Abstract
Background:
An epidemiological association between excess weight and increased risk of cancer has been described in melanoma, for which the physiopathological mechanisms are still unknown. The study of tumor microenvironment and of the role of adipocytes in cancer development, progression and metastasis has recently received great interest. However, the role of peritumoral adipocytes has been characterized only in a few types of cancer, and in melanoma it still remains to be defined.
Methods:
We investigated the interactions between adipocytes and melanoma cells using an in vitro co-culture system. We studied the morphological and functional properties of 3T3-L1 adipocytes before and after co-culture with A375 melanoma cells, in order to assess the role of adipocytes on melanoma migration.
Results:
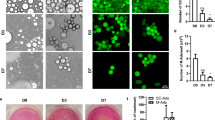
Morphological analysis showed that after 6 days of co-culture 3T3-L1 adipocytes were reduced in number and size. Moreover, we observed the appearance of dedifferentiated cells with a fibroblast-like phenotype that were not present in controls and that had lost the expression of some adipocyte-specific genes, and increased the expression of collagen, metalloproteinases and genes typical of dedifferentiation processes. Through the Matrigel Invasion Test, as well the Scratch Test, it was possible to observe that co-culture with adipocytes induced in melanoma cells increased migratory capacity, as compared with controls. In particular, the increase in migration observed in co-culture was suppressed after adding the protein SFRP-5 in the medium, supporting the involvement of the Wnt5a pathway. The activation of this pathway was further characterized by immunofluorescence and western blot analysis, showing in melanocytes in co-culture the activation of β-catenin and LEF-1, two transcription factors involved in migration processes, neo-angiogenesis and metastasis.
Conclusions:
These data allow us to hypothesize a dedifferentiation process of adipocytes toward fibroblast-like cells, which can promote migration of melanoma cells through activation of Wnt5a and the intracellular pathways of β-catenin and LEF-1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Renehan A, Zwahlen M, Egger M . Adiposity and cancer risk: new mechanistic insights from epidemiology. Nature Rev Cancer 2015; 15: 484–498.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M . Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008; 371: 569–578.
Wang J, Yang DL, Chen ZZ, Gou BF . Associations of body mass index with cancer incidence among populations, genders, and menopausal status: a systematic review and meta-analysis. Cancer Epidemiol 2016; 42: 1–8.
Freisling H, Arnold M, Soerjomataram I, O'Doherty MG, Ordóñez-Mena JM, Bamia C et al. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: meta-analysis of individual participant data of seven prospective cohorts in Europe. Br J Cancer 2017; 116: 1486–1497.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K et al.Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med 2016; 375: 794–798.
Sergentanis TN, Antoniadis AG, Gogas HJ, Antonopoulos CN, Adami H, Ekbom A et al. Obesity and risk of malignant melanoma: a meta-analysis of cohort and case–control studies. Eur J Cancer 2013; 49: 642–657.
Calle EE, Kaaks R . Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004; 4: 579–591.
Nieman KM, Romero IL, Van Houten B, Lengyel E . Adipose tissue and adipocytes support tumorigenesis and metastasis. Bioch Biophys Acta 2013; 1831: 1533–1541.
Fischer-Posovszky P, Wabitsch M, Hochberg Z . Endocrinology of adipose tissue—an update. Horm Metab Res 2007; 39: 314–321.
Pollak M . The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 2012; 12: 159–169.
Park J, Euhus DM, Scherer PE . Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev 2011; 32: 550–570.
Whipple CA . Tumor talk: understanding the conversation between the tumor and its microenvironment. Cancer Cell Microenviron 2015; 26: e773.
Balkwill FR, Capasso M, Hagemann T . The tumor microenvironment at a glance. J Cell Sci 2012; 125: 5591–5596.
Tan J, Buache E, Chenard MP, Dali-Youcef N, Rio MC . Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int J Dev Biol 2011; 55: 851–859.
Zoico E, Darra E, Rizzatti V, Budui S, Franceschetti G, Mazzali G et al. Adipocytes Wnt-5a mediated dedifferentiation: a possible target in pancreatic cancer microenvironment. Oncotarget 2016; 7: 20223–20235.
Kwan HY, Fu X, Liu B, Chao X, Chan CL, Cao H et al. Subcutaneous adipocytes promote melanoma cell growth by activating the Akt signaling pathway: role of palmitic acid. J Biol Chem 2014; 289: 30525–30537.
Bochet L, Lehuede C, Dauvillier S, Wang YY, Dirat B, Laurent V et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res 2013; 73: 5657–5668.
Cinti S . Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab 2009; 297: E977–E986.
Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol 2008; 215: 210–222.
Szabó P, Kolář M, Dvořánková B, Lacina L, Gabius HJ, Strnad H et al. Mouse 3T3 fibroblasts under the influence of fibroblasts isolated from stroma of human basal cell carcinoma acquire properties of multipotent stem cells. Biol Cell 2011; 103: 233–248.
Da Forno PD, Jh Pringle, Hutchinson P, Osborn J, Huang Q, Potter L et al. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res 2008; 14: 5825–5832.
Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 2002; 1: 279–288.
Spranger S, Bao R, Gajewski TF . Melanoma-intrinsic b-catenin signalling prevents anti-tumour immunity. Nature 2015; 523: 231–235.
Zhan T, Rindtorff N, Boutros M . Wnt signaling in cancer. Oncogene 2017; 36: 1461–1473.
Klaus A, Birchmeier W . Wnt signalling and its impact on development and cancer. Nat Rev Cancer 2008; 8: 387–398.
Trevellin E, Scarpa M, Carraro A, Lunardi F, Kotsafti A, Porzionato A et al. Esophageal adenocarcinoma and obesity: peritumoral adipose tissue plays a role in lymph node invasion. Oncotarget 2015; 6: 11203–11215.
Zoico E, Budui SL, Rizzatti V, Franceschetti G, Darra E, Pedrazzani C et al. Immunophenotipical changes and browning phenomena in the peri-tumoral adipose tissue of colorectal cancer patients. Obesity doi:10.1002/oby.22008.
Zhang D, Wang Y, Shi Z, Liu J, Sun P, Hou X et al. Metabolic reprogramming of cancer-associated fibroblasts by IDH3α downregulation. Cell Rep 2015; 10: 1335–1348.
Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res 2012; 72: 5130–5140.
Ishii G, Ochiai A, Neri S . Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev 2016; 99: 186–196.
Catalán V, Gomez-Ambrosi J, Rodriguez A, Perez-Hernandez AI, Gurbindo J, Ramirez B et al. Activation of noncanonical Wnt signaling through WNT5a in visceral adipose tissue of obese subjects is related to inflammation. J Clin Endocrinol Metab 2014; 99: E1407–E1417.
Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE et al. β-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell 2011; 20: 741–754.
Ekström E, Bergenfelz C, von Bülow V, Serifler F, Carlemalm E, Jönsson G et al. WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol Cancer 2014; 13: 88.
Linnskog R, Jönsson G, Axelsson L, Prasad CP, Andersson T . Interleukin-6 drives melanoma cell motility through p38α-MAPK-dependent up-regulation of WNT5A expression. Mol Oncol 2014; 8: 1365–1378.
Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 2011; 71: 2455–2465.
Acknowledgements
We thank Professor Mark Newman who corrected the English of the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Zoico, E., Darra, E., Rizzatti, V. et al. Role of adipose tissue in melanoma cancer microenvironment and progression. Int J Obes 42, 344–352 (2018). https://doi.org/10.1038/ijo.2017.218
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.218
This article is cited by
-
Melanoma cells induce dedifferentiation and metabolic changes in adipocytes present in the tumor niche
Cellular & Molecular Biology Letters (2023)
-
Focus on dedifferentiated adipocytes: characteristics, mechanisms, and possible applications
Cell and Tissue Research (2019)