Abstract
The chemokine CXCL13 is overexpressed in the intestine during inflammation. To mimic this condition, we created transgenic mice-expressing CXCL13 in intestinal epithelial cells. CXCL13 expression promoted a marked increase in the number of B cells in the lamina propria and an increase in the size and number of lymphoid follicles in the small intestine. Surprisingly, these changes were associated with a marked increase in the numbers of RORγt+NKp46−CD3−CD4+ and RORγt+NKp46+ cells. The RORγt+NKp46−CD3−CD4+ cells expressed CXCR5, the receptor for CXCL13, and other markers of lymphoid tissue-inducer cells, such as LTα, LTβ, and TNF-related activation-induced cytokine (TRANCE). RORγt+NKp46−CD3−CD4+ gut LTi cells produced IL-22, a cytokine implicated in epithelial repair; and expressed the IL-23 receptor, a key regulator of IL-22 production. These results suggest that overexpression of CXCL13 in the intestine during inflammatory conditions favors mobilization of B cells and of LTi and NK cells with immunomodulatory and reparative functions.
Similar content being viewed by others
Introduction
The mucosal immune system of the gastrointestinal tract is a complex network of distinct lymphoid compartments, organized to ensure defense against pathogen invasion and maintenance of immune tolerance. This system comprises lamina propria (LP) cells, and organized lymphoid structures such as Peyer's patches (PP) and isolated lymphoid follicles (ILF), which function as sites for efficient interaction of antigens, antigen-presenting cells, and lymphocytes.1, 2, 3, 4
ILF result from maturation of pre-existing lymphoid structures called cryptopatches (CP).5 The general assumption is that CP, which are mainly composed of lineage negative (lin−) cells, contain the organizers of inducible tertiary lymphoid structures in the gut.1, 6 ILF resemble PP in spatial organization and cellularity, consisting of a cluster of B cells surrounded by dendritic cells (DC) and few interspersed T cells. In the small intestine, ILF are found predominantly in the distal part of the ileum,7 but their number and size are dependent on the mouse strain.8, 9 Unlike PP, ILF maturation is a post-gestational event that takes place after colonization of the intestine with bacteria10 and is inducible by luminal stimuli.5, 11, 12, 13 ILF formation, similar to PP, requires a lymphotoxin-β receptor (LTβR)-sufficient mesenchymal stromal cell interacting with a hematopoietic cell-expressing lymphotoxin (LT).14 Mice deficient for LTα or LTβR fail to develop ILF.10 Ligation of the LTβR present on stromal cells induces secretion of factors, including chemokines, starting a positive loop of recruitment and stimulation of lymphocytes. The current hypothesis is that this hematopoietic cell is related to fetal lymphoid tissue-inducer (LTi) cells and may be involved in the formation of ILF in the small intestine of the adult mouse.1, 11, 15 However, to date, little is known about their ability to produce cytokines and the mechanisms guiding their migration.
Genetic analyses have uncovered a role for other factors in the formation of ILF. For instance, mice deficient in tumor necrosis factor receptor-I, interleukin (IL)-7, and the retinoic acid-related orphan receptor RORγt, fail to develop ILF.10, 14, 15, 16 Other molecules involved in lymphoid organogenesis in the gut are the chemokine receptors CCR6 and CXCR5. CCR6-deficient mice have underdeveloped PP17 and fail to form mature ILF, in part due to a defect in B-cell influx.18 Although mice deficient in CXCL13 and its receptor CXCR5 present severe defects in the formation of lymph nodes and PP,19 the role of CXCL13 and CXCR5 in the development of ILF has not yet been clarified. Adult CXCR5-deficient mice have been reported to have normal number of solitary intestinal lymphoid tissue, but have reduced number of B cells.20
Lymphoid chemokines, such as CCL19, CCL21, and CXCL13, have an important role in the formation of secondary lymphoid organs and PP.21, 22, 23, 24, 25 Expression of CXCL13, CCL19, and CCL21 has also been reported in ILF,16 but it is unclear what role these chemokines have in ILF formation. CXCL13 appears to contribute to the formation of secondary lymphoid organs in two ways: mediating the influx of mature lymphocytes into the newly forming follicles and regulating the recruitment of LTi cells to the anlagen. Fetal LTi cells express CXCR5,26 but the expression of this chemokine receptor on adult gut LTi cells has not been reported.
Abnormal CXCL13 expression has been detected in various human diseases including rheumatoid arthritis,27 multiple sclerosis,28 Sjögren's disease,29 Hashimoto's thyroiditis,30 myasthenia gravis,31 and in chronic renal inflammation.32 In all these conditions expression of CXCL13 is associated with the presence of B-cell aggregates suggesting that CXCL13 may be directly responsible for their formation. Analysis of transgenic mice-expressing CXCL13 ectopically strongly supports this hypothesis. Transgenic mice-expressing CXCL13 in the pancreas develop intra-pancreatic lymph node-like aggregates rich in B cells,33, 34 suggesting that dysregulation in the expression of CXCL13 may be sufficient to promote development of ectopic lymphoid follicles.
CXCL13 is normally expressed at low levels in the mouse intestine.35 However, inflammatory conditions induced by dextran sulfate sodium35 or trinitrobenzene sulfuric acid treatment36 markedly upregulate its expression. In humans, CXCL13 and CXCR5 are normally expressed in lymphoid follicles in the small and large intestine.37 CXCL13 and CXCR5 expression has also been detected in human inflammatory conditions such as chronic gastritis induced by Helicobacter pylori.38, 39, 40 and in ulcerative colitis.37
To investigate the biological consequences of increased production of CXCL13, we created transgenic mice expressing CXCL13 in intestinal epithelial cells. CXCL13 expression promoted a marked increase in the number of B cells in the lamina propria and an increase in the size and number of lymphoid follicles in the small intestine. Surprisingly, these changes were associated with a marked increase in the numbers of lin−CD3−CD4+ cells that comprised NKp46+ and NKp46− cells expressing the retinoic acid-related orphan receptor RORγt. The RORγt+NKp46− cells expressed CXCR5, the receptor for CXCL13, and other LTi cell markers such as LTα, LTβ, and TRANCE. Unexpectedly this RORγt+NKp46− population also produced IL-22, a cytokine implicated in epithelial repair; and expressed the receptor for IL-23, a key regulator of IL-22 production. These results suggest that overexpression of CXCL13 in the intestine during inflammatory conditions may favor mobilization of B cells, and of LTi and NK cells with immunomodulatory and reparative functions.
Results
CXCL13 expression in the gut promotes marked accumulation of B cells
To mimic the elevated levels of CXCL13 observed in gut inflammatory conditions, we generated transgenic mice carrying the murine CXCL13 cDNA driven by the the mouse villin promoter41 (Supplementary Figure 1a online). Two independent lines (3 and 5) of Villin-CXCL13 mice (VCXCL13 mice) were expanded for analysis. The transgenic (TG) mice were healthy, had a normal lifespan, and did not show gross abnormalities. To examine transgene expression in these animals, we measured CXCL13 protein levels by ELISA in protein extracts from duodenum, jejunum, ileum, and colon. CXCL13 was readily detected in samples from intestinal segments from transgenic mice from both lines (Supplementary Figure 1b online). Necropsy of adult transgenic mice showed that they had normal spleens, and a normal complement of peripheral lymph nodes (cervical, mesenteric, para-aortic, axillary, peripancreatic, and brachial lymph nodes). However, all VCXCL13 mice examined lacked PP and some animals lacked popliteal and/or inguinal lymph nodes ( Table 1). The relative number of B cells in the blood, spleen, and mesenteric lymph nodes of VCXCL13 mice were similar to those of wild-type (WT) mice (Supplementary Figure 1c online).
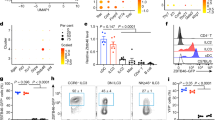
Next, we examined the cellularity of the intestine of WT and VXCL13 mice by flow cytometry. Expression of CXCL13 promoted a dramatic increase (11- to 15-fold) in the number of B cells in the intestine (Figure 1a). The vast majority of CD19+ B cells present in the small intestine of VCXCL13 TG mice expressed IgM (Figure 1b) and were B2 B cells (Figure 1c). No statistically significant differences were observed in the number of B1 B cells between WT and VCXCL13 mice. The T cell and myeloid compartments were not affected by expression of CXCL13 (Figure 1a and data not shown). Together these results indicate that increased expression of CXCL13 in the intestine promotes marked changes in the number of B cells in the intestine.
CXCL13 expression in the gut promotes B-cell accumulation. (a-b) Flow cytometric analysis of B and T cells (a) and IgM+ cells (b) in small intestine of wild-type (WT) and VCXCL13 transgenic (TG) mice. Analysis was carried out on CD45+ cells. The bar graphs on the right show absolute number of cells (WT n=6, TG3 n=3, TG5 n=7; **P=0.005, ***P<0.0001). (c) Flow cytometric analysis of B1/B2 B cells. B1 B cells are defined as B220+CD11b+CD5+ cells. Analysis was done on CD45+ cells. The bar graph on the right shows absolute number of cells (WT n=2, TG3 n=3, TG5 n=4; **P=0.005).
CXCL13 expression causes a marked increase in the number and size of ILF
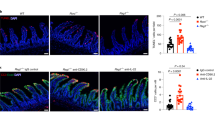
To analyze the distribution of the B cells in the intestine we performed immunostaining. Analysis of the small intestine of VCXCL13 mice showed the presence of large B220+ B-cell aggregates resembling ILF and an increase in numbers of scattered B cells (Figure 2a). These enlarged ILF were less compact than those found in WT mice, but similar to ILF found in WT mice, they contained B cells, CD11c+ DC, IL7Rα+ cells and scattered T cells (Figure 2b).
CXCL13 promotes formation of large ILF. (a) Horizontal sections of the small intestine from wild-type (WT) and transgenic (TG) mice stained with anti-B220 antibodies (green). B cells cluster forming large aggregates in TG mice. (b) Immunostaining for B220 (green) and CD3, CD11c, and IL7Rα (red) in the small intestine of WT and VCXCL13 (TG) mice. Scale bars=100 μm.
Next, we quantified the size and the number of ILF in the intestine. To do so, we defined ILF as structures containing both IL7Rα+ and B220+ cells (Figure 3a and not shown). The mean size of the aggregates in both the jejunum and ileum of VCXCL13 mice was significantly higher than in WT littermates (Figure 3b). Surprisingly, the number of ILF was also dramatically increased in jejunum and ileum (Figure 3c). No differences were observed in the duodenum (Figure 3c). These results indicate that expression of CXCL13 in the intestinal epithelium increases the number and the size of ILF in jejunum and ileum.
CXCL13 expression increases the size and the number of ILF in the gut. (a) Immunostaining for IL7Rα was used to identify ILF in horizontal sections of the gut of wild-type (WT) and transgenic (TG) mice. Scale bars=100 μm. (b) ILF size in jejunum and ileum of WT and VCXCL13 (TG) mice. (c) Quantification of ILF in different segments of the small intestine (d=duodenum, j=jejunum, i=ileum, ***P<0.0001).
CXCL13 expression in epithelial cells induces mobilization of B cells to the intestine
The increase in the number and size of ILF could be due to an increase in the recruitment and/or accumulation of B cells in the intestine. To investigate if expression of CXCL13 promoted the migration of B cells towards the intestine we crossed VCXCL13 mice with Rag−/− mice to generate VCXCL13/Rag−/− mice and performed adoptive transfer studies. B cells (107 cells) were isolated from spleens of WT mice and transferred into VCXCL13/Rag−/− mice and littermate controls (Rag−/− mice). One week after transfer, spleen and lamina propria cells were isolated and analyzed by flow cytometry to investigate the presence of B cells. As shown in Figure 4a, we did not see statistically significant differences in the number of CD19+ B cells found in the spleen of both recipients. However, analysis of lamina propria cells showed that VCXCL13/Rag−/− mice had an increase in both relative and absolute number of CD19+ B cells (Figure 4b), suggesting that CXCL13 expression promotes increased recruitment of B cells into the intestine.
CXCL13 expression promotes increased migration of B cells to the intestine. Flow cytometric analysis of CD19+ B cells in the spleen (a) and LPL of small intestine (b) from Rag−/− (n=5) and VCXCL13/Rag−/− (n=5) recipient mice 1 week after transfer of B cells (**P=0.001).
CXCL13 expression in the gut increases the number of CD3−CD4+IL7Rα+ cells
Formation of ILF appears to be dependent on the presence of CD3−CD4+IL7Rα+ cells that resemble fetal LTi.1, 11, 15 The increased number of ILF in the gut of VCXCL13 mice prompted us to investigate if the expression of CXCL13 resulted in the expansion of such a cell population. In WT mice CD3−CD4+ cells represented ∼2% of CD45+ cells present in the lamina propria of the small intestine (Figure 5a). These CD3−CD4+ cells were IL7Rα+ and c-kit+ and did not express the myeloid markers CD11c and CD11b (Figure 5c and data not shown). Remarkably, we found that the absolute number of CD3−CD4+IL7Rα+ cells was significantly increased in VCXCL13 mice (Figure 5b). These findings indicate that expression of CXCL13 affects the number of CD3−CD4+IL7Rα+ cells in the gut.
CXCL13 increases the number of LTi cells in the gut. (a) Flow cytometric analysis of CD3−CD4+ cells in the LPL from small intestine of wild-type (WT) and transgenic (TG) mice. Analysis was done on CD45+ cells. (b) Absolute numbers of CD3−CD4+IL7Rα+ cells (n=5; **P<0.0097). (c) IL7Rα, c-kit and CD11c expression in CD3−CD4+ cells isolated from the VCXCL13 gut. The dashed line represents staining with the isotype control. (d-f) Flow cytometric analysis of LPL from small intestine of RORγt (n=4) and RORγt/VCXCL13 (n=5) mice. Shown are the relative (d–f plots) and absolute numbers (d–f bar graphs) of CD45+RORγtGFP+ cells (d), RORγtGFP+NKp46+ and RORγtGFP+NKp46− cells (e), and CD3−CD4+ cells (f). Cells in (e) were gated on the R1 subset and cells in (f) were gated on the R2 subset (**P=0.001, ***P=0.0004, *P=0.02).
CXCL13 expression in the gut increases the number of RORγtGFP+ cells
Fetal CD3−CD4+IL7Rα+ LTi cells express the Rorc(γt) gene. To test if CXCL13 expression promoted an increase in the number of RORγt+ cells found in the adult intestine, we crossed VCXCL13 mice with mice in which the EGFP reporter gene was inserted in the Rorc(γt) locus15 (referred to here as RORγt mice). Double heterozygous RORγt/VCXCL13 mice were generated for the analysis presented here. We then evaluated the presence of RORγtGFP+ cells in the lamina propria of RORγt/VCXCL13 mice and RORγt littermates by flow cytometry. We found that in RORγt mice, the RORγtGFP+ population represented ∼6% of the total CD45+ cells (Figure 5d). Although the relative number of such cells was decreased in RORγt/VCXCL13 mice, their absolute number was significantly increased compared with RORγt controls (Figure 5d). These results indicate that expression of CXCL13 induced the expansion of RORγt+ cells in the intestine.
RORγtGFP+NKp46−CD3−CD4+ LTi cells in the gut produce IL-22
It has been recently shown that RORγt cells present in the adult spleen42 and gut43, 44 express the NK cell marker NKp46 and that they produce IL-22. Additional analyses of the RORγtGFP+ population showed that it could be further divided into two subsets on the basis of NKp46 expression (Figure 5e). Both RORγtGFP+NKp46+ and RORγtGFP+NKp46− subsets were increased in the intestine of the RORγt/VCXCL13 mice. Most RORγt+ cells were NKp46+ and did not express surface markers CD3 or CD4. The CD3−CD4+ cells were present within the RORγtGFP+NKp46− population and their numbers were also elevated in the RORγt/VCXCL13 mice (Figure 5f).
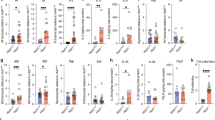
To investigate the mechanisms underlying the recruitment and function of the RORγtGFP+ cells we sorted them from the intestine of RORγt/VCXCL13 mice and analyzed mRNA expression by Q-PCR (Figure 6a). We found that the RORγtGFP+NKp46−CD3−CD4+ cells preferentially expressed LTα, LTβ, and TRANCE molecules expressed by LTi cells (Figure 6b). Based on this gene expression profile we have termed these RORγtGFP+NKp46−CD3−CD4+ cells, LTi cells. Next, we examined the surface expression of the chemokine receptors CXCR5 and CCR6 on LTi cells by flow cytometry. We found that gut LTi cells expressed CXCR5 and CCR6 (Figure 6c), and that over 30% of them expressed both chemokine receptors (Figure 6d). Takatori et al.42 have shown that spleen LTi cells express IL-17 and IL-22 mRNA. We then examined expression of IL-17 and IL-22 in freshly sorted LTi and B cells by Q-PCR. We found that similar to splenic LTi, gut LTi expressed low, but detectable amounts of IL-17. In contrast, IL-22 was abundantly expressed in freshly sorted LTi cells (Figure 6e). As IL-22 production is stimulated by IL-23,45, 46 we next asked whether IL-23R was expressed by gut LTi cells. As shown in Figure 6f, IL-23R mRNA was detected in LTi cells but not in B cells. To investigate if gut LTi cells secreted IL-22, we placed LTi and B cells in culture and measured IL-22 by ELISA in supernatants 24 h later. IL-22 was detected in the supernatants of LTi cells, but not in B-cell supernatants. Addition of IL-23 to the culture media promoted a significant increase in the levels of IL-22 in the culture supernatants from LTi cells, but not from B cells. These results confirm IL-22 production by LTi cells and show that they respond to IL-23.
LTi cells in the gut produce IL-22. (a) Representative plots showing the surface markers used to sort NKp46 cells and LTi cells from the gut of RORγt/VCXCL13 mice. The NKp46 population is RORγtGFP+NKp46+ and the LTi population is RORγtGFP+NKp46−CD3−CD4+. (b) Q-PCR analysis on total RNA isolated from sorted NKp46 and LTi cells. (c and d) Surface expression of CXCR5 and CCR6 on LTi cells. (c) The shaded histogram indicates staining with isotype-matched control antibodies. (d) Note that 30% of LTi cells express both chemokine receptors. (e and f) IL-17, IL-22 (e) and IL-23R (f) mRNA expression in sorted LTi and B cells. (g) Sorted LTi and B cells were cultured in the absence (unstimulated) or presence of IL-23 for 24 h and IL-22 was measured in culture supernatants by ELISA.
Discussion
In this study, we show that expression of the chemokine CXCL13 by epithelial cells in the intestine promotes an increase in the number and the size of ILF in the gut. These changes were associated with increased accumulation of B cells and RORγt+ cells that expressed CXCR5, the receptor for CXCL13. The findings reported here besides documenting a role for CXCL13 in regulation of B cell trafficking into the lamina propria, directly implicate CXCL13 and CXCR5 in the trafficking of RORγt+ immunomodulatory cells capable of producing IL-22.
ILF formation is the result of B cell influx into pre-existing lymphoid structures called CP, mainly composed of lin− cells.5 The initial description of CP as clusters of cells containing a subset of lin−CD3−CD4+ cells expressing c-kit and IL-7Rα47 suggested the presence of precursor cells in the adult gut. These cells phenotypically resemble fetal LTi cells15 and may represent their adult counterpart. Here, we show a significant increase in the number of such cells in transgenic mice overexpressing CXCL13. Furthermore, phenotyping of these cells showed that they were NKp46−CD3−CD4+IL-7Rα+ and that they expressed markers present in fetal LTi, such as RORγt, LTα, LTβ, and TRANCE. Expression of these markers was not detected in the CD3+CD4+ T-cell population (data not shown) or in the RORγt+NKp46+ population. In addition, these RORγt+NKp46−CD3−CD4+ cells expressed CXCR5, adding support to the concept that LTi-cell mobilization is mediated by CXCR5 and CXCL13. Importantly, such LTi cells were located within large aggregates of B cells and were surrounded by varying number of DC and scattered T cells. The coexistence of LTi cells, B cells and DC within the ILF, rather than their homogeneous distribution throughout the lamina propria suggests the existence of additional signals other than CXCL13 for the formation of ILF. The nature of such signals is unknown at present.
Ectopic expression of CXCL13 in the pancreatic islets of transgenic mice results in the accumulation of B and T lymphocytes and the formation of tertiary lymphoid structures.33, 34 Early in development (E18.5) such pancreatic aggregates contain CD3−CD4+IL7Rα+ cells,48 but is unclear if such cells persist in the adult mouse. Unlike the pancreatic aggregates, those induced by CXCL13 expression in the intestine are less compact, have fewer T cells and contain a higher number of LTi cells. Thus, this report describes for the first time that CXCL13 promotes accumulation of LTi cells in the adult.
Transgenic expression of CXCL13 in the epithelial cells of the intestine caused a dramatic influx of B cells in the gut and formation of increased numbers of ILF that were larger than those of WT mice. Interestingly, these changes were associated with defects in PP and lymph node formation suggesting a developmental defect. Embryonic expression of CXCL13 driven by the villin promoter may have desensitized or misdirected LTi cells and prevented their proper localization in the PP anlagen. The lack of such cells during a critical developmental period could have prevented the activation of stromal cells by LTβR ligands. Indeed, antagonism of LTβR signaling during uterine life leads to defects in PP organogenesis.14 Animals that are treated in the uterus with an LTβR antagonist do not develop PP, but ILF formation can occur once the animals mature.14 This suggests that the mechanisms for PP and ILF formation may be different. PP formation may require classical fetal LTi and LTβR signaling, whereas ILF formation may not necessarily require LTβR signaling if an extraneous source of chemokines, such as CXCL13 or CCL21 can be provided. As several cell types, including epithelial cells, can express such chemokines, it is possible that lymphoid aggregates can be formed in the absence of LTβR signaling. In fact, well-organized lymphoid aggregates are present in the thyroid of animals expressing CCL21 in thyrocytes,49, 50 even in the absence of Id2-dependent LTi and LTβR signaling. Genetic deletion of LTβR in VCXCL13 mice will be required to test the role of LTβR signaling in ILF formation promoted by overproduction of CXCL13. The generation of such mutant mice should also help define the role of LTβR signaling, LTi and B cells in the production of IgA in the intestine. Recent work by the Fagarasan group11 suggests a critical role for LTi and LTβr signaling in T-cell independent production of IgA by ILF.
Our results suggest that adult gut LTi cells may also be an important source of immunomodulatory molecules. We show here that gut LTi, similar to RORγt+NKp46+ NK cells,43, 44, 51 represent a source of IL-22 in the intestinal mucosa. As shown in Figure 6, freshly isolated gut LTi cells express IL-22 and IL-23R mRNA. IL-22 protein is secreted by gut LTi in culture and addition of IL-23 promotes an increase in the levels of IL-22 in the supernatants. These results confirm and expand results obtained by Takatori et al.,42 who showed expression of IL-22 mRNA by gut LTi after stimulation with IL-7 and IL-23. Maximal production of IL-22 by gut LTi and RORγt+NKp46+ NK cells in vivo may thus require concomitant activation of DC and phagocytic cells, the main cellular sources of IL-23.52
IL-22 is a member of the IL-10 cytokine family, that is expressed by Th17 CD4+ T cells, NK cells, NKT cells, and CD8+ cells.43, 44, 53 The increased production of IL-22 in the mucosa by RORγt+NKp46+ NK cells and gut LTi cells may have important functions, because it has been shown that ligation of the IL-22R expressed by gut epithelial cells53, 54 promotes the expression of antimicrobial proteins and molecules involved in tissue repair45, 46, 55, 56 and protects from inflammatory bowel disease in both innate and T-cell-mediated models of colitis.53 Thus, by providing cues for the recruitment of LTi cells, CXCL13 may be an important component in pathways leading to epithelial homeostasis and repair.
Our results suggest that adult LTi cell recruitment may be regulated by chemokine ligands acting on at least two chemokine receptors: CXCR5 and CCR6. A role for CXCR5 in LTi mobilization has been inferred by its expression in fetal LTi57 and by the fact that its genetic ablation induces defects in PP and ILF formation.17, 18 Of interest is the observation that adult gut LTi express CCR6. CCR6 is also expressed by 70% of cells found in CP, mainly lin−c-kit+ precursor cells,58 which suggests that similar to CXCR5, CCR6 may participate in the recruitment of B cells and LTi cells to ILF. We now show that CCR6, besides being a marker for RORγt-expressing Th17 cells,59 is expressed by gut LTi cells. The co-expression of CXCR5 and CCR6 on LTi cells suggest the existence of redundant pathways for their recruitment. It will be of interest to examine if intestinal overexpression of CCL20, the ligand for CCR6, or deletion of both chemokine receptors, will promote changes in the number of LTi cells and ILF.
We have shown here that expression of CXCL13 induces accumulation of LTi cells in the gut, but it is unclear whether this is due to their continuous recruitment, extension of lifespan, or proliferation in situ. RORγt cells are present in the intestinal lamina propria from the fetus to adulthood, but it is unclear if the cells found in adults are fetal LTi cells that have persisted postnatally, or whether they have been recruited from elsewhere.15 As the cells in the lymphoid organ anlagen do not proliferate,60 their accumulation, as observed here, may be the result of active recruitment. The recent observation that transplantation of RORγt+ bone marrow cells from adult mice can induce ILF formation in RORγt−/− adult recipients provide direct evidence that LTi cells can be recruited into the intestine and suggest that they give origin to ILF.11
In summary, our results show that expression of the chemokine CXCL13 in the intestinal epithelium promotes recruitment of B cells and of IL-22-producing LTi cells and formation of large lymphoid follicles. Together the results suggest that increased expression of CXCL13 observed during inflammation may be an important factor for the generation of protective mucosal responses.
Methods
Mice. The plasmid containing the mouse villin promoter (pBS-Villin) has been previously described.41 The mouse CXCL13 cDNA (ATG to STOP) was cloned downstream of the villin promoter.41 The transgene was isolated from the resulting plasmid by Sal I digestion, gel purified, and injected into mouse eggs using standard techniques. Identification of the transgenic mice was accomplished by PCR amplification of mouse tail DNA using the following primers: 5′-TAGGAAGCCAGTTTCCCTTC-3′ and 5′-CTTGGGGAGTTGAAGACAG-3′. Rag−/− and RORγt+/− mice were obtained from The Jackson Laboratory (Bar Harbor, Maine). In all experiments, genetically modified mice were compared with their corresponding control littermates. Mice were kept under pathogen-free conditions. All experiments involving animals were performed following guidelines of the Animal Care and Use Committee of the Mount Sinai School of Medicine.
Isolation of lamina propria leukocytes (LPL). Lamina propria leukocytes were isolated as previously described with some modifications.61 Briefly, the small intestine was removed, flushed with ice-cold calcium- and magnesium-free Hank's balanced salt solution, and freed of fat, mesentery, and PP. The tissue was then cut into small pieces about 2 cm in length and washed two times with Hank's balanced salt solution and then, incubated with PBS with 1.3 mm EDTA for 1 h at 37°C. Fragments of intestine were further incubated in 7 ml of 2 mg ml–1 collagenase D (Roche, Indianapolis, IN) in RPMI for 1 h at 37°C and lamina propria leukocytes were isolated after 38% Percoll density gradient.
Flow cytometric analysis. Cells from LPL, spleen or mesenteric lymph nodes were blocked with Fc block (eBioscience, San Diego, CA) for 20 min at 4°C and stained with different antibodies against several markers. The antibodies used were from BD Biosciences (San Jose, CA) and eBioscience. The CCR6 antibody was from R&D Systems (Minneapolis, MN). Events were acquired on a Becton Dickinson (Franklin Lakes, NJ) FACScan and analyzed using FlowJo software (Tree Star, Ashland, OR). Analyses were performed in alive cells. To sort cells from the small intestine of RORγt/VCXCL13 mice, cells were depleted of B cells using MACS beads (Miltenyi Biotec, Auburn, CA) following manufacturer's instructions. Cells were sorted on a FACSAria II cell sorter (Becton Dickinson) according to standard protocols.
Measurement of CXCL13 and IL-22 levels by enzyme-linked immunosorbent assay (ELISA). Total CXCL13 concentration in intestinal tissue extracts was determined using an ELISA kit from R&D Systems (Minneapolis, MN), following the manufacturer's instructions. Two different pieces from each segment were analyzed. The amount of chemokine is expressed as pg mg–1 tissue protein. IL-22 protein levels were determined in the supernatants of cells sorted from the intestine of RORγt/VCXCL13 mice. Briefly 2 × 104 LTi and B cells were cultured in the absence or presence of 20 ng ml–1 of IL-23 (R&D Systems) in RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine, and 100 U ml–1 Pen-Strep, for 24 h at 37°C Supernatants of these cultures were removed and IL-22 protein content was assessed by ELISA (R&D Systems).
RNA analysis. Total RNA was extracted using the RNeasy mini Kit (Qiagen, Valencia, CA), according to the manufacturer's instructions. Quantitative PCR (Q-PCR) was conducted in duplicate. Relative expression levels were calculated as 2^(Ct Ubiquitin-Ct gene) using Ubiquitin RNA as the endogenous control. Primers were designed using primer express 2.0 software (Applied Biosystems, Foster City, CA). Primer sequences are ubiquitin (Forward=TGGCTATTAATTATTCGGTCTGCAT, Reverse=GCAAGTGGCTAGAGTGCAGAGTAA); CXCR5 (F=ATATGGATGACCTGTACAAGGAACTG, R=CCTCGACTGTAGAGCAGAAGTTACTG); CCR6 (F=CCCCGTGTTGTATGCGTTTATTG, R=GCATTATCATTTTCGACGGTCTC); IL-17A (F=GAAGCTCAGTGCCGCCA, R=TTCATGTGGTGGTCCAGCTTT); IL-22 (F=CATGCAGGAGGTGGTACCTT, R=CAGACGCAATTTCTCAG); IL-23R (F=GGTCTTCTTGGCCATCATGT, R=AGCCACTTTGGGATCATCAG); CCR7 (F=TCCAGGCACGCAACTTTGA, R=CCACCACGGCAATGATCAC); LTα (F=CCAGGACAGCCCATCCACT, R=GTACCCAACAAGGTGAGCAGC); LTβ (F=ACCTCATAGGCGCTTGGATG, R=ACGCTTCTTCTTGGCTCGC) and TRANCE (F=GGTTAACCAAGATGGCTTCTATTACC, R=CGCTTCCCGATGTTTCATG).
Immunostaining of frozen sections. Small intestines were cleaned, opened longitudinally and flushed with cold PBS. The whole tissue was divided in segments (duodenum, jejunum, ileum), stapled on cardboard and fixed in PFA 4% for 1 h at 4°C, followed by overnight incubation in 20% sucrose. Small flattened sheets of tissue (25 mm) were placed in cryomold cassettes, covered with OCT freezing medium, and quick-frozen in methylbutane on dry ice. Horizontal sections of 12 μm thickness through the crypt zone were cut with a cryostat, air dried, and stained. Primary antibodies used were: anti-CD3, B220, IL7Rα CD11c from eBioscience. Secondary antibodies used were Alexa Fluor 488 and 594 goat anti-rat IgG from Molecular Probes (Invitrogen, Eugene, OR) and Cy3 goat anti-Armenian hamster and Cy5 goat anti-rat (Jackson ImmunoResearch Laboratories, West Grove, PA). Briefly, primary antibodies were incubated for 1 h at room temperature, followed by incubation with the appropriate fluorescent-labeled secondary antibodies for 30 min. The slides were then washed and mounted with Fluoromount-G (SouthernBiotech, Birmingham, AL). Analysis was performed using a Nikon fluorescence microscope. In some instances, images were overlaid and processed using Adobe Photoshop version 7.0. Microscopic quantification and measurement of ILF was performed by automated image analysis with the Volocity software (Improvision, Waltham, MA). Cryptopatches (IL7Rα+B220−) were excluded from the analysis.
Cell purification and transfer. Single-cell suspension of splenocytes from C57Bl/6 mice was prepared and incubated with anti-B220 beads (Miltenyi Biotec). Cells were then washed and passed through a magnetic cell-sorting column (Miltenyi Biotec). The resulting fraction contained >95% B220+ B cells. B220+ B cells (11 × 106) were resuspended in 100 μl of PBS and injected into the retro-orbital sinus of recipient animals.
Statistical analysis. Statistical analysis of the data was performed using Prism 5.0a (GraphPad Software). Data in the text were given as means±s.e. unless otherwise stated. Unpaired Student's t-test was used to determine statistical significance. Differences were considered significant when P<0.05.
Disclosure
The authors declare no conflict of interest.
References
Eberl, G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat. Rev. Immunol. 5, 413–420 (2005).
Forster, R., Pabst, O. & Bernhardt, G. Homeostatic chemokines in development, plasticity, and functional organization of the intestinal immune system. Semin. Immunol. 20, 171–180 (2008).
Newberry, R.D. & Lorenz, R.G. Organizing a mucosal defense. Immunol. Rev. 206, 6–21 (2005).
Brandtzaeg, P., Kiyono, H., Pabst, R. & Russell, M.W. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal. Immunol. 1, 31–37 (2008).
Pabst, O. et al. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J. Immunol. 177, 6824–6832 (2006).
Lugering, A. & Kucharzik, T. Induction of intestinal lymphoid tissue: the role of cryptopatches. Ann. N. Y. Acad. Sci. 1072, 210–217 (2006).
Newberry, R.D., McDonough, J.S., McDonald, K.G. & Lorenz, R.G. Postgestational lymphotoxin/lymphotoxin beta receptor interactions are essential for the presence of intestinal B lymphocytes. J. Immunol. 168, 4988–4997 (2002).
Pabst, O. et al. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur. J. Immunol. 35, 98–107 (2005).
Lorenz, R.G. & Newberry, R.D. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann. N. Y. Acad. Sci. 1029, 44–57 (2004).
Hamada, H. et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 168, 57–64 (2002).
Tsuji, M. et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity 29, 261–271 (2008).
Bouskra, D. et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510 (2008).
Fagarasan, S. et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 298, 1424–1427 (2002).
Lorenz, R.G., Chaplin, D.D., McDonald, K.G., McDonough, J.S. & Newberry, R.D. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J. Immunol. 170, 5475–5482 (2003).
Eberl, G. & Littman, D.R. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgamma t+ cells. Science 305, 248–251 (2004).
McDonald, K.G., McDonough, J.S. & Newberry, R.D. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J. Immunol. 174, 5720–5728 (2005).
Varona, R. et al. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J. Clin. Invest. 107, R37–45 (2001).
McDonald, K.G. et al. CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles. Am. J. Pathol. 170, 1229–1240 (2007).
Ansel, K.M. et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406, 309–314 (2000).
Velaga, S. et al. Chemokine receptor CXCR5 supports solitary intestinal lymphoid tissue formation, B cell homing, and induction of intestinal IgA responses. J. Immunol. 182, 2610–2619 (2009).
Okada, T. et al. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J. Exp. Med. 196, 65–75 (2002).
Warnock, R.A. et al. The role of chemokines in the microenvironmental control of T vs. B cell arrest in Peyer's patch high endothelial venules. J. Exp. Med. 191, 77–88 (2000).
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 99, 23–33 (1999).
Drayton, D.L., Liao, S., Mounzer, R.H. & Ruddle, N.H. Lymphoid organ development: from ontogeny to neogenesis. Nat. Immunol. 7, 344–353 (2006).
Mebius, R.E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3, 292–303 (2003).
Mebius, R.E., Rennert, P. & Weissman, I.L. Developing lymph nodes collect CD4+CD3− LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997).
Heller, F. et al. The contribution of B cells to renal interstitial inflammation. Am. J. Pathol. 170, 457–468 (2007).
Aloisi, F. et al. Lymphoid chemokines in chronic neuroinflammation. J. Neuroimmunol. 198, 106–112 (2008).
Barone, F. et al. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjogren's syndrome. Arthritis. Rheum. 52, 1773–1784 (2005).
Aust, G. et al. The role of CXCR5 and its ligand CXCL13 in the compartmentalization of lymphocytes in thyroids affected by autoimmune thyroid diseases. Eur. J. Endocrinol. 150, 225–234 (2004).
Le Panse, R. et al. Regulatory and pathogenic mechanisms in human autoimmune myasthenia gravis. Ann. N. Y. Acad. Sci. 1132, 135–142 (2008).
Vermi, W. et al. Role of dendritic cell-derived CXCL13 in the pathogenesis of Bartonella henselae B-rich granuloma. Blood 107, 454–462 (2006).
Luther, S.A., Lopez, T., Bai, W., Hanahan, D. & Cyster, J.G. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity 12, 471–481 (2000).
Martin, A.P., Canasto-Chibuque, C., Shang, L., Rollins, B.J. & Lira, S.A. The chemokine decoy receptor M3 blocks CC chemokine ligand 2 and CXC chemokine ligand 13 function in vivo. J. Immunol. 177, 7296–7302 (2006).
Shang, L. et al. Expression of the chemokine binding protein M3 promotes marked changes in the accumulation of specific leukocytes subsets within the intestine. Gastroenterology (2009). (In press)
Abad, C. et al. cDNA array analysis of cytokines, chemokines, and receptors involved in the development of TNBS-induced colitis: homeostatic role of VIP. Inflamm. Bowel. Dis. 11, 674–684 (2005).
Carlsen, H.S., Baekkevold, E.S., Johansen, F.E., Haraldsen, G. & Brandtzaeg, P. B cell attracting chemokine 1 (CXCL13) and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue. Gut. 51, 364–371 (2002).
Mazzucchelli, L. et al. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J. Clin. Invest. 104, R49–54 (1999).
Galamb, O. et al. Helicobacter pylori and antrum erosion-specific gene expression patterns: the discriminative role of CXCL13 and VCAM1 transcripts. Helicobacter 13, 112–126 (2008).
Hofman, V.J. et al. Gene expression profiling in human gastric mucosa infected with Helicobacter pylori. Mod. Pathol. 20, 974–989 (2007).
Pinto, D., Robine, S., Jaisser, F., El Marjou, F.E. & Louvard, D. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J. Biol. Chem. 274, 6476–6482 (1999).
Takatori, H. et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206, 35–41 (2009).
Luci, C. et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 10, 75–82 (2009).
Sanos, S.L. et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 10, 83–91 (2009).
Liang, S.C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Zheng, Y. et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007).
Kanamori, Y. et al. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J. Exp. Med. 184, 1449–1459 (1996).
Luther, S.A., Ansel, K.M. & Cyster, J.G. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J. Exp. Med. 197, 1191–1198 (2003).
Marinkovic, T. et al. Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J. Clin. Invest. 116, 2622–2632 (2006).
Furtado, G.C. et al. Lymphotoxin beta receptor signaling is required for inflammatory lymphangiogenesis in the thyroid. Proc. Natl. Acad. Sci. USA 104, 5026–5031 (2007).
Satoh-Takayama, N. et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008).
Kastelein, R.A., Hunter, C.A. & Cua, D.J. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 25, 221–242 (2007).
Zenewicz, L.A. et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29, 947–957 (2008).
Wolk, K. et al. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004).
Aujla, S.J. et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14, 275–281 (2008).
Ouyang, W., Kolls, J.K. & Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467 (2008).
Honda, K. et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer's patch organogenesis. J. Exp. Med. 193, 621–630 (2001).
Lugering, A. et al. Lymphoid precursors in intestinal cryptopatches express CCR6 and undergo dysregulated development in the absence of CCR6. J. Immunol. 171, 2208–2215 (2003).
Hirota, K. et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 204, 2803–2812 (2007).
Eberl, G. & Littman, D.R. The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer's patches. Immunol. Rev. 195, 81–90 (2003).
Lefrancois, L., Barrett, T.A., Havran, W.L. & Puddington, L. Developmental expression of the alpha IEL beta 7 integrin on T cell receptor gamma delta and T cell receptor alpha beta T cells. Eur. J. Immunol. 24, 635–640 (1994).
Acknowledgements
This work was supported by NIH Grants P01 DK072201 (SAL and LM), New York Crohn's Foundation and CCFA (LM). Federica Marchesi was funded by the International Union Against Cancer (UICC) and a fellowship from the Innochem Project. We thank Limin Shang and Luciana R Muniz for technical help.
Author information
Authors and Affiliations
Corresponding author
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/mi
Supplementary information
Rights and permissions
About this article
Cite this article
Marchesi, F., Martin, A., Thirunarayanan, N. et al. CXCL13 expression in the gut promotes accumulation of IL-22-producing lymphoid tissue-inducer cells, and formation of isolated lymphoid follicles. Mucosal Immunol 2, 486–494 (2009). https://doi.org/10.1038/mi.2009.113
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2009.113
This article is cited by
-
Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer
Cellular & Molecular Immunology (2019)
-
The EBI2-oxysterol axis promotes the development of intestinal lymphoid structures and colitis
Mucosal Immunology (2019)
-
IL-23 activates innate lymphoid cells to promote neonatal intestinal pathology
Mucosal Immunology (2015)
-
Genetic polymorphisms and tissue expression of interleukin-22 associated with risk and therapeutic response of gastric mucosa-associated lymphoid tissue lymphoma
Blood Cancer Journal (2014)
-
TNFα-dependent development of lymphoid tissue in the absence of RORγt+ lymphoid tissue inducer cells
Mucosal Immunology (2014)