Abstract
The purpose of our study was to evaluate the effect of photodynamic therapy (PDT), using erythrosine as a photosensitizing agent and a dental halogen curing unit as a light source, on Streptococcus mutans in a biofilm phase. The S. mutans biofilms were formed in a 24-well cell culture cluster. Test groups consisted of biofilms divided into four groups: group 1: no photosensitizer or light irradiation treatment (control group); group 2: photosensitizer treatment alone; group 3: light irradiation alone; group 4: photosensitizer treatment and light irradiation. After treatments, the numbers of colony-forming unit (CFU) were counted and samples were examined by confocal laser scanning fluorescence microscopy (CLSM). Only group 4 (combined treatment) resulted in significant increases in cell death, with rates of 75% and 55% after 8 h of incubation, and 74% and 42% at 12 h, for biofilms formed in brain–heart infusion (BHI) broth supplemented with 0% or 0.1% sucrose, respectively. Therefore, PDT of S. mutans biofilms using a combination of erythrosine and a dental halogen curing unit, both widely used in dental clinics, resulted in a significant increase in cell death. The PDT effects are decreased in biofilms that form in the presence of sucrose.
Similar content being viewed by others
Introduction
Dental caries result from an imbalance in the physiological equilibrium between tooth minerals and oral microbial biofilms.1 Biofilm bacteria, such as streptococci (e.g., Streptococcus mutans and Streptococcus sobrinus) and Lactobacillus species, secrete organic acids and this process leads to demineralization of the teeth. S. mutans has also been implicated as a cariogenic bacteria because of its relatively high numbers in plaque prior to the appearance of carious lesions, its capacity for rapid degradation of carbohydrates with the formation of abundant acid, and its ability to tolerate low pH environments.2,3
Populations of surface-attached microorganisms comprising either single or multiple species are commonly referred to as biofilms.4 Microbial biofilms in the oral cavity are involved in the etiology of various oral diseases, including caries, periodontal and endodontic disease, bacterial infection, and dental implant failures.5 One remarkable feature of oral biofilm is the production of copious quantities of extracellular polysaccharides, which is regarded as a contributing factor to the increasing resistance of oral bacteria to antibiotics.4 The bacteria encase themselves in the oral biofilm in a hydrated matrix of polysaccharide and protein, forming a slimy layer6 that is associated with the chronic nature of subsequent infections and with their inherent resistance to antibiotic chemotherapy.
Current techniques to remove biofilms involve either periodic mechanical disruption of the oral microbial biofilm, such as by tooth brushing, or the use of antiseptics. However, prognosis with the former depends on patient compliance, while the latter could produce drug-resistant organisms and disrupt the normal bacterial flora. Therefore, alternative techniques for biofilm removal from a tooth surface are required. Photodynamic therapy (PDT) is one technique that uses a combination of an appropriate photosensitizer and a light source. PDT might represent an excellent alternative, or additional therapy, for the control of biofilms, because it is recognized as a safe treatment strategy that is both minimally invasive and nontoxic.5
Previous studies have shown that a number of types of oral bacteria, including periodontal pathogenic bacteria, cariogenic bacteria and bacteria associated with endodontic lesions, are susceptible to PDT.7,8,9,10,11,12 However, PDT has been not used for elimination of bacteria within carious lesions. Information regarding irradiation time, irradiation distance and specificity of the light source is still needed for the dental clinical application of PDT. Although some research related to the susceptibility of bacteria associated with caries to PDT has been reported for the use of lasers or LEDs (light-emitting diodes), the systems used were expensive and specifically designed for PDT.13,14 No research has yet been reported to show the effects of PDT on S. mutans using erythrosine and the dental halogen curing unit conventionally used in dental clinics.
The purpose of our study was to evaluate the PDT effects of a combination of erythrosine and a standard dental halogen curing unit on viability of S. mutans in the biofilm phase.
Materials and methods
Bacterial strains and culture conditions
This study used the S. mutans ATCC25175 strain. Bacteria were incubated in brain–heart infusion broth (BHI) (Becton, Dickinson and Company, Sparks, MD, USA) under aerobic conditions, supplemented with 5% CO2 at 37 °C for 18 h. The turbidity of the suspensions was measured by a spectrophotometer (Smart Plus 2700; Young-woo inst., Seoul, Korea). A standard curve relating the culture turbidity and bacterial cell numbers was established and utilized for cell dilutions. The bacteria were diluted to 107 colony-forming units per mL (CFU·mL−1) with phosphate-buffered saline (PBS) and this suspension was used as an inoculum for biofilm formation.
Biofilm formation
S. mutans biofilms were formed in a 24-well cell culture cluster (Corning Costar, 3524, flat bottom; Corning Glass, Corning, NY, USA) by incubating a 1-mL S. mutans suspension (990 µL BHI and 10 µL cultured bacteria; final concentration of 104 CFU·mL−1) with different concentrations of sucrose (0%, 0.1% and 1%) under 5% CO2 at 37 °C for different incubation times (0, 4, 8, 12, 16 and 20 h). Subsequently, 1 mL of the medium was removed, the wells were washed twice with PBS to remove unbound bacteria, and then 1 mL PBS was added to each well in which the biofilm was formed.
Photosensitizer
Erythrosine was used as a photosensitizer. A stock solution of 1 mmol·L−1 erythrosine (Sigma-Aldrich, St Louis, MO, USA) was prepared in PBS. This solution was filtered-sterilized, and stored at −20 °C in the dark. Working solutions were obtained by diluting the stock solutions with PBS to 20 µmol·L−1.
Light source
A conventional halogen curing unit (XL 3000; 3M ESPE, St Paul, MN, USA) was used as a light source. The diameter of the light beam was 8 mm. The power output from the light was 600 mW·cm−2; this was checked by a radiometer (Light Intensity Meter; Dentamerica, San Jose, CA, USA).
Photodynamic treatment of biofilms
Test groups consisted of biofilms subjected to:
Group 1: no photosensitizer or light irradiation treatment;
Group 2: photosensitizer (20 µmol·L−1 erythrosine) alone;
Group 3: light irradiation alone;
Group 4: combined photosensitizer (20 µmol·L−1 erythrosine) and light irradiation treatment.
All biofilms were incubated for 8 and 12 h, since the quantity of formed biofilms was highest at these incubation times. A 20.4 µL volume of erythrosine (20 µmol·L−1 final concentrations) was then added into the wells of groups 2 and 4. Next, the biofilms of groups 3 and 4 were irradiated under the dental halogen curing unit for 30 s. The distance between the light tip and sample was 1 cm. Subsequently, each well containing the biofilms was sonicated twice for 10 s (VC 100; Sonics & Materials Inc., Danbury, CT, USA). Each sample was then diluted with PBS and 50 µL diluted suspension was spread on triplicate blood agar plates (Hanil-KOMED, Seongnam, Gyeonggi-do, Korea). The plates were incubated for 72 h at 37 °C under 5% CO2 and the numbers of CFU on the plates were counted.
Confocal laser scanning fluorescence microscopy
For visualization, biofilms were allowed to form for 12 h in BHI supplemented with 0.1% sucrose at 37 °C. Two test groups (groups 1 and 4) were then selected for confirmation by confocal laser scanning fluorescence microscopy (CLSM). The formed biofilms were washed very gently three times with sterile distilled water to remove unbound bacteria. The viability of bacteria within the biofilms was then determined by staining the biofilms with LIVE/DEAD BacLight Bacterial Viability Kits (Molecular Probe, Eugene, OR, USA). This kit includes two fluorescent nucleic acid stains: SYTO9 and propidium iodide: 3 µL of each stain was added to 1 mL distilled water and then 100 µL of each staining solution was added to the formed biofilms before the biofilms dried. The staining dish was covered and the biofilms were stained for 15 min at room temperature in a dark room, protected from light. The biofilms were then rinsed gently with 100 µL distilled water and all excess stain and rinse water was removed from the base of well. Each biofilm was then scraped with a sterile explorer, transferred to a glass slide using a micropipette, and covered with a cover glass. Stained biofilms were examined with an Olympus Fluoview FV300 confocal microscope (Olympus, Tokyo, Japan), using an EGFP filter (488/507 nm) for detection of SYTO9 and a propidium iodide filter (530/615 nm) for detecting propidium iodide.
Statistical analysis
In this study, assays for the PDT effect were performed in triplicate, and all procedures were repeated independently at least twice on different days. Data are the mean±s.d. of a representative experiment; similar results were obtained for all experiments. The time based CFU values and means and standard deviations of CFU values for the four test groups were compared by one-way ANOVA (SPSS, version 19.0; SPSS Inc., Armonk, New York, USA). P values less than 0.05 were considered statistically significant. The Bonferroni method was performed for multiple comparison procedures. The repeated measure ANOVA was performed for confirmation of the significance of the bacterial growth curve pattern.
Results
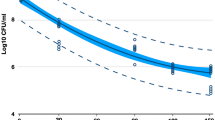
Figure 1 shows time based CFU values for different concentrations of sucrose. The CFU values of the biofilms formed after 4 and 8 h were significantly lower in BHI supplemented with 0% sucrose than the values obtained with 0.1% and 1% sucrose, respectively. However, no significant difference was noted between the biofilms supplemented with 0.1% and 1% sucrose, except at 8 h, where the ability of S. mutans to colonize was promoted in the presence of increasing amounts of sucrose. Increasing the incubation time to 12 h also increased the quantity of formed biofilms, but after this time, the S. mutans biofilms reached the stationary phase of growth. The repeated measure ANOVA showed a statistical significance of the pattern of the bacterial growth curve (P=0.013).
Time based CFU analysis. The ability of Streptococcus mutans to colonize was increased in the presence of sucrose. The quantity of biofilms formation was increased up to 12 h incubation time. Error bars present standard deviations. Comparison of 0% sucrose (*), 0.1% sucrose (†), 1% sucrose (‡), statistically significant with P<0.05. CFU, colony-forming unit.
Table 1 shows the PDT effects after 8 and 12 h incubation in 0% and 0.1% sucrose. The CFU values were higher with 0.1% sucrose than with 0% sucrose at both incubation times. The CFU values were slightly decreased in both groups 2 (photosensitizer alone) and 3 (light treatment alone). Nevertheless, comparison of groups 1–3 revealed that either the addition of photosensitizer or light irradiation alone had no significant antibacterial effect. Only the combined treatment of light irradiation in the presence of the photosensitizer (group 4) resulted in a significant decrease in CFU counts at all incubation times and sucrose concentrations.
Figures 2 and 3 show a direct comparison of the efficacy of PDT against S. mutans biofilms in terms of the percentage of cell death based on colony counts for the control group (group 1) after incubation for 8 h (Figure 2) and 12 h (Figure 3). Using a cutoff level of P<0.005, only group 4 (combined treatment) showed any significant increase in cell death. In addition, the PDT effect was decreased in the presence of added sucrose.
Pilot tests were performed to confirm that sonication had no effect on cell viability in the biofilms. Cell viability was not affected by the presence of the photosensitizer, the light irradiation, or the sonication time.
The effects of PDT on S. mutans biofilm were visualized with CLSM. As shown in Figure 4, the majority of the cells showed green fluorescence in the absence of light irradiation and erythrosine, indicating a high level of cell viability. However, biofilms treated with light irradiation and erythrosine showed increased red fluorescence, indicating increased numbers of dead cells.
CLSM images of biofilms at 12 h incubation time in 0.1% sucrose. Green fluorescence represents viable bacteria and red fluorescence represents affected bacteria. (a) Group 1: non-treated biofilms with PDT. (b) Group 4: treated biofilms with PDT. The scale bar for (a) and (b) is 50 µm. CLSM, confocal laser scanning fluorescence microscopy; PDT, photodynamic therapy.
Discussion
PDT might be an optional technique for the reduction of biofilms that cause oral disease, and it is recognized as a safe treatment strategy that is minimally invasive and nontoxic.5 Three components—a light source, a photosensitizer, and tissue oxygen—are indispensable for a successful treatment prognosis following PDT. The absorption of light by a photosensitizer molecule in the ground state results in excitation to the singlet state when the molecule absorbs the energy of the photon of light. The singlet state molecule may decay back to the ground state by emitting a photon as light energy (fluorescence) or by internal conversion, with energy lost as heat. Alternatively, the singlet state molecule may convert into an excited triplet state molecule. Molecules in the triplet state can react further by one or both of two pathways, known as the type I and type II photoprocesses. The type I reaction involves electron transfer reactions from the photosensitizer triplet state, with the participation of a substrate, to produce radical ions that can react with oxygen to produce cytotoxic species such as superoxide and hydroxyl radicals. The type II reaction involves energy transfer system from the photosensitizer triplet state to ground state molecular oxygen to produce excited singlet oxygen, which can react with and destroy biological materials such as proteins, nucleic acids and lipids, thereby leading to cytotoxicity. Singlet oxygen is probably the major damaging species in PDT and has a diffusion distance of approximately 100 nm.5
A photosensitizer has the capability to absorb light of a specific wavelength and transform it into useful energy. In PDT, this would further involve the production of cytotoxic agents. The key character of a photosensitizer is its ability to produce cytotoxic agents to induce the desired biological effect.15 The light source also influences on the effect of PDT, and the efficacy depends on output and emission wavelength, etc. Many light sources are used in PDT, such as dental halogen curing units, LEDs and lasers.
The ideal photosensitizer and light source should be nontoxic to humans and should have an appropriate combination of emission and absorption wavelengths. We used erythrosine as a photosensitizer and a dental halogen curing unit as a light source, since these are both already used extensively and safely in the oral environment. Erythrosine is currently used clinically as a plaque-disclosing agent and in PDT, where it has been shown to induce bacterial cell death of >1.5 log10 in S. mutans biofilms in in vitro studies.16,17 Erythrosine has several advantages over other photosensitizers, including its nontoxicity to the host and its approval for usage in food products. A halogen lamp is not an optimal light source because of its low light power density (mW·cm−2) and low light energy fluence (J·cm−2). However, this type of lamp is widely used as a curing unit in dental clinics; therefore, no additional device is required for use as a light source for PDT. The conventional lamp has an emission wavelength range of 400–520 nm, which is similar to the region of absorption of erythrosine (500–550 nm). Therefore, the combination of erythrosine and a dental halogen curing unit can be used for PDT. To date, several researchers have carried out studies on the PDT effect against streptococcal species associated with dental caries and showed the possibility for targeting these cariogenic bacteria.2,13,18,19,20,21,22,23 However, the present study is the first to show yet reported a PDT effect on S. mutans using erythrosine and a dental halogen curing unit.
In the present study, S. mutans biofilms formed in 24-well microtiter plates to the greatest extent within 12 h. After this time, the growth pattern of S. mutans biofilms indicated that the stationary phase had been reached. At 16 h, the CFU values for biofilms exposed to all sucrose concentrations were lower than those seen after 12 h incubation times. These results appeared to be due to detachment of the biofilms by sloughing due to their large bulk. These detached bacterial cells were not counted in the CFU values as the cells were no longer attached to the microtiter plate. Therefore, in this study, we selected two incubation time points (8 and 12 h) in the growth phase of biofilms to confirm the PDT effects on S. mutans biofilms because the greatest amount of biofilm formed at 12 h, while 8 h was still within the log phase of the bacterial growth pattern.
We used CLSM for the visualization of the PDT effects on S. mutans biofilm formation. Previously, several researchers have also used CLSM to confirm biofilm formation and PDT effect.14,16,24,25 CLSM provides a divided image for simultaneous observation of surviving bacteria and affected bacteria. We distinguished between bacteria with damaged and undamaged cell membranes using the LIVE/DEAD BacLight Bacterial Viability Kit (Invitrogen, Karlsruhe, Germany), which provides a novel two-color fluorescence assay of bacterial viability. The SYTO9 dye in this kit penetrates both viable and nonviable bacteria, while the propidium iodide dye penetrates bacteria with damaged membranes and quenches SYTO9 fluorescence. Dead cells that take up propidium iodide fluoresce red and while viable cells fluoresce green.14
We confirmed a PDT effect on S. mutans biofilms: Figure 2 (biofilms formed after 8 h of incubation) shows cell death of 75% and 55 % in biofilms with treated 0% and 0.1% sucrose, respectively, in group 4 (combined light and photosensitizer treatment). Likewise, Figure 3 (biofilms formed after 12 h incubation time) shows cell death of 74% and 42% in similarly treated biofilms. These differences were statistically significant, whereas the CFU values for groups 2 and 3 showed slight decreases that were not statistically significant. Therefore, no phototoxicity was evident in biofilms exposed to halogen light irradiation (group 3) or to the photosensitizer under natural light (group 2). Figures 2 and 3 also show a decrease in the PDT effect in response to sucrose addition, which might be a consequence of increased production of extracellular polysaccharides.
In this study, CLSM images of biofilms showed mostly green fluorescence (viable cells) in non-treated biofilms, whereas biofilms treated with PDT showed increased red fluorescence (dead cells), indicating a pronounced effect of PDT on cell viability of S. mutans biofilms. However, quantitative comparisons of the PDT effect were not possible, since the biofilms formed in the microtiter plates were transferred to glass slides for CLSM imaging. We also confirmed a significant increase in cell death in S. mutans biofilms, based on both CFU values and CLSM images. PDT appears promising as a method for the control of biofilms in caries lesions. The use of a conventional photosensitizer and light source in this study also indicates that a PDT effect is obtainable without extra costs to the dental clinic.
This study had a number of limitations. One is that the transfer of the biofilms from well plates to glass slides for CLSM imaging could have affected the integrity of the biofilm. However, we chose to do this, because the material of the well plate is plastic, and this could affect the reflection of light during the CSLM imaging, whereas glass would not have this effect. We also noted that the most outstanding feature of CLSM was the presence of different layers within the intact biofilms, but we did not analyze the efficacy of PDT within these distinct layers of biofilms; further research is needed using CLSM at this level. The scope of the present study confirms the in vitro effects of PDT on S. mutans viability in the biofilm phase. However, the susceptibility of the biofilms formed in vitro in this study may be different from that of biofilms formed within the oral cavity with respect to both the pattern of biofilm formation and the included bacterial species. The effect of PDT could be quite different on oral biofilms; therefore, further research is needed to confirm the clinical efficacy of PDT using a photosensitizer and a conventional halogen curing lamp.
Overall, this study showed that PDT can reduce the extent of S. mutans biofilms and might be a potential therapy for use in dental clinics. However, more clinical and laboratory research is needed to establish the optimum treatment parameters for the anticariogenic potential of PDT.
Conclusion
Several techniques have been attempted for removal of oral biofilm as an etiology of various oral diseases, including caries, but with limited success. For this reason, alternative techniques are required. PDT is a technique that uses the combination of an appropriate photosensitizer and light source and might represent a minimally invasive and nontoxic method for control of oral biofilm formation. The present study confirmed the positive effects of PDT effects for reduction of biofilms of S. mutans, a major cariogenic bacterium, using erythrosine and halogen curing unit, which are both widely used in most dental clinics. We demonstrated a significant decrease in in vitro S. mutans biofilm formation in response to this simple PDT technique and verified its potential for use in the control of biofilms that cause caries lesions. Because the photosensitizer and light source used in this study are erythrosine and a halogen curing unit, which are conventionally used in dental clinics, this PDT effect would be obtainable in most clinics without additional costs.
References
Selwitz RH, Ismail AI, Pitts NB . Dental caries. Lancet 2007; 369(9555): 51–59.
Zanin IC, Goncalves RB, Junior AB et al. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study. J Antimicrob Chemother 2005; 56(2): 324–330.
Svensater G, Welin J, Wilkins JC et al. Protein expression by planktonic and biofilm cells of Streptococcus mutans. FEMS Microbiol Lett 2001; 205(1): 139–146.
O’Toole GA, Kolter R . Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol 1998; 28(3): 449–461.
Soukos NS, Goodson JM . Photodynamic therapy in the control of oral biofilms. Periodontol 2000 2011; 55(1): 143–166.
Stewart PS, Costerton JW . Antibiotic resistance of bacteria in biofilms. Lancet 2001; 358(92767): 135–138.
Giusti JS, Santos-Pinto L, Pizzolito AC et al. Antimicrobial photodynamic action on dentin using a light-emitting diode light source. Photomed Laser Surg 2008; 26(4): 281–287.
Burns T, Wilson M, Pearson GJ . Effect of dentine and collagen on the lethal photosensitization of Streptococcus mutans. Caries Res 1995; 29(3): 192–197.
Dobson J, Wilson M . Sensitization of oral bacteria in biofilms to killing by light from a low-power laser. Arch Oral Biol 1992; 37(11): 883–887.
Okamoto H, Iwase T, Morioka T . Dye-mediated bactericidal effect of He-Ne laser irradiation on oral microorganisms. Lasers Surg Med 1992; 12(4): 450–458.
Soukos NS, Wilson M, Burns T et al. Photodynamic effects of toluidine blue on human oral keratinocytes and fibroblasts and Streptococcus sanguis evaluated in vitro. Lasers Surg Med 1996; 18(3): 253–259.
Garcez AS, Nunez SC, Hamblin MR et al. Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesion. J Endod 2008; 34(2): 138–142.
Costa AC, Chibebe Junior J, Pereira CA et al. Susceptibility of planktonic cultures of Streptococcus mutans to photodynamic therapy with a light-emitting diode. Braz Oral Res 2010; 24(4): 413–418.
Sharma M, Visai L, Bragheri F et al. Toluidine blue-mediated photodynamic effects on staphylococcal biofilms. Antimicrob Agents Chemother 2008; 52(1): 299–305.
Sharman WM, Allen CM, van Lier JE . Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today 1999; 4(11): 507–517.
Wood S, Metcalf D, Devine D et al. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother 2006; 57(4): 680–684.
Metcalf D, Robinson C, Devine D et al. Enhancement of erythrosine-mediated photodynamic therapy of Streptococcus mutans biofilms by light fractionation. J Antimicrob Chemother 2006; 58(1): 190–192.
Wilson M, Burns T, Pratten J . Killing of Streptococcus sanguis in biofilms using a light-activated antimicrobial agent. J Antimicrob Chemother 1996; 37(2): 377–381.
Gad F, Zahra T, Hasan T et al. Effects of growth phase and extracellular slime on photodynamic inactivation of gram-positive pathogenic bacteria. Antimicrob Agents Chemother 2004; 48(6): 2173–2178.
Bevilacqua IM, Nicolau RA, Khouri S et al. The impact of photodynamic therapy on the viability of Streptococcus mutans in a planktonic culture. Photomed Laser Surg 2007; 25(6): 513–518.
Vahabi S, Fekrazad R, Ayremlou S et al. The effect of antimicrobial photodynamic therapy with radachlorin and toluidine blue on Streptococcus mutans: an in vitro study. J Dent (Tehran) 2011; 8(2): 48–54.
Zanin IC, Lobo MM, Rodrigues LK et al. Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode. Eur J Oral Sci 2006; 114(1): 64–69.
Bolean M, Paulino Tde P, Thedei G Jr et al. Photodynamic therapy with rose bengal induces GroEL expression in Streptococcus mutans. Photomed Laser Surg 2010; 28 (Suppl 1): S79–S84.
Kreth J, Tam K, Merritt J et al. Quantitiative analyses of Streptococcus mutans biofilms with quartz crystal microbalance, microjet impingement and confocal microscopy. Biofilms 2004; 1(4): 277–284.
Li LN, Guo LH, Lux R et al. Targeted antimicrobial therapy against Streptococcus mutans establishes protective non-cariogenic oral biofilms and reduces subsequent infection. Int J Oral Sci 2011; 2(2): 66–73.
Acknowledgements
This work is supported by Cooperative Research (CR1102) of Gangneung-Wonju National University Dental Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Lee, YH., Park, HW., Lee, JH. et al. The photodynamic therapy on Streptococcus mutans biofilms using erythrosine and dental halogen curing unit. Int J Oral Sci 4, 196–201 (2012). https://doi.org/10.1038/ijos.2012.63
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijos.2012.63
Keywords
This article is cited by
-
Antimicrobial photodynamic therapy against Lactobacillus casei using curcumin, nano-curcumin, or erythrosine and a dental LED curing device
Lasers in Medical Science (2023)
-
Porphyrins developed for photoinactivation of microbes in wastewater
Environmental Science and Pollution Research (2022)
-
Treatment of dental plaque biofilms using photodynamic therapy: a randomised controlled study
European Archives of Paediatric Dentistry (2021)
-
Effects of antibacterial photodynamic therapy on salivary mutans streptococci in 5- to 6-year-olds with severe early childhood caries
Lasers in Medical Science (2019)
-
Photodynamic Inactivation Mediated by Erythrosine and its Derivatives on Foodborne Pathogens and Spoilage Bacteria
Current Microbiology (2015)