was used to instil 10 μl inoculum into the LGT or nares, respectively. At the indicated time points, animals were humanely killed by CO2 inhalation. Cardiac puncture was performed to drain blood and obtain sera. The genital tract was dissected out and cut at the point where the cervix joins the uterine body to separate the “upper” (uterine horns and uterine body) and “lower” (cervix and vagina) sections. Tissues were flash frozen in liquid N2 and stored at −80oC.
References
Wira, C.R., Fahey, J.V., Sentman, C.L., Pioli, P.A. & Shen, L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol. Rev. 206, 306–335 (2005).
Burgener, A. et al. A systems biology examination of the human female genital tract shows compartmentalization of immune factor expression. J. Virol. 87, 5141–5150 (2013).
Givan, A.L. et al. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am. J. Reprod. Immunol. 38, 350–359 (1997).
Pudney, J., Quayle, A.J. & Anderson, D.J. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol. Reprod. 73, 1253–1263 (2005).
Hook, E.W. 3rd & Holmes, K.K. Gonococcal infections. Ann. Intern. Med. 102, 229–243 (1985).
Eschenbach, D.A. & Holmes, K.K. Acute pelvic inflammatory disease: current concepts of pathogenesis, etiology, and management. Clin. Obstet. Gynecol. 18, 35–56 (1975).
McCormack, W.M. et al. Acute pelvic inflammatory disease: characteristics of patients with gonococcal and nongonococcal infection and evaluation of their response to treatment with aqueous procaine penicillin G and spectinomycin hydrochloride. Sex. Transm. Dis. 4, 125–131 (1977).
Flynn, L., Byrne, B., Carton, J., Kelehan, P., O'Herlihy, C. & O'Farrelly, C. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from non-pregnant human endometrium. Am. J. Reprod. Immunol. 43, 209–217 (2000).
Kamat, B.R. & Isaacson, P.G. The immunocytochemical distribution of leukocytic subpopulations in human endometrium. Am. J. Pathol. 127, 66–73 (1987).
Salamonsen, L.A. & Woolley, D.E. Menstruation: induction by matrix metalloproteinases and inflammatory cells. J. Reprod. Immunol. 44, 1–27 (1999).
Edwards, J.L. Neisseria gonorrhoeae survival during primary human cervical epithelial cell infection requires nitric oxide and is augmented by progesterone. Infect. Immun. 78, 1202–1213 (2010).
Jerse, A.E. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect. Immun. 67, 5699–5708 (1999).
Liu, Y., Feinen, B. & Russell, M.W. New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front. Microbiol. 2, 52 (2011).
Zhu, W., Chen, C.J., Thomas, C.E., Anderson, J.E., Jerse, A.E. & Sparling, P.F. Vaccines for gonorrhea: can we rise to the challenge? Front. Microbiol. 2, 124 (2011).
Imarai, M. et al. Regulatory T cells are locally induced during intravaginal infection of mice with Neisseria gonorrhoeae. Infect. Immun. 76, 5456–5465 (2008).
Song, W. et al. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17beta-estradiol-treated mice. Vaccine 26, 5741–5751 (2008).
Streeter, P.R. & Corbeil, L.B. Gonococcal infection in endotoxin-resistant and endotoxin-susceptible mice. Infect. Immun. 32, 105–110 (1981).
Corbeil, L.B., Wunderlich, A.C. & Braude, A.I. Technique for transcervical intrauterine inoculation of the mouse. Lab. Anim. Sci. 28, 314–316 (1978).
Braude, A.I. Maxwell Finland lecture. Resistance to infection with the gonococcus. J. Infect. Dis. 145, 623–624 (1982).
Ngampasutadol, J. et al. Human factor H interacts selectively with Neisseria gonorrhoeae and results in species-specific complement evasion. J. Immunol. 180, 3426–3435 (2008).
Ngampasutadol, J. et al. Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection. Proc. Natl. Acad. Sci. USA 102, 17142–17147 (2005).
Cornelissen, C.N. et al. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 27, 611–616 (1998).
Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 28, 521–574 (2007).
Kaushic, C., Ashkar, A.A., Reid, L.A. & Rosenthal, K.L. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 77, 4558–4565 (2003).
Uronen, H. et al. Gram-negative bacteria induce proinflammatory cytokine production by monocytes in the absence of lipopolysaccharide (LPS). Clin. Exp. Immunol. 122, 312–315 (2000).
Packiam, M., Veit, S.J., Anderson, D.J., Ingalls, R.R. & Jerse, A.E. Mouse strain-dependent differences in susceptibility to Neisseria gonorrhoeae infection and induction of innate immune responses. Infect. Immun. 78, 433–440 (2010).
Frazer, L.C., O'Connell, C.M., Andrews, C.W. Jr., Zurenski, M.A. & Darville, T. Enhanced neutrophil longevity and recruitment contribute to the severity of oviduct pathology during Chlamydia muridarum infection. Infect. Immun. 79, 4029–4041 (2011).
Hedges, S.R., Mayo, M.S., Mestecky, J., Hook, E.W. 3rd & Russell, M.W. Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect. Immun. 67, 3937–3946 (1999).
Kasper, D.L., Rice, P.A. & McCormick, W.M. Bactericidal antibody in genital infection due to Neisseria gonorrhoeae. J. Infect. Dis. 135, 243–251 (1977).
Kiyono, H. & Fukuyama, S. NALT- versus Peyer's-patch-mediated mucosal immunity. Nat. Rev. Immunol. 4, 699–710 (2004).
Rendi, M.H., Muehlenbachs, A., Garcia, R.L. & Boyd, K.L. Female reproductive system In Comparative Anatomy and Histology, Piper, M. & Treuting, S.D. & Liggitt, Denny & Charles W, Frevert, (eds). Elsevier, (2012).
Knobil, E. William Harvey and the physiology of reproduction. Physiologist 24, 3–7 (1981).
Verma, V. Ultrastructural changes in human endometrium at different phases of the menstrual cycle and their functional significance. Gynecol. Obstet. Invest. 15, 193–212 (1983).
Netea, M.G., Kullberg, B.J. & Van der Meer, J.W. Circulating cytokines as mediators of fever. Clin. Infect. Dis. 31, S178–S184 (2000).
Macdonald, R.R. Cyclic changes in cervical mucus. 1. Cyclic changes in cervical mucus as an indication of ovarian function. J. Obstet. Gynaecol. Br. Commonw. 76, 1090–1094 (1969).
Corbeil, L.B., Chatterjee, A., Foresman, L. & Westfall, J.A. Ultrastructure of cyclic changes in the murine uterus, cervix, and vagina. Tissue Cell 17, 53–68 (1985).
Timmerman, M.M., Shao, J.Q. & Apicella, M.A. Ultrastructural analysis of the pathogenesis of Neisseria gonorrhoeae endometrial infection. Cell. Microbiol. 7, 627–636 (2005).
Linden, S.K., Sutton, P., Karlsson, N.G., Korolik, V. & McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 1, 183–197 (2008).
McGuckin, M.A., Linden, S.K., Sutton, P. & Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 9, 265–278 (2011).
Fazeli, A., Bruce, C. & Anumba, D.O. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum. Reprod. 20, 1372–1378 (2005).
Marie, C., Pitton, C., Fitting, C. & Cavaillon, J.M. Regulation by anti-inflammatory cytokines (IL-4, IL-10, IL-13, TGFbeta) of interleukin-8 production by LPS- and/or TNFalpha-activated human polymorphonuclear cells. Mediators Inflamm. 5, 334–340 (1996).
Kaushic, C., Zhou, F., Murdin, A.D. & Wira, C.R. Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect. Immun. 68, 4207–4216 (2000).
Ison, C.A., Hadfield, S.G., Bellinger, C.M., Dawson, S.G. & Glynn, A.A. The specificity of serum and local antibodies in female gonorrhoea. Clin. Exp. Immunol. 65, 198–205 (1986).
Mestecky, J., Moldoveanu, Z. & Russell, M.W. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am. J. Reprod. Immunol. 53, 208–214 (2005).
Buchanan, T.M., Eschenbach, D.A., Knapp, J.S. & Holmes, K.K. Gonococcal salpingitis is less likely to recur with Neisseria gonorrhoeae of the same principal outer membrane protein antigenic type. Am. J. Obstet. Gynecol. 138, 978–980 (1980).
Lencer, W.I. & Blumberg, R.S. A passionate kiss, then run: exocytosis and recycling of IgG by FcRn. Trends Cell Biol. 15, 5–9 (2005).
Li, Z., Palaniyandi, S., Zeng, R., Tuo, W., Roopenian, D.C. & Zhu, X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc. Natl. Acad. Sci. USA 108, 4388–4393 (2011).
Gallichan, W.S. & Rosenthal, K.L. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology 224, 487–497 (1996).
Usala, S.J., Usala, F.O., Haciski, R., Holt, J.A. & Schumacher, G.F. IgG and IgA content of vaginal fluid during the menstrual cycle. J. Reprod. Med. 34, 292–294 (1989).
Acknowledgements
We thank Nelly Leung, Kate Banks, and Frank Guiliano for their technical support and helpful discussions throughout this study. This work has been funded by the Canadian Institutes of Health Research (CIHR) operating grant MOP-15499 and the USA National Institutes of Health (NIH) R01A1103400-01A1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Islam, E., Shaik-Dasthagirisaheb, Y., Kaushic, C. et al. The reproductive cycle is a pathogenic determinant during gonococcal pelvic inflammatory disease in mice. Mucosal Immunol 9, 1051–1064 (2016). https://doi.org/10.1038/mi.2015.122
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2015.122
This article is cited by
-
Murine host response to Neisseria gonorrhoeae upper genital tract infection reveals a common transcriptional signature, plus distinct inflammatory responses that vary between reproductive cycle phases
BMC Genomics (2018)
-
The murine vaginal microbiota and its perturbation by the human pathogen group B Streptococcus
BMC Microbiology (2018)