Abstract
Understanding the role of homing receptors could aid vaccine strategies for developing distal mucosal immunity. Infection studies have revealed that immune intestinal B cells use α4β7 homing receptors, but their role in subsequent oral immunization with soluble antigens is unknown. To assess the influence of L-selectin and α4β7 on distal B cells following oral cholera toxin (CT) immunization, L-selectin-deficient (L-Sel−/−) IgA anti-CT-B-specific B cells were enhanced 30-, 9.2-, and 3.5-fold in head and neck lymph nodes (HNLNs), nasal-associated lymphoid tissue, and nasal passages (NPs), respectively, vs. L-Sel+/+ mice. Cell-sorted intestinal and NP IgA antibody-forming cells (AFCs) were mostly α4β7+, unlike HNLN L-Sel−/− IgA and IgG anti-CT-B AFCs that were αEβ7+, contrasting with L-Sel+/+ HNLN IgA AFCs that were mostly α4β7+. In vitro studies revealed that L-Sel−/− HNLN B cells preferentially expressed αE following polyclonal stimulation. These studies show that HNLN B cells express αEβ7 in the absence of L-selectin to sustain distal IgA responses.
Similar content being viewed by others
Introduction
The mucosal immune system is comprised of inductive tissues where antigen (Ag) is first taken up and processed for recognition by naive lymphocytes. Subsequently, these B and T lymphocytes proliferate and differentiate, ultimately emigrating to mucosal effector tissues or what is referred to as the common mucosal immune system.1, 2, 3, 4 and 5 While past interests were in devising oral vaccines for respiratory diseases and adapting the common mucosal immune system to obtain protective immunity,6, 7 studies have shown that intranasal (IN) delivery is feasible with most Ags,8 as evidenced by recent influenza vaccines.9 Consequently, mucosal immunization studies have focused mostly on evaluating the site of vaccination, e.g., oral immunization and gastrointestinal tract immunity, and nasal immunization and upper and lower respiratory tract responses.
Understanding how immune lymphocytes migrate and which homing receptors are necessary to seed these distal mucosal compartments, specifically the upper respiratory tract, could enable improved vaccine efficacy. In the light of this, lymphocyte expression of the mucosal or intestinal homing receptor α4β7 has been accepted as the principal mode of interaction with the mucosal addressin cell adhesion molecule-1 (MAdCAM-1).10, 11, 12, 13, 14 and 15 Such an interaction appears to play a secondary role for lymphocytes migrating to non-intestinal mucosal tissues, as the ability to secrete immune antibodies (Abs) in nasal and reproductive tract secretions was significantly delayed in L-selectin-deficient (L-Sel−/−) mice following IN immunization with the potent mucosal adjuvant, cholera toxin (CT16, 17). Furthermore, the nasal-associated lymphoid tissue (NALT18), as well as the head and neck lymph nodes (HNLNs19), primarily expresses peripheral node addressin with variable expression of MAdCAM-1, and the majority of lymphocyte binding in these sites is mediated by L-Sel–peripheral node addressin interactions. These L-Sel–peripheral node addressin interactions have also been shown to be important for lymphocyte homing to the nasal passages (NPs) and lungs in humans and sheep.20, 21 and 22 Such results suggest that peripheral homing receptor-addressin interactions play an important role in lymphocyte trafficking to the non-intestinal inductive sites. However, other homing receptor interactions may have an important role in trafficking to these sites, especially after mucosal immunization. For instance, IN immunization with CT results in the stimulation of a novel gut (distal) B-lymphocyte subset expressing αEβ7, 16 and this unique subset of cells has also been found in the HNLNs, subsequent to long-term CT immunization.17 Furthermore, the NALT and HNLN express varied levels of MAdCAM-1, and it is conceivable that some B-lymphocyte trafficking to these sites might occur via α4β7 interactions.
Studies in humans have revealed that L-Sel, as well as α4β7, is expressed by B lymphocytes stimulated via oral immunization,4, 5, 23 suggesting a role for L-Sel in the production of immunity in the intestinal effector site. Likewise, L-Sel−/− mice orally immunized with a Salmonella vaccine fail to induce gut IgA responses to the Salmonella's passenger Ag, even though peripheral Ab and T-cell responses are induced.24 Although our previous studies have shown that early induction of non-intestinal immunity is dependent upon L-Sel, 16 it remains to be determined how the effect of the loss of L-Sel will impact immune responses in the gut after soluble Ag delivery. Results from rotavirus infection studies clearly show the dependence on gut α4β7high B cells to mediate protection, 25 but differences in the routes of Ag composition (live vs. soluble) and deposition may help explain why differences in homing receptors’ dependency are observed between infections and immunizations with soluble Ags.
In the light of our past studies, we questioned whether oral CT delivery in L-Sel−/− mice would negatively impact the stimulation of gut (or proximal) IgA responses, as observed with our Salmonella vaccine studies24 and with the IN CT immunization study,16, 17 or whether secretory IgA (S-IgA) responses would be delayed, as previously reported for IN CT immunization. Another intriguing aspect noted in our previous IN CT study was the sustained IgA production by αEβ7+ B cells in L-Sel−/− mice at distal mucosal sites typically not evident with IN immunizations.17 Thus, we also questioned whether distal (upper respiratory tract) immunity will be supported by these αEβ7+ B cells in L-Sel−/− mice following oral CT immunization.
Results
Oral CT immunization of L-Sel−/− mice shows sustained IgA Ab responses
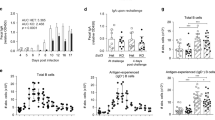
Previous work examined the homing receptors and addressin usage that supports upper respiratory tract responses. It has been shown that peripheral node addressin expression either associates with MAdCAM-1 or remains unassociated, suggesting the importance of L-Sel to upper respiratory tract immunity.18, 19 Moreover, the relevance of L-Sel for upper respiratory tract responses shows that mucosal immunity is compromised in L-Sel−/− non-intestinal mucosal tissues following nasal CT-B immunization.16, 17 Thus, we questioned whether oral CT (soluble Ag) immunization would result in IgA responses. L-Sel−/− and L-Sel+/+ mice were orally immunized with CT on days 0, 7, and 14, and anti-CT-B titers were measured on day 16 to obtain an early measurement. The results showed that fecal IgA (P=0.021; Figure 1a) and serum IgA (P=0.006; Figure 1c) anti-CT-B responses were elevated when compared to L-Sel+/+ mice. The fecal IgA anti-CT-B titers remained elevated for at least 2 additional weeks and, by day 42, responses diminished when compared with L-Sel+/+ mice (P<0.001). L-Sel−/− serum IgA responses remained elevated throughout all the experiments and, for the most part, these IgA titers were significantly greater than those observed in L-Sel+/+ mice (P⩽0.006). Interestingly, nasal washes from L-Sel−/− mice showed a 16-fold increase in CT-B-specific S-IgA titers, suggesting that in the absence of L-Sel, distal immunity could be augmented. The observed elevation in serum IgA anti-CT-B Abs appeared to be due to increased respiratory IgA titers, as evidenced by the elevated S-IgA present in L-Sel−/− nasal washes (P=0.010) on day 42 (Figure 1b). Serum IgG anti-CT-B responses were similar for both groups for each time point examined (Figure 1d).
Rapid onset mucosal IgA in L-Sel−/− mice. Depicted are (a) fecal IgA and serum (c) IgA and (d) IgG anti-CT-B titers following oral immunization of L-Sel−/− mice with CT. L-Sel−/− and L-Sel+/+ mice were orally immunized on days 0, 7, and 14. Serum and mucosal secretions were collected and assessed for immune Abs by CT-B ELISA. Although L-Sel−/− fecal IgA anti-CT-B Abs decreased at 42 days, (b) S-IgA in nasal washes from L-Sel−/− mice remained elevated. Results up to 42 days after primary immunization are depicted as the mean of 10 mice ±s.e.m., and statistical differences between L-Sel−/− and L-Sel+/+ mice were determined: *P<0.001; **P=0.006; ***P=0.021. Ab, antibody; CT, cholera toxin; ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; L-Sel, L-selectin.
To assess the source of enhanced mucosal and serum IgA responses, CT-B-specific enzyme-linked immunosorbent spot (ELISPOT) assay was performed on lymphocyte fractions from the intestinal lamina propria (iLP) and NP 16 days post-primary immunization. The number of IgA and IgG CT-B-specific antibody-forming cells (AFCs) in the NP and iLP was not significantly different between L-Sel−/− and L-Sel+/+ mice (Figure 2a,b). Except for a slight reduction in the total IgA AFCs in the L-Sel−/− NP (Figure 2a), the total number of IgA and IgG AFCs was also not significantly different (Figure 2a,b). Likewise, there were no significant differences in IgA or IgG CT-B-specific as well as total IgA or IgG AFCs in the NALT and Peyer's patch (PP) (Figure 2c,d). Only the cervical lymph node (CLN) in L-Sel−/− mice showed an increase in Ag-specific IgA AFCs (P=0.002) as well as total IgA (P<0.001) and total IgG (P=0.031; Figure 2e,f). The total IgA AFCs (P=0.003) were also increased in the submaxillary gland lymph node (SMLN) (Figure 2e).
Oral immunization of L-Sel−/− mice with CT results in increased IgA AFC responses in CLN 16 days post-primary immunization. L-Sel−/− and L-Sel+/+ mice were orally immunized, as previously described, and on day 16, total lymphocytes from (a, b) NP and small iLP, (c, d) NALT and PP, and (e, f) SMLN, PRLN, and CLN were collected and assayed for anti-CT-B and total (a, c, e) IgA and (b, d, f) IgG AFC responses. CT-B-specific and total IgA AFC responses were slightly enhanced in L-Sel−/− CLN as well as total IgG AFCs. The number of IgA and IgG CT-B-specific AFCs in the other L-Sel−/− HNLN, NALT, PP, NP, and iLP were not significantly different from L-Sel+/+ mice. Results depict the mean of three experiments ±s.e.m. per tissue, and statistical differences between L-Sel−/− and L-Sel+/+ mice were determined: *P<0.001; **P⩽0.003; ***P=0.031. AFC, antibody-forming cell; CLN, cervical lymph node; CT, cholera toxin; HNLN, head and neck lymph node; Ig, immunoglobulin; iLP, intestinal lamina propria; L-Sel, L-selectin; NALT, nasal-associated lymphoid tissue; NP, nasal passage; PP, Peyer's patch; PRLN, parotid gland lymph node; SMLN, submaxillary gland lymph node.
Upper respiratory tract immune responses are increased in L-Sel−/− mice by 6 weeks post-primary immunization
To determine whether the observed upper respiratory tract is more responsive with time, B-cell ELISPOT experiments were conducted 4 weeks after the last oral CT dose. No significant differences in the number of IgA or IgG anti-CT-B iLP AFCs were observed between L-Sel+/+ and L-Sel−/− mice at this time point (Figure 3a,b). As before, distal immunity was notably augmented in L-Sel−/− mice, as evidenced by the increased L-Sel−/− NP IgA CT-B-specific AFC responses, which were significantly greater than responses obtained with the L-Sel+/+ mice by 3.5-fold (P<0.001). This apparently weak distal B-cell response in L-Sel+/+ mice was expected and did not deviate from the 16-day responses. In contrast, the L-Sel−/− NP IgA AFC responses were significantly enhanced by 15-fold by 6 weeks (P<0.001).
Oral immunization of L-Sel−/− mice with CT results in enhanced IgA AFC responses in NP 42 days post-primary immunization and supported by increases in NALT and HNLN IgA AFCs. L-Sel−/− and L-Sel+/+ mice were orally immunized, as previously described, and on day 42, total lymphocytes from (a, b) NP and iLP and (c, d) NALT, HNLN, and PP were collected and assayed for anti-CT-B and total (a, c) IgA and (b, d) IgG AFC responses. Each L-Sel−/− HNLN was combined and assessed for anti-CT-B activity. CT-B-specific IgA AFC responses were elevated in L-Sel−/− mice when compared to L-Sel+/+ mice. IgA and IgG iLP CT-B-specific AFC responses were not significantly different between L-Sel+/+ and L-Sel−/− mice; however, dramatic increases in L-Sel−/− NALT and HNLN CT-B-specific IgA and IgG AFC responses were observed when compared to L-Sel+/+ mice. Total IgA and IgG AFCs in L-Sel−/− HNLN and total IgG AFCs in L-Sel−/− NALT were also elevated when compared to L-Sel+/+ mice. Depicted are the mean AFC responses of three experiments ±s.e.m. per tissue, and statistical differences between L-Sel−/− and L-Sel+/+ mice were determined: *P<0.001; **P⩽0.014; ***P=0.025. AFC, antibody-forming cell; CT, cholera toxin; HNLN, head and neck lymph node; Ig, immunoglobulin; iLP, intestinal lamina propria; L-Sel, L-selectin; NALT, nasal-associated lymphoid tissue; NP, nasal passage; PP, Peyer's patch; PRLN, parotid gland lymph node; SMLN, submaxillary gland lymph node.
To determine whether regional inductive mucosal tissues supported these CT-B-specific Ab responses, lymphocytes isolated from NALT, HNLN, and PP were analyzed by B-cell ELISPOT (Figure 3c,d). Specific and total elevations in IgA AFCs were observed in L-Sel−/− NALT by 9.2-fold (P=0.014) and 6.5-fold (P=0.008) and in HNLN by 30-fold (P<0.001) and 6.7-fold (P<0.001), respectively, when compared to L-Sel+/+ mice (Figure 3c). L-Sel−/− IgG CT-B-specific AFCs were also significantly increased in the NALT by 2.4-fold (P=0.009) and in the HNLN by 19-fold (P<0.001) when compared to L-Sel+/+ mice (Figure 3d). Although the IgG AFC responses in the PP did not significantly differ between L-Sel+/+ and L-Sel−/− mice, the L-Sel+/+ PP showed slightly greater (P=0.025) CT-B-specific IgA AFC responses when compared to L-Sel−/− mice. Thus, these data show that specific elevations in upper respiratory tract (distal) AFC responses were sustained in L-Sel−/− mice orally immunized with CT, and these responses were greatly augmented when compared to L-Sel+/+ mice.
CT-B-specific αEβ7+ B cells are present in the gut but not in the NP at 6 weeks post-primary immunization
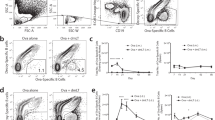
NP and iLP B-cell subsets were evaluated by flow cytometry from orally CT-immunized L-Sel+/+ and L-Sel−/− mice on day 42 (Figure 4). The majority of B220+ lymphocytes in L-Sel+/+ and L-Sel−/− NP were found to be α4β7low and, for the most part, not αEβ7+. In contrast, those in the L-Sel+/+ and L-Sel−/− iLP were mostly α4β7low, although a minor subset stained αEβ7+. Yet this population was found to be more prominent in the L-Sel−/− iLP than in the L-Sel+/+ iLP by approximately 2.4-fold (Figure 4c,d).
Increased presence of αEβ7+ iLP B cells in the L-Sel−/− mice 6 weeks post-primary oral immunization with CT. L-Sel+/+ and L-Sel−/− NP and iLP B cells were analyzed for expression of (a) L-Sel and α4β7, (b) α4β7 and αE, and (c) β7 and αE. αEβ7+ B cells remained as a minor subset in the NP, but in the L-Sel−/− iLP they showed (d) ∼2.4-fold greater percentage than that in L-Sel+/+ iLP. (c) Boxed histographs represent the populations sorted for B-cell ELISPOT in Figure 5. Data are representative of three experiments except in d, which is the mean±s.e.m. of three experiments. CT, cholera toxin; ELISPOT, enzyme-linked immunosorbent spot; iLP, intestinal lamina propria; L-Sel, L-selectin; NP, nasal passage.
To discern whether the αEβ7+ B cells were Ag-specific, B220+ B cells from the L-Sel+/+ and L-Sel−/− iLP and NP were sorted for β7low vs. αEβ7+ (see Figure 4c,d for examples of sorted populations), and the sorted subsets were evaluated in CT-B-specific ELISPOT assay. Not surprisingly, the IgA anti-CT-B activity was mostly found within the β7low subset; however, a significant portion of the IgA anti-CT-B Abs were produced by the αEβ7+ B cells in both L-Sel−/− and L-Sel+/+ iLP (Figure 5a). No iLP IgG anti-CT-B AFCs were detected (Figure 5b). Examination of NP revealed that no IgA CT-B-specific activity was associated with the αEβ7+ B cells from L-Sel+/+ or L-Sel−/− NP (Figure 5c), but instead all the reactivity appeared in the β7low subset. However, some IgG CT-B-specific AFCs were found associated with the NP αEβ7+ subset (Figure 5d).
Effector CT-B-specific B cells were mostly L-Sellow/β7low in the iLP and NP at 42 days post-primary immunization. Cell-sorting experiments were conducted sorting (a, b) iLP and (c, d) NP lymphocytes for β7 vs. β7high and αE expression and assessed by CT-B-specific (left panel) and total (right panel) (a, c) IgA and (b, d) IgG ELISPOT. No β7high B cells were found in either L-Sel+/+ or L-Sel−/− NP; all the iLP β7high B cells were αEβ7+, not α4β7high, and the β7low B cells were all L-Sellow/β7low. The majority of the CT-B-specific iLP IgA AFCs were β7low. For both L-Sel+/+ and L-Sel−/− mice, the L-Sellow/β7low B-cell subset contained all of the NP IgA anti-CT-B activity, and for L-Sel−/− mice, αEβ7+ B cells contained the IgG anti-CT-B and the total IgG AFCs. Results depict the mean of three experiments ±s.e.m., and statistical differences between β7low and αEβ7+ B cells were determined: *P⩽0.002; **P⩽0.008; ***P<0.026; ****P<0.05. AFC, antibody-forming cell; CT, cholera toxin; ELISPOT, enzyme-linked immunosorbent spot; Ig, immunoglobulin; iLP, intestinal lamina propria; L-Sel, L-selectin; NALT, nasal-associated lymphoid tissue; NP, nasal passage.
Cell-sorted αEβ7+ B cells contain the majority of the IgA CT-B-specific activity in L-Sel−/− HNLN
Fluorescence-activated cell-sorting analyses for L-Sel, α4β7, and αEβ7 expression were performed on B220-gated B cells obtained from the NALT, HNLN, and PP (Figure 6). At 6 weeks post-primary immunization, levels of L-Sel were diminished on L-Sel+/+ NALT B cells, but both L-Selhigh α4β7low and L-Sellow α4β7low (double-low) were present in CLN, SMLN, and PP, whereas L-Sel−/− B cells were all α4β7low (Figure 6a). Interestingly, on examination of both L-Sel+/+ and L-Sel−/− mucosal inductive tissues, each was found to contain αEβ7+ B cells (Figure 6b). Additional studies were then undertaken to discern whether this αEβ7+ B-cell subset was present at earlier time points. Less than 3% of the B cells from naive mice CLN, SMLN, and PP (Figure 6d–f) and ∼5% of the B cells from the naive mice NALT were αEβ7+ (Figure 6g). Over the course of the oral immunizations, increases in αEβ7+ B cells were detected mostly in the L-Sel−/− mice, peaking at about 35 days post-primary immunization. Collectively, these results suggest that these increases in αEβ7+ B cells would be CT-B-specific.
αEβ7+ B cells appear late in mucosal inductive tissues after primary oral immunization with CT in both L-Sel+/+ and L-Sel−/− mice. L-Sel+/+ and L-Sel−/− NALT, CLN, SMLN, and PP B cells were analyzed for expression of (a) L-Sel and α4β7 and (b) β7 and αE at 6 weeks post-primary immunization. Expression for αEβ7 was evident in each of the mucosal inductive tissues examined. (c) Cell-sorting profiles show that the sorted β7low vs. αEβ7 B cells were greater than 95% pure. (d–g) A kinetic analysis was performed to determine when these αEβ7+ B cells are induced in the (d) CLN, (e) SMLN, (f) PP, and (g) NALT. Less than 5% αEβ7+ B cells are present in naive mucosal inductive tissues, but these increase with time, peaking at day 35 post-primary immunization. Each time point represents pooled tissues from five individual mice, and differences between days 0 and 35 were evaluated: *P<0.001. CLN, cervical lymph node; CT, cholera toxin; ELISPOT, enzyme-linked immunosorbent spot; Ig, immunoglobulin; iLP, intestinal lamina propria; L-Sel, L-selectin; NALT, nasal-associated lymphoid tissue; NP, nasal passage; PP, Peyer's patch; SMLN, submaxillary gland lymph node.
To assess whether these αEβ7+ B cells secrete anti-CT-B Abs, B220+ B cells from PP and HNLN were sorted for β7low vs. αEβ7+ and evaluated by CT-B-specific ELISPOT. In L-Sel+/+ PP, only the α4β7low B-cell subset showed CT-B-specific IgA AFCs, unlike the L-Sel−/− PP in which the responses were equally distributed between α4β7low and αEβ7+ B cells (Figure 7a). The CT-B-specific IgG PP responses also differed between mouse strains. The PP IgG AFCs were equally distributed between both subsets in L-Sel+/+ mice, but only α4β7low subset in the L-Sel−/− mice contained the CT-B-specific B cells (Figure 7b). As shown in Figure 3, minimal-to-no AFC responses were detected in L-Sel+/+ HNLN. Thus, the lack of IgA and IgG AFC responses in the HNLN was not surprising (Figures 3 and 7c,d), as oral immunization is not normally effective in stimulating upper respiratory tract responses. Interestingly, however, increased AFC responses were detected in L-Sel−/− HNLN (Figure 3), and cell-sorting analyses revealed that the majority of these CT-B-specific IgA AFCs were αEβ7+ (Figure 7c). The IgG AFC responses were not significantly different between α4β7low and αEβ7+ B-cell subsets (Figure 7d).
The increased numbers of B cells in the L-Sel−/− HNLN at 42 days post-primary immunization are attributed to increased functional activity by αEβ7+ B cells exhibiting both IgA and IgG anti-CT-B activity. Cell-sorting experiments were conducted on (a, b) PP and (c, d) HNLN lymphocytes for β7 vs. β7high and αE expression and assessed by CT-B-specific (left panel) and total (right panel) (a, c) IgA and (b, d) IgG ELISPOT. (a, b) For PP CT-B-specific responses, all the L-Sel+/+ IgA AFCs were α4β7low, and IgG AFCs were equally divided between α4β7low and αEβ7+ subsets. The L-Sel−/− PP IgA AFCs were equally divided between both subsets, and no αEβ7+ IgG anti-CT-B AFCs were detected. (c, d) For the HNLN CT-B-specific responses, very few to no IgA or IgG AFCs were detected in L-Sel+/+ mice. In contrast, the majority of the IgA AFCs were αEβ7+, and the IgG AFC responses were composed of either αEβ7+ or α4β7low subset. Thus, sustained and elevated IgA anti-CT-B responses are attributed to the αEβ7+ Ag-reactive B cells found late in L-Sel−/− HNLN following oral immunization with CT. The results depict the mean of three experiments ±s.e.m., and statistical differences between β7low and αEβ7+ B cells were determined: *P⩽0.008; **P=0.02; ***P⩽0.048. AFC, antibody-forming cell; Ag, antigen; CT, cholera toxin; ELISPOT, enzyme-linked immunosorbent spot; HNLN, head and neck lymph node; Ig, immunoglobulin; L-Sel, L-selectin; PP, Peyer's patch.
L-Sel−/− mice show increased propensity for αE integrin expression on B cells
As the percentage of L-Sel−/− αEβ7+ B cells increased with time in the HNLN, we questioned whether these B cells show an increased likelihood to express αEβ7. Whole HNLN, PP, and splenic lymphocytes from L-Sel−/− or L-Sel+/+ mice were cultured for 1–3 days, activated with lipopolysaccharide (LPS) or LPS+CT, or left unactivated. Cells were collected and analyzed for αEβ7 expression by flow cytometry. Unstimulated L-Sel−/− HNLN B cells at 48 h showed elevated αEβ7 expression when compared to L-Sel+/+ HNLN B cells (Figure 8a); no significant differences were seen with PP or splenic B cells. The addition of LPS did not significantly enhance αEβ7 expression (Figure 8b), but CT co-stimulation showed significantly greater αEβ7+ B cells for the L-Sel−/− HNLN, PP, and spleens than identical B cells from L-Sel+/+ mice (Figure 8c,d). A kinetic analysis clearly showed that the L-Sel−/− HNLN B cells showed the greatest propensity for αEβ7 expression (Figure 8d), but L-Sel+/+ HNLN B cells did not, suggesting that αEβ7 may be driven to expression in the absence of L-Sel.
L-Sel−/− HNLN shows increased expression of αEβ7+ B cells. Total HNLN, PP, and Spl lymphocytes were cultured (a) without activation or activated with (b) LPS or (c) LPS plus CT for 48 h. B220+ TCR-β− lymphocytes were then analyzed for αE and β7 expression. Increased percentages of αEβ7+ B cells were observed with the (a) HNLN lymphocytes left unstimulated and (c) HNLN, PP, and Spl lymphocytes stimulated with LPS plus CT. (d) A kinetic analysis was performed and showed that L-Sel−/− HNLN αEβ7+ B cells showed preferential enhancement at 24, 48, and 72 h with LPS plus CT treatment. Depicted data are the means of six individual mice ±s.e.m., and statistical differences between L-Sel−/− and L-Sel+/+ mice were determined: *P⩽0.001; **P⩽0.015. CT, cholera toxin; HNLN, head and neck lymph node; LPS, lipopolysaccharide; L-Sel, L-selectin; PP, Peyer's patch; Spl, splenic; TCR-β, T-cell receptor-β.
Discussion
The results obtained in this study differ from what was observed upon nasal CT immunization16 in that proximal tissues in L-Sel−/− mice were responsive to oral CT, reaffirming the relevance of α4β7–MAdCAM-1 interactions.23, 25 Of interest, however, were the distal responses obtained in these mice; in particular, NP CT-B-specific IgA responses became elevated with time in L-Sel−/− mice but not in normal C57BL/6 mice. On investigating mucosal inductive site responses, the NALT and PP responses did not differ between L-Sel+/+ and L-Sel−/− mice during the early responses. Clearly, however, by 6 weeks, increases in CT-B-specific AFC responses in the NALT and more prominently in the HNLN of orally immunized L-Sel−/− mice were observed. Remarkably, the absence of L-Sel resulted in long-term elevated NP AFC responses, which may be supported by NALT and/or HNLN, whereas in L-Sel+/+ mice, these distal immune responses were significantly less robust and remained unchanged or reduced by 6 weeks post-immunization. These differences between mouse species seemed not to be attributed to selective regurgitation of CT following oral immunization, as this was not observed. Rather, these latter results were consistent with previous reports showing that oral immunization is not as effective for stimulating long-lived B cells in distal mucosal tissues.26, 27 Why then is there selective enhancement in the L-Sel−/− upper respiratory tract?
The most obvious explanation for this result is that responses to immunization in the iLP appear to rely on αEβ7 expression by B and T cells.25, 28, 29 Therefore, oral immunization possibly stimulated an αEβ7+ subset of B cells that could traffic to the nasal effector sites from the intestinal inductive sites even without expression of L-Sel. Consistent with this explanation, our flow cytometry analyses revealed that the subpopulations of B lymphocytes induced in the effector sites of orally immunized mice were similar to those observed after IN CT immunization.16, 17 L-Selhigh/αEβ7low and L-Sellow/αEβ7low (double-low) B lymphocytes were found in NP and iLP, whereas the αEβ7+ population was more prominent in iLP. Cell-sorting experiments revealed that the β7low population provided the majority of CT-B-specific and total IgA response in all effector sites, excluding the possibility that αEβ7+ B cells compensated in the mucosal effector tissues in the absence of L-Sel. Obviously, oral immunization with CT did not stimulate the α4β7high subset that migrated to the non-intestinal effector sites, as was expected from the previously described rotavirus studies.25
However, the most prominent functional differences observed were with the distal B-cell responses, particularly in the upper respiratory tract. Investigation of the responses occurring in the intestinal and non-intestinal inductive sites may provide more clues as to the mechanism of immune responses following oral CT immunization in L-Sel−/− mice. AFC responses were beginning to increase in L-Sel−/− HNLN by 16 days and continued until at least 42 days. Additionally, both L-Sel+/+ and L-Sel−/− NALT AFC responses were not significantly different at 16 days; however, both CT-B-specific and total IgA responses were significantly increased in L-Sel−/− NALT at 42 days post-primary immunization. Although no differences in gut IgA responses were observed, AFC responses in the L-Sel+/+ PP were slightly enhanced when compared to L-Sel−/− PP. Therefore, in the absence of L-Sel, there was selective enhancement of immune responses in the nasal inductive and effector sites. This attribute of impacting distal immunity was one of the original tenets upon which the theory of the common mucosal immune system1, 2 and 3 was founded but later was shown to be somewhat more variable.4, 5 What is striking about the results presented here is the observation that, indeed, the distal immune responses were augmented and retained for at least 42 days, whereas this was not evident in L-Sel+/+ mice.
How the augmentation in the L-Sel−/− upper respiratory tract may come about may be explained in part by the observed results in the HNLN. Although the L-Sel−/− HNLN showed reduced cellularity, overall it had an increased “concentration” of AFCs, suggesting that lymphocyte trafficking to these sites is reduced with a concomitant stimulation of resident B-cell populations, as others have suggested.30 This was largely based on observations made with L-Sel−/− PLN showing increased numbers of germinal centers30 following peripheral immunization, again suggesting stimulation of resident B cells. Moreover, the results from the oral CT studies were very similar to those previously observed at 42 days post-IN immunization,17 suggesting that the head and neck have a larger contribution by L-Sel.18, 19 It has also been shown that activated B lymphocytes can shed L-Sel31 and that re-expression of L-Sel is important for the trafficking of memory B lymphocytes.32 Thus, as these B cells seem to be restricted or fixed within these tissues, they perhaps behave as memory B cells. Because of this memory phenotype, they are unable to migrate from the HNLN or NALT as effectively as L-Sel+/+ mice, thereby restricting their differentiation to occur locally, which could then account for their increased numbers. Consistent with this idea that these B cells may be more of a memory phenotype is the observation that at 42 days post-primary immunization, the majority of the L-Sel−/− were GL7− (data not shown). Activated B cells have been found to show increased expression of GL7.33, 34
The most prominent observation of this study was the increased IgA AFC responses in the HNLN in which these B cells were almost entirely αEβ7+. This finding suggests that αEβ7 can play an important role in the generation of B-cell immunity. This result was surprising, as αEβ7 has not previously been shown to mediate lymphocyte trafficking, but rather appears to play a role in lymphocyte retention in the iLP35, 36 and in the skin.37, 38 However, a novel ligand for αEβ7 has been observed, 39 and little is known about the role of αEβ7 on B lymphocytes. Although we observed increased presence of αEβ7+ B cells in the HNLN, NALT, and NP at 42 days, no CT-B-specific AFCs were detected in the NALT, and only the L-Sel−/− NP αEβ7+ B cells contained IgG CT-B-specific AFCs. In contrast, HNLN αEβ7+ B cells compensated for the absence of L-Sel mostly during the late-phase responses, as no CT-B-specific αEβ7+ B cells were detectable at 16 days in NALT, HNLN, or NP. These CT-B-specific αEβ7+ B cells appear to be generated locally in the HNLN, as basal levels of αEβ7+ B cells could be detected by day 16, although we cannot exclude their potential derivations from the gut. Indeed, the percentage of αEβ7 lymphocytes was increased in L-Sel−/− by day 42 post-immunization, and its contribution to the CT-B-specific response was limited to the gut and the HNLN and, interestingly, not the NP. Further evidence that the αEβ7 is induced preferentially by L-Sel−/− mice is from the in vitro studies in which unstimulated or LPS plus CT-stimulated L-Sel−/− HNLN B cells showed increased αEβ7 with time when compared to L-Sel+/+ mice, which simply did not show modulation. This latter finding supports the cell-sorting experiments for IgA AFC in which no αEβ7+ B cells from the HNLN or PP were present in L-Sel+/+ mice, whereas this propensity for αEβ7 expression was clearly evident in L-Sel−/− HNLN and PP. Collectively, these studies show that alternative integrins can facilitate mucosal immunity as in the present study, which shows that αEβ7 is induced and contributes to sustaining distal IgA responses. Current work is seeking to understand which adjuvants38 may enhance αEβ7 expression to enable development of vaccine strategies to enhance distal mucosal immunity.
Methods
Mice and immunizations. Specific pathogen-free C57BL/6N (L-Sel+/+) female mice were purchased from the National Cancer Institute (Frederick Cancer Research Facility, Frederick, MD) at 5–6 weeks of age and maintained in the Animal Resources Center at Montana State University (Bozeman, MT). Breeding pairs of L-Sel−/− mice on a B6 background were maintained in the Animal Resources Center, as previously described.16, 17, 24 All mice were kept under pathogen-free conditions in individually ventilated cages under HEPA-filtered barrier conditions and fed sterile food and water ad libitum. The mice were free of bacterial and viral pathogens, as determined by Ab screening and by histopathologic analysis of major organs and tissues. The mice used in these experiments were between 5 and 8 weeks of age. Mice were orally gavaged with 200 μl of 50% saturated NaHCO3 solution and, after 15 min, were immunized with 10 μg CT (List Biological Laboratories, Campbell, CA) in 200 μl sterile phosphate-buffered saline (PBS) and additional boosts on days 7 and 14 with 10 μg CT, similar to that previously described.40
Collection of serum and mucosal samples. Blood was collected from mice through saphenous vein bleeding. Fresh fecal pellets were collected from individual mice and solubilized in 50 μg ml−1 of soybean trypsin inhibitor (Sigma-Aldrich, St Louis, MO) in sterile PBS (10 × v/w) by continual vortexing for 30 min at 4 °C and then subjected to microcentrifugation. Nasal washes were performed on euthanized mice by intubating their tracheas to access the nasopharyngeal cavity using a small internal diameter (0.010 inch) tygon tubing (Cole-Parmer, Vernon Hills, IL). The nasal cavity was flushed with 300 μl of sterile PBS, and nasal washes were collected from the nares into microcentrifuge tubes. Sera and supernatants from mucosal samples were frozen until assayed.
Anti-CT-B ELISA. The method for anti-CT-B enzyme-linked immunosorbent assay (ELISA) was identical to that previously described.17 Falcon Micro-test III Flexible assay microtiter plates (BD Biosciences, Oxnard, CA) were coated with 50 μl per well of 5 μg ml−1 B subunit of CT (CT-B; List Biological Laboratories) in sterile PBS. Serum or mucosal samples diluted in ELISA buffer were added at 50 μl per well, and plates were incubated at 4 °C overnight. Plates were washed and 50 μl per well of detecting horseradish peroxidase conjugates of goat anti-mouse IgG (γ-chain specific) or goat anti-mouse IgA (α-chain specific) Abs (1 μg ml−1; Southern Biotechnology Associates, Birmingham, AL) was added, and the plates were then incubated at 37 °C for 1.5 h. Horseradish peroxidase was visualized by the addition of 50 μl per well of ABTS (2,2′-azino-bis-[3-ethylbenzthiazoline-6-sulfonic acid]) substrate (Moss, Pasadena, CA). Optical density was determined by reading the plates at 415 nm, and end-point titers were expressed as the reciprocal of the last sample dilution giving an absorbance >0.1 over the value of negative control wells (in the absence of biological fluid) after a 1-h incubation. Similar dilutions of mucosal samples and serum from non-immune mice showed no Ab titer to CT-B.
Isolation of inductive tissue lymphocytes. Parotid gland LN, SMLN (sometimes also referred to as the superficial cervical or mandibular gland LN41, 42), CLN (sometimes referred to as deep CLN41), and PP were isolated from immunized L-Sel+/+ and L-Sel−/− mice. Each set of lymphoid tissue was pooled from five mice and washed in RPMI-1640 medium. NALT tissues were collected by removing the soft palates, as previously described,17, 18 followed by collagenase digestion (200 U ml−1 collagenase type IV solution; Sigma-Aldrich) in RPMI-1640 media containing 0.08 U ml−1 DNase (Promega, Madison, WI). The palates were vigorously agitated on a magnetic stir plate for 45 min at 37 °C; the resulting cell suspensions were removed and filtered through Nitex fabric (Fairview Fabrics, Hercules, CA) and the cells were washed and resuspended in complete medium (RPMI-1640+10% fetal bovine serum (Hyclone, Logan, UT)+10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer+10 mM non-essential amino acids+10 mM sodium pyruvate+10 U ml−1 penicillin/streptomycin) or fluorescence-activated cell-sorting buffer (Dulbecco's PBS+2% fetal bovine serum).
For flow cytometry and ELISPOT analysis, CLN, SMLN, and parotid gland LN were removed and subjected to Dounce homogenization.19 The resulting cell suspensions were filtered through Nitex fabric (Fairview Fabrics), washed with RPMI-1640 medium, and centrifuged at 1,500 r.p.m. for 5 min. Cell pellets were resuspended in fluorescence-activated cell-sorting buffer or complete medium.
Isolation of effector tissue lymphocytes. NPs devoid of NALT were removed from the head by scraping the turbinates from the nasal cavity, followed by digestion with 200 U ml−1 collagenase type IV solution containing 0.08 U ml−1 DNase at 37 °C for 30 min, as previously described.16, 17 For iLP lymphocytes, intestines were extracted from the mouse, the PPs were carefully removed, and fecal material and mucus were flushed from the intestine using RPMI-1640 medium. Intestines devoid of isolated lymphoid follicles43 were processed, as previously described,16, 17 and NP and iLP lymphocytes were resuspended in a 40% Percoll solution (Pharmacia, Uppsala, Sweden) and then layered over a 60% Percoll solution and subjected to gradient centrifugation. Lymphocytes were removed from the interface layer, washed, and resuspended in complete medium.
CT-B-specific and total Ab ELISPOT. Mixed cellulose ester membrane-bottomed microtiter plates (Multi-Screen-HA; Millipore, Bedford, MA) were coated with 5 μg ml−1 CT-B (List Biological Laboratories) or goat anti-mouse IgA or IgG (H-chain-specific) Abs (Southern Biotechnology Associates) in sterile PBS overnight at room temperature. The plates were blocked at 37 °C for 2 h with complete medium. A total of 100 μl of cells from each tissue at varying concentrations (2 × 106–1.25 × 105 lymphocytes per ml) was added to the wells, and the plates were incubated at 37 °C overnight. Cells were removed, and the plates were washed as previously described.17 For detection of mouse Ab responses, 100 μl of 1.0 μg ml−1 goat anti-mouse IgG and IgA-horseradish peroxidase conjugates (Southern Biotechnology Associates) was added to the wells, and the plates were incubated overnight at 4 °C. After washing, the wells were developed with 100 μl of AEC (3-amino-9-ethylcarbazole) (Moss), and the reaction was allowed to continue until spots developed (∼30 min). The reaction was stopped with H2O, the plates were allowed to dry overnight, and spot-forming cells were enumerated by counting under a low-power dissecting microscope (Leica, Buffalo, NY).
Cell-surface staining for analysis and B-lymphocyte cell sorting. Abs for staining B lymphocytes were obtained from BD PharMingen (San Diego, CA): FITC-M290 anti-CD103 (αE integrin), PE-FIB504 rat anti-β7, PE-DATK 32 rat anti-mouse α4β7, CyChrome-rat anti-mouse B220 (RA3-6B2), and APC-MEL 14 rat anti-mouse L-selectin. PE-goat anti-mouse IgA (Southern Biotechnology Associates) was used in some of the cell-sorting experiments. FL1, FL2, FL3, and FL4 parameters were set with CaliBrite beads (BD PharMingen), and compensations were set with FACSComp software (CellQuest, Becton Dickinson, San Jose, CA). Four-color analysis was performed using a FACSCalibur (BD Biosciences), and 10,000 events per sample were collected. Cell sorting for B220+ B-cell subsets was performed using a FACSVantage with Turbo-Sort (BD Biosciences), and sorted B-cell subsets were evaluated in CT-B-specific and total IgA or IgG ELISPOT assays.
In vitro activation of B cells with LPS and CT. To assess whether CT induces αE expression, whole-cell cultures of HNLN, PP, and spleens were performed from individual L-Sel−/− and C57BL/6N mice. Single-cell suspensions were prepared, as described above, and were either left unactivated or cultured with 4.0 μg ml−1 LPS (Escherichia coli O55:B5, List Biologicals, Campbell, CA) or 1.0 μg ml−1 LPS+5.0 μg ml−1 CT for 24, 48, or 72 h. Cells were harvested and stained for coexpression of αE and β7 by B220+ TCR-β− B cells.
Statistical analysis. Results were analyzed using analysis of variance followed by a multigroup comparison test, and P-values ⩽0.05 are indicated.
Disclosure
The authors declared no conflict of interest.
References
Rudzik, R., Clancy, R.L., Perey, D.Y., Day, R.P. & Bienenstock, J. Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer's patch and bronchial lymphocytes. J. Immunol. 114, 1599–1604 (1975).
Mestecky, J., McGhee, J.R., Michalek, S.M., Arnold, R.R., Crago, S.S. & Babb, J.L. Concept of the local and common mucosal immune response. Adv. Exp. Med. Biol. 107, 185–192 (1978).
McDermott, M.R. & Bienenstock, J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J. Immunol. 122, 1892–1898 (1979).
Quiding-Jarbrink, M., Nordstrom, L., Granstrom, G., Kilander, A., Jertbom, M., Butcher, E.C. et al. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J. Clin. Invest. 99, 1281–1286 (1997).
Kantele, A., Hakkinen, M., Moldoveanu, Z., Lu, A., Savilahti, E., Alvarez, R.D. et al. Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: evidence for compartmentalization within the common mucosal immune system in humans. Infect. Immun. 66, 5630–5635 (1998).
Top, F.H. Jr, Buescher, E.L., Bancroft, W.H. & Russell, P.K. Immunization with live type 7 and type 4 adenovirus vaccines. II. Antibody response and protective effects against acute respiratory disease due to adenovirus type 7. J. Infect. Dis. 124, 155–160 (1971).
Takafuji, E.T., Gaydos, J.C., Allen, R.G. & Top, F.H. Jr Simultaneous administration of live, enteric-coated adenovirus types 4, 7, and 21 vaccines: safety and immunogenicity. J. Infect. Dis. 140, 48–53 (1979).
van Ginkel, F.W., Jackson, R.J., Yuki, Y. & McGhee, J.R. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 165, 4778–4782 (2000).
Belshe, R.B. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 103, 177–185 (2004).
Berlin, C., Berg, E.L., Briskin, M.J., Andrew, D.F., Kilshaw, P.J., Holzmann, B. et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993).
Bargatze, R.F., Jutila, M.A. & Butcher, E.C. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity 3, 99–108 (1995).
Hamann, A., Andrew, D.P., Jablonski-Westrich, D., Holzmann, B. & Butcher, E.C. Role of α4-integrins in lymphocyte homing to mucosal tissues in vivo. J. Immunol. 152, 3282–3293 (1994).
Briskin, M.E., Winsor-Hines, D., Shyjan, A., Cochran, N., Bloom, S., Wilson, J. et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am. J. Pathol. 151, 97–110 (1997).
Holzmann, B., McIntyre, B.W. & Weissman, I.L. Identification of a murine Peyer's patch-specific lymphocyte homing receptor as an integrin molecule with an alpha chain homologous to human VLA-4α. Cell 56, 37–46 (1989).
Berg, E.L., McEvoy, L.M., Berlin, C., Bargatze, R.F. & Butcher, E.C. selectin-mediated lymphocyte rolling on MAdCAM-1. Nature 366, 695–698 (1993).
Csencsits, K.L., Walters, N. & Pascual, D.W. Cutting edge: dichotomy of homing receptor dependence by mucosal effector B cells: αE versus L-selectin. J. Immunol. 167, 2441–2445 (2001).
Csencsits, K.L. & Pascual, D.W. Absence of L-selectin delays mucosal B cell responses in nonintestinal effector tissues. J. Immunol. 169, 5649–5659 (2002).
Csencsits, K.L., Jutila, M.A. & Pascual, D.W. Nasal-associated lymphoid tissue: phenotypic and functional evidence for the primary role of peripheral node addressin in naive lymphocyte adhesion to high endothelial venules in a mucosal site. J. Immunol. 163, 1382–1389 (1999).
Csencsits, K.L., Jutila, M.A. & Pascual, D.W. Mucosal addressin expression and binding interactions with native lymphocytes vary among the cranial, oral, and nasal-associated lymphoid tissues. Eur. J. Immunol. 32, 3029–3039 (2002).
Abitorabi, M.A., Mackay, C.R., Jerome, E.H., Osorio, O., Butcher, E.C. & Erle, D.J. Differential expression of homing molecules on recirculating lymphocytes from sheep gut, peripheral, and lung lymph. J. Immunol. 156, 3111–3117 (1996).
Picker, L.J., Martin, R.J., Trumble, A ., Newman, L.S., Collins, P.A., Bergstresser, P.R. et al. Differential expression of lymphocyte homing receptors by human memory/effector T cells in pulmonary versus cutaneous immune effector sites. Eur. J. Immunol. 24, 1269–1277 (1994).
Quiding, M., Lakew, M., Granstrom, G., Nordstrom, I., Holmgren, J. & Czerkinsky, C. Induction of specific antibody responses in the human nasopharyngeal mucosa. Adv. Exp. Med. Biol. 371B, 1445–1450 (1995).
Kantele, A., Kantele, J.M., Savilahti, E., Westerholm, M., Arvilommi, H., Lazarovits, A. et al. Homing potentials of circulating lymphocytes in humans depend on the site of activation: oral, but not parenteral, typhoid vaccination induces circulating antibody-secreting cells that all bear homing receptors directing them to the gut. J. Immunol. 158, 574–579 (1997).
Pascual, D.W., White, M.D., Larson, T. & Walters, N. Impaired mucosal immunity in L-selectin-deficient mice orally immunized with a Salmonella vaccine vector. J. Immunol. 167, 407–415 (2001).
Williams, M.B. et al. The memory B cell subset responsible for the secretory IgA response and protective humoral immunity to rotavirus expresses the intestinal homing receptor, α4β7 . J. Immunol. 161, 4227–4235 (1998).
Rudin, A., Johansson, E.L., Bergquist, C. & Holmgren, J. Differential kinetics and distribution of antibodies in serum and nasal and vaginal secretions after nasal and oral vaccination of humans. Infect. Immun. 66, 3390–3396 (1998).
Rudin, A., Riise, G.C. & Holmgren, J. Antibody responses in the lower respiratory tract and male urogenital tract in humans after nasal and oral vaccination with cholera toxin B subunit. Infect. Immun. 67, 2884–2890 (1999).
Williams, M.B. & Butcher, E.C. Homing of naive and memory T lymphocyte subsets to Peyer's patches, lymph nodes, and spleen. J. Immunol. 159, 1746–1752 (1997).
Rott, L.S., Rose, J.R., Bass, D., Williams, M.B., Greenberg, H.B., Butcher, E.C. Expression of mucosal homing receptor α4β7 by circulating CD4+ cells with memory for intestinal rotavirus. J. Clin. Invest. 100, 1204–1208 (1997).
Steeber, D.A., Green, N.E., Sato, S. & Tedder, T.F. Humoral immune responses in L-selectin-deficient mice. J. Immunol. 157, 4899–4907 (1996).
Bradley, L.M., Watson, S.R. & Swain, S.L. Entry of naive CD4T cells into peripheral lymph nodes requires L-selectin. J. Exp. Med. 180, 2401–2406 (1994).
Kraal, G., Weissman, I.L. & Butcher, E.C. Memory B cells express a phenotype consistent with migratory competence after secondary but not short-term primary immunization. Cell. Immunol. 115, 78–87 (1998).
László, G., Hathcock, K.S., Dickler, H.B. & Hodes, R.J. Characterization of a novel cell-surface molecule expressed on subpopulations of activated T and B cells. J. Immunol. 150, 5252–5262 (1993).
Cervenak, L., Magyar, A., Boja, R. & László, G. Differential expression of GL7 activation antigen on bone marrow B cell subpopulations and peripheral B cells. Immunol. Lett. 78, 89–96 (2001).
Lefrancois, L., Parker, C.M., Olson, S., Muller, W., Wagner, N., Schon, M.P. et al. The role of β7 integrins in CD8T cell trafficking during an antiviral immune response. J. Exp. Med. 189, 1631–1638 (1999).
Austrup, F., Rebstock, S., Kilshaw, P.J. & Hamann, A. Transforming growth factor-β1-induced expression of the mucosa-related integrin αE on lymphocytes is not associated with mucosa-specific homing. Eur. J. Immunol. 25, 1487–1491 (1995).
Pauls, K., Schon, M., Kubitza, R.C., Homey, B., Wiesenbom, A., Lehmann, P. et al. Role of integrin αE(CD103)β7 for tissue-specific epidermal localization of CD8+ T lymphocytes. J. Invest. Dermatol. 117, 569–575 (2001).
Sigmundsdóttir, H., Johnston, A., Gudjónsson, J.E. & Valdimarsson, H. Differential effects of interleukin 12 and interleukin 10 on superantigen-induced expression of cutaneous lymphocyte-associated antigen (CLA) and αEβ7 integrin (CD103) by CD8+ T cells. Clin. Immunol. 111, 119–125 (2004).
Strauch, U.G., Mueller, R.C., Li, X.Y., Cernadas, M., Higgins, J.M., Binion, D.G. et al. Integrin αE(CD103)β7 mediates adhesion to intestinal microvascular endothelial cell lines via an E-cadherin-independent interaction. J. Immunol. 166, 3506–3514 (2001).
Pascual, D.W., Hone, D.M., Hall, S., van Ginkel, F.W., Yamamoto, M., Walters, N. et al. Expression of recombinant enterotoxigenic colonization factor antigen I by Salmonella typhimurium elicits a biphasic T helper cell response. Infect. Immun. 67, 6249–6256 (1999).
Tilney, N.L. Patterns of lymphocyte drainage in the adult laboratory rat. J. Anat. 109, 369–383 (1971).
Hebel, R. & Stromberg, M.W. Anatomy and Embryology of the Laboratory Rat (BioMed, Worthsee, Germany, 1986).
Hamada, H., Hiroi, T., Nishiyama, Y., Takahashi, R., Masunaga, Y., Hachimura, S. et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 168, 57–64 (2002).
Acknowledgements
We thank Nancy Kommers for her assistance in preparing this manuscript. This work was supported by US Public Health Grant AI-55563 and in part by the Montana Agricultural Station and US Department of Agricultural Formula Funds. The VMB flow cytometry facility was supported in part by the NIH/National Center for Research Resources, Centers of Biomedical Excellence P20 RR-020185, and an equipment grant from the M.J. Murdock Charitable Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pascual, D., Riccardi, C. & Csencsits-Smith, K. Distal IgA immunity can be sustained by αEβ7+ B cells in L-selectin−/− mice following oral immunization. Mucosal Immunol 1, 68–77 (2008). https://doi.org/10.1038/mi.2007.2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2007.2