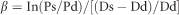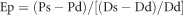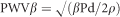Abstract
Arterial stiffness can predict cardiovascular events, and the aim of this study was to produce age- and sex-specific reference values for echo-tracking carotid stiffness in healthy subjects. A total of 900 subjects (500 males, mean age 45.8±19 years) were enrolled. Common carotid artery stiffness and compliance, using a high-definition echo-tracking ultrasound system, were evaluated. To compare stiffness parameters across the different age groups, individual scores were transformed into T-scores, indicating how many standard deviation (s.d.) units an individual’s score was above or below the mean that was observed in the group including same-sex individuals aged 36 to 44 years. Carotid stiffness was similar among genders, except compliance, which was lower in women (P<0.0001). These characteristics were also maintained when the studied population was divided into seven age groups. Stiffness parameters increased significantly with age, but the opposite occurred for compliance. The T-score was found to increase significantly across all age groups, with a steeper increase in stiffness around the age of 60 years in women. For each T-score s.d., the corresponding carotid absolute values for arterial stiffness and compliance were obtained. In a multivariate model, carotid stiffness parameters were constantly and independently associated with age, mean arterial pressure, pulse pressure, heart rate and body mass index. Our study provides a normogram of carotid arterial stiffness and compliance indices obtained with the echo-tracking method in a large population of healthy subjects stratified by gender and age that can be used in clinical practice.
Similar content being viewed by others
Introduction
Arterial stiffness has emerged as a major independent predictor of cardiovascular risk and has been recognized as a marker of target organ damage.1 It can be measured via systemic or local arterial compliance or regionally via carotid–femoral pulse wave velocity (PWV). PWV is considered to be the ‘gold standard’ method for determining regional arterial stiffness and has been proven to be an independent predictor of cardiovascular events.1 However, this methodology has certain limitations, such as inaccuracy of distance measurements for the carotid–femoral distance measurement, and it is affected by properties of mixed elastic and muscular parts of the arterial tree. The less used echo-tracking system allows the assessment of local arterial stiffness by deriving the pressure–diameter curve of the artery (the measurements of changes in diameter over the cardiac cycle and the local pulse pressure (PP) should be determined simultaneously) and calculating local arterial stiffness from the time delay between two adjacent distension waveforms. Local arterial stiffness can be determined at different arterial sites, but the carotid artery is of special interest because it is more easily assessed and has been shown to be more stable, enabling evaluation of the stiffness of elastic arteries.2, 3 Recently, Engelen et al.4 reported the reference values of local carotid stiffness in a large group of healthy subjects. However, the measurements were derived from different systems and different anatomical locations of the carotid artery. Van Sloten et al.5 demonstrated the predictive value of carotid stiffness for cardiovascular events. In addition, an ultrasound-derived carotid stiffness estimation can be assessed simultaneously with the presence of plaques and precise measurements of intima–media thickness (IMT).
The aim of the present study was to produce reference values of local carotid arterial stiffness parameters using a single echo-tracking system and a standardized anatomical location in a large population of healthy subjects and to assess the relative impact of age, gender, anthropometric and hemodynamic indices, as well as lifestyle habits.
Methods
A total of 968 subjects were prospectively enrolled and recruited from a single center (San Antonio Hospital, San Daniele del Friuli, Udine, Italy), and a final number of 900 were considered healthy. Participants were selected from subjects who were investigated for work eligibility, subjects who were healthy blood donors, subjects who underwent electrocardiogram for obtaining access to spa facilities and subjects who were university members of the third age. All subjects underwent physical examination and anthropometry; they also completed questionnaires regarding their medical history, physical activity, coffee intake, alcohol use and smoking.6 None of the postmenopausal women were on estrogen replacement therapy. Weight (in kilograms) and height (in meters) were measured using standard techniques, and body mass index (BMI) was calculated as body weight divided by height squared. Body surface area was calculated using the DuBois formula (0.20247 × height0.725 × weight0.425).
To generate a healthy sample, subjects were excluded if they had diabetes mellitus, hypertension (systolic blood pressure (SBP) ⩽140 mm Hg and diastolic blood pressure (DBP) ⩽90 mm Hg), nephropathy, cardiovascular disease including valve diseases or any other condition requiring chronic medication.7 All subjects underwent blood pressure and heart rate (HR) measurements in the supine position with an oscillometric semiautomatic sphygmomanometer. These were measured two times in the right arm after 10 min of rest in a quiet room and then before the carotid study; the two readings were taken 30 min apart. Phase V Korotkoff sounds were considered to be DBP, except for subjects with sounds tending towards zero, in whom phase IV was taken. PP (SBP−DBP) and mean arterial pressure (MAP) (((2 × DBP)+SBP)/3) were calculated.
Carotid artery stiffness parameters
Local arterial stiffness was evaluated at the level of the left common carotid artery 1 to 2 cm before its bifurcation using a high-definition echo-tracking ultrasound system (Alpha 10; Hitachi Aloka, Tokyo, Japan) as described previously.7, 8 (The measurements carried out at the right and left common carotid were similar.) A wide-band multifrequency 5–13 MHz linear probe was used. Echo-tracking uses the raw radiofrequency signals that are based on the video signals with an accuracy of 0.01 mm. The optimal angle between the ultrasound beam and the vessel wall for diameter change measurements by echo-tracking is 90°. However, blood flow velocity perpendicular to the beam cannot be detected. A different ultrasound beam was used to overcome this problem that is independently steerable. Figure 1 (top left panel) shows a long-axis view of the common carotid artery and the ultrasound beam configuration with the independent beam steering function. The solid line shows the ultrasound beam direction for velocity measurements; the dotted line shows the beam direction for diameter change measurements. Both beams were steered to intersect at the center of the range gate. The ultrasound beam steering angle can be changed from −30° to +30°, with 5° angular increments. The echo-tracking gates were manually set at the boundaries between the intima and media of the anterior and posterior walls. The rate gate for velocity measurements was automatically positioned at the center of the diameter using echo-tracking gates. Flow velocity was corrected for the angle between the ultrasound beam and the blood flow velocity vector. Carotid arterial pressure and diameter change waveforms were similar during systole, whereas the carotid arterial pressure–diameter relationship showed slight nonlinearity and hysteresis during diastole (Figure 1, right panel).9 Brachial cuff pressure was measured just before starting ultrasound imaging and was entered into the system for calculation of carotid stiffness indices. At least five consecutive beats were averaged to obtain a representative waveform.
Measurement of arterial stiffness parameters with echo-tracking. Left panel: Echo-tracking gate at the level of the right common carotid artery and the change in diameter during the cardiac cycle. Right panel: Arterial stiffness parameters. AC, arterial compliance; β, beta-index; Ep, pressure–strain elastic modulus; PWV-β, one-point pulse wave velocity. A full color version of this figure is available at the Hypertension Research journal online.
The following indices were calculated for the common carotid artery (Figure 1, left panel):
-
1
β-stiffness (stiffness parameter), index of arterial stiffness: β = In
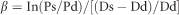
-
2
Ep (pressure–strain elastic modulus), index of vessel stiffness:
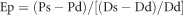
-
3
PWV (one-point PWV):
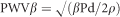
-
4
AC (arterial compliance), index of blood vessel compliance:
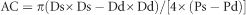
where Ps and Pd are systolic and diastolic brachial pressure, respectively (used as a surrogate for carotid SBP and DBP), Ds and Dd are carotid arterial systolic and diastolic diameter, respectively, and ρ is blood density (1050 kg m−3).
Reproducibility was assessed with determination of interobserver and intraobserver variability in 50 randomly selected healthy subjects. Intraobserver intersession variability was assessed by one observer by performing two sessions on different days in the same subject. The time interval between sessions ranged from 2 days to 1 week. Interobserver intrasession variability was evaluated by two observers who measured echo-tracking in each subject consecutively.
One-point carotid PWV obtained by the echo-tracking system implemented in the ultrasound machine was compared with the gold standard carotid–femoral PWV assessed with the SphygmoCor system (Model SCOR-Px, Software version 7.01; AtCor Medical, Sydney, NSW, Australia).10
Statistical analysis
Summary statistics are presented as the median, mean and s.d. and 5–95% confidence interval. The study population was divided into seven age groups (group 1: 16–19 years, n=58; group 2: 20–29 years, n=172; group 3: 30–39 years, n=147; group 4: 40–49 years, n=159; group 5: 50–59 years, n=133; group 6: 60–69 years, n=107; and group 7: ⩾70 years, n=124) and then stratified by gender. An unpaired Student’s t-test was used to compare gender differences, and analysis of variance (ANOVA) was performed to evaluate differences among age groups. Pearson's partial correlation was used to assess clinically relevant variables in men and women separately, which were then entered into the multivariate model. To easily compare stiffness and compliance parameters across the different age groups, individual scores were transformed into T-scores, indicating how many s.d. units an individual’s score was above or below the mean that was observed in the referred group, including same-sex individuals who were aged 36 to 44 years. The formula to calculate the T-scores was as follows:
T-score=(individual stiffness parameter value−stiffness parameter mean value in age group 36–44 years)÷stiffness parameter s.d. value in age group 36–44 years.
Inter- and intraobserver variability was examined using correlation coefficients (Pitman’s test of differences in variation) and mean differences, and limits of agreement are reported. Statistical significance was set at P<0.05.
All statistical analyses were performed using SYSTAT v.12.0 (Systat Software, Chicago, IL, USA).
Results
General characteristics
A total of 900 healthy subjects (500 males; mean age 45.8±19 years; range 16–94 years) were included in the study. Demographic and clinical characteristics, blood chemistry and one-point carotid stiffness compliance of the population are reported in Table 1. Females were slightly older with lower blood pressure and higher HR; carotid stiffness parameters were significantly higher in females, but after adjustment for age and HR, only arterial compliance was still significantly lower in females. IMT was lower in females, and the difference reached statistical significance when the data were adjusted for age and HR. Blood chemistry was characterized by higher levels of lipid profiles in males as well as higher hemoglobin and serum creatinine.
After stratification by gender and age, height progressively decreased as age increased, and weight increased up to 60 years in men and then decreased, whereas in women, it started to decrease at 50 years. BMI increased up to 50 years in both genders and then stabilized in men and continued to increase in women (Table 2). SBP was lower in women until the age of 50 years when it became similar to that of men. DBP was comparable between genders in the two youngest groups; then, it was lower in women and became similar again in the oldest group (Table 3). MAP and PP were lower in women than in men across all age groups (Table 3). One-point carotid stiffness parameters increased significantly with age in both genders, and AC was constantly and significantly lower in women in the two youngest groups (Table 4). IMT increased with age (group 1: 0.44±0.07 mm and 0.43±0.05 mm in males and females, respectively, NS; group 2: 0.47±0.1 mm and 0.48±0.08 mm, NS; group 3: 0.58±0.1 mm and 0.49±0.09 mm, P<0.0001; group 4: 0.59±0.1 mm and 0.59±0.1 mm, NS; group 5: 0.69±0.17 mm and 0.63±0.14 mm, NS; group 6: 0.71±0.16 mm and 0.69±0.16 mm, NS; and group 7: 0.82±0.17 mm and 0.82±0.16 mm, NS) (ANOVA P<0.0001 for both genders).
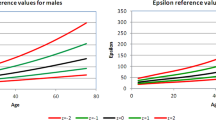
The T-score was found to increase significantly across all age groups in both genders, with women being slightly stiffer. In particular, there was a sharp increase in stiffness ~60 years of age in women and 65 years of age in men, with carotid stiffness in elderly women exceeding values for men. The opposite was observed for AC. Stiffness parameters did not change between the 16 and 25 years age group in women, whereas a progressive increase with age was observed in men from the age of 16 years (Figure 2).
Factors affecting carotid stiffness
In a simple correlation, one-point carotid stiffness parameters correlated positively with age, blood pressure parameters, HR, weight and BMI and negatively with height in men (Table 5) and with age, blood pressure parameters, weight and BMI in women, whereas HR correlated positively with PWV and negatively with height (Table 5). Gender correlated positively with β-index (r=0.106, P=0.014), Ep (r=0.07, NS) and PWV (r=0.076, NS) and negatively with AC (r=−0.199, P<0.0001). Physical activity correlated positively with AC (r=0.306) and negatively with β-stiffness (r=−0.328), Ep (r=−0.335) and PWV (r=−0.374) (P<0.0001 for all). Only 10% (n=92) of subjects were smokers, whereas 96% were coffee drinkers and light alcohol consumers; these parameters did not correlate with carotid arterial stiffness (NS). A multivariate model was constructed using all variables that were statistically significant in simple correlation analyses. Age, BMI, PP, MAP, HR and weight were independently correlated with one-point carotid stiffness. AC was correlated with age, PP, MAP, HR, physical activity and smoking. IMT was associated with age, BMI, PP and HR (Table 6).
Reproducibility
Interobserver correlation
The results of Pitman’s test of difference in variants for local arterial stiffness/compliance were as follows: β-stiffness r=−0.153 (NS), Ep r=0.173 (NS), PWV r=0.018 (NS) and AC r=0.146 (NS). On Bland–Altman analysis, the limits of agreement (reference range for difference) were −0.2027 to 1.830, with a mean difference of −0.098 (confidence interval (CI): −0.354 to 0.157) for β-stiffness; limits of agreement (reference range for difference) of −29.718 to 31.456, with a mean difference of 0.914 (CI: −3.150 to 4.978) for Ep; limits of agreement (reference range for difference) of −1.050 to 0.984, with a mean difference of −0.033 (CI −0.168 to 0.102) for PWV; and limits of agreement (reference range for difference) of −0.185 to 0.215, with a mean difference of 0.015 (CI −0.012 to 0.041) for AC (Supplementary Figure 1, top 4 graphs).
Intraobserver correlation
The results of Pitman’s test of difference in variants for local arterial stiffness/compliance were as follows: β-stiffness r=−0.387 (P=0.003), Ep r=−0.104 (NS), PWV r=−0.181 (NS) and AC r=0.013 (NS). In the Bland–Altman analysis, the limits of agreement (reference range for difference) were −4.048 to 3.460, with a mean difference of −0.294 (CI −0.792 to 0.204) for β-stiffness; limits of agreement (reference range for difference) of −69.263 to 72.238, with a mean difference of 1.487 (CI −7.899 to 10.874) for Ep; limits of agreement (reference range for difference) of −1.424 to 1.265, with a mean difference of −0.079 (CI −0.258 to 0.099) for PWV; limits of agreement (reference range for difference) of −0.305 to 0.308, with a mean difference of 0.002 (CI −0.039 to 0.043) for AC (Supplementary Figure 1, bottom 4 graphs).
Discussion
At present, there are no reference values in the literature for local carotid stiffness and compliance parameters derived from echo-tracking ultrasound methods. In this study, we determined the age- and sex-specific reference values for local carotid stiffness and compliance parameters in a group of healthy subjects aged 16 to 100 years using the one-point ultrasound wall-tracking method. As expected, a progressive increase in carotid stiffness with age and the opposite for compliance was recorded in both genders. The studied indices included β-stiffness (a measure of arterial wall stiffness independent of blood pressure),11 Ep (a measure of the mechanical properties of the arterial wall),12 AC (describing the absolute change in diameter or area for a given pressure change)13 and PWV (derived from β-stiffness and providing information on arterial stiffness at a specific region of interest).2 In a previous study from our group, one-point carotid PWV was found to correlate with carotid–femoral PWV measured with the SphygmoCor device in a heterogeneous population, identifying the cutoff of 6.65 m s−1 as the best predictor of carotid–femoral PWV >12 m s−1.9 SphygmoCor is considered the ‘gold standard’ for arterial stiffness measurements, and its predictive value for cardiovascular events and mortality is well established.14 The guidelines for the management of arterial hypertension15 as well as the guidelines for the diagnosis and treatment of aortic diseases16 suggest measuring both vascular stiffness and target organ damage.
Local carotid arterial stiffness provides similar information to carotid–femoral PWV on the impact of aging on arterial stiffness in normal subjects, although some argue that this is not the case for hypertensive and/or diabetic patients.17 However, recent evidence demonstrated an association between carotid stiffness (also measured by echo-tracking),18 target organ involvement7, 8, 19, 20 and cardiovascular events in different pathophysiological scenarios also including healthy subjects.5, 21, 22, 23, 24
In the present study, carotid stiffness, β-stiffness, Ep and PWV were similar in men and women for all of the age groups, whereas AC was consistently lower in women. These are not unexpected data, and the lower AC is related to a smaller body size in women and smaller arterial diameter. However, because arterial stiffness is similar between genders, carotid distensibility that better reflects the arterial wall characteristics is not different.25 Boutouyrie et al.26 described regional stiffness, carotid–femoral PWV, in a large cohort of subjects and reported higher arterial stiffness in men than in women but the difference almost disappeared after adjusting for age. More recently, Vermeersch et al.27 showed that carotid–femoral PWV increased at the same rate in men and women and did not differ between genders in any of the age groups. They also found that local stiffness (carotid artery) increased more rapidly than carotid–femoral aortic stiffness in women than in men over the studied age range when considering all of the variables (compliance, distensibility and PWV), suggesting a different response of the two arteries to increasing age in the two genders.
Normal reference values for one-point carotid stiffness parameters are of clinical utility when evaluating an individual subject as compared with what is expected in a reference population. To compare stiffness parameters across different age groups, T-scores were considered instead of mean stiffness values because they make it easier to determine how much individual stiffness values vary or deviate from those expected in healthy adults. In addition, for each T-score s.d., the corresponding carotid stiffness and compliance absolute values were obtained. All parameters showed a non-linear positive relationship between age and carotid stiffness and a negative relationship with compliance in both genders, with a steeper increase after 60 years of age in women. An earlier and steeper increase in carotid stiffness in women was also reported by Engelen et al.4 Mean age at natural menopause is around 51 years,28 and both aortic and carotid stiffness were found to be higher as the time since menopause increased, either in patients or normotensive postmenopausal women.29 In our study, local carotid stiffness was somewhat lower in women than in men until the age of 60 years but increased thereafter and exceeded that of men, suggesting that menopause is a major contributor to the age-dependent increase in arterial stiffness. It is well known that estrogen is a potent vasodilator with antiatherosclerotic effects, and estrogen deficiency in postmenopausal women leads to rapid arterial stiffening.30 In our study, BMI was also shown to have a role; until the age of 59 years, women were constantly lighter than men, but thereafter, no differences in weight were observed between genders. Therefore, it is likely that menopause, along with an increase in BMI, may have contributed to the sharp increase in local arterial stiffness after 60 years of age. In an analysis of data derived from the Multi-Ethnic Study of Atherosclerosis, Stern et al.31 found that women between 45 and 54 years of age had lower arterial stiffness than men, but beyond the age of 55 years, arterial distensibility was similar between sexes. These data also suggest that postmenopausal women bear a greater cardiac afterload than men and may be more predisposed to heart failure.32
Several studies demonstrated that elevated resting HR is a predictor of adverse cardiovascular events, even after adjustment for physical activity.33 Similarly, in our population of healthy subjects, HR correlated positively with arterial stiffness and negatively with arterial compliance. The association between HR and arterial stiffness and arterial compliance is based on decreased dynamic compliance, shorter time available for arterial wall recoil, a greater number of pulsatile strain cycles and elastic fiber fracture, all of which contribute to age-related arterial stiffening and promote atherosclerotic lesion formation.34, 35, 36
PP showed a J-curve pattern from adolescence to old age, as a result of greater pressure amplification from the aorta to peripheral arteries in younger subjects and increased aortic stiffening in older adults.37 However, MAP increased progressively from the youngest group to the elderly. Blood pressure parameters, such as MAP or steady components, and PP or pulsatile components were independently associated with carotid stiffness/compliance.
In our study, age, BMI, blood pressure parameters and HR were the key determinants of local arterial stiffness and compliance, with the exception of BMI and weight in relation to AC where gender, physical activity and smoking were included in the multiple regression model. Recently, Brunner et al.38 reported the same correlation between adiposity and arterial stiffness in both genders, demonstrating that the BMI to carotid–femoral PWV relationship accounted for 12% of the increment in cardiovascular risk resulting from higher BMI. In the univariate correlation analysis, female gender and physical activity were positively and negatively correlated with carotid stiffness, respectively; the opposite was observed for AC. On multivariate analysis, female gender and physical activity were independently associated only with AC in addition to age, PP, MAP, HR and smoking. A favorable and independent association has been reported between regular physical activity and central arterial compliance, counterbalancing the effect of aging.39, 40 The mechanism by which aerobic exercise improves arterial compliance resides in the intrinsic characteristics of the artery, including the composition of the arterial wall (elastin and collagen fibers and structural determinants) and functional properties (e.g., vasoconstrictor effect of smooth muscle cells). One-point carotid compliance is also affected by smoking. The two most toxic compounds of cigarettes are nicotine and carbon monoxide, where nicotine causes endothelial cell proliferation and intimal hyperplasia and carbon monoxide increases circulating endothelial cells.41, 42
The IMT data of our study are consistent with those reported in the literature, in particular with regard to a positive correlation with age. In a multicenter experience comparing mean values between genders, IMT was significantly higher in men than in women, but when the difference between genders was tested within age groups, IMT was similar.43 Independent determinants were found to be SBP, BMI, PP and HR in a negative way. The negative relation between HR and IMT could be an interaction between different factors such as SBP, BMI and age. In fact, age is positively related to BP and weight and negatively related to HR with a decrease in HR of 1 pulse per minute per year. It could also be partially mediated by SBP; in fact, a lower HR with a prolonged diastolic time will shift the reflecting wave into a systolic phase, increasing the central arterial stiffness that in turn contributes to an increase in IMT.44 In another setting, the association between IMT and metabolic syndrome (hypertension, dyslipidemia, hyperglycemia and central obesity) was demonstrated, and arterial hypertension was demonstrated to be the most important determinant of IMT.45 The correlation between IMT and stiffness suggests a close relationship between structural and morphological modifications.46, 47, 48
Reproducibility in terms of inter- and intraobserver variability was good and similar to that reported by Magda et al.,49 although it was slightly lower in this study.
Limitations
Some limitations should be acknowledged. First, peripheral blood pressure measurements are known to overestimate central blood pressure, especially in young populations, and brachial pressure was used to calculate one-point carotid stiffness parameters. Second, all calculations were based on the assumption reported by Sugawara et al.9 who demonstrated a good linear relationship between carotid arterial pressure and diameter. Third, brachial pressure measurements were taken immediately before starting the carotid study, assuming that hemodynamic parameters did not change significantly during the exam. Fourth, the present results are derived from a single-center experience.
Conclusion
Our study provides a sonographic normogram of carotid arterial stiffness/compliance indices obtained with the echo-tracking method in a large population of healthy subjects stratified by gender and age, including anthropometry, hemodynamic parameters and lifestyle habits. These results may improve ultrasonic determination and interpretation of local arterial stiffness measures.
References
Lantelme P, Laurent S, Besnard C, Bricca G, Vincent M, Legedz L, Milon H . Arterial stiffness is associated with left atrial size in hypertensive patients. Arch Cardiovasc Dis 2008; 101: 35–40.
Harada A, Okada T, Niki K, Chang D, Sugawara M . On-line noninvasive one-point measurements of pulse wave velocity. Heart Vessels 2002; 17: 61–68.
Zambanini A, Cunningham SL, Parker KH, Khir AW, McG Thom SA, Hughes AD . Wave-energy patterns in carotid, brachial, and radial arteries: a noninvasive approach using wave-intensity analysis. Am J Physiol Heart Circ Physiol 2005; 289: H270–H276.
Engelen L, Bossuyt J, Ferreira I, van Bortel LM, Reesink KD, Segers P, Stehouwer CD, Laurent S, Boutouyrie P . Reference values for local arterial stiffness. Part A: carotid artery. J Hypertens 2015; 33: 1981–1996.
van Sloten TT, Sedaghat S, Laurent S, London GM, Pannier B, Ikram MA, Kavousi M, Mattace-Raso F, Franco OH, Boutouyrie P, Stehouwer CDA . Carotid stiffness is associated with incident stroke: a systematic review and individual participant data meta-analysis. J Am Coll Cardiol 2015; 66: 2116–2125.
Palatini P, Graniero GR, Mormino P, Nicolosi L, Mos L, Visentin P, Pessina AC . Relation between physical training and ambulatory blood pressure in stage I hypertensive subjects. Results of the HARVEST Trial. Hypertension and Ambulatory Recording Venetia Study. Circulation 1994; 90: 2870–2876.
Vriz O, Bossone E, Bettio M, Pavan D, Carerj S, Antonini-Canterin F . Carotid artery stiffness and diastolic function in subjects without known cardiovascular disease. J Am Soc Echocardiogr 2011; 24: 915–921.
Vriz O, Zito C, di Bello V, La Carrubba S, Driussi C, Carerj S, Bossone E, Antonini-Canterin F . Non-invasive one-point carotid wave intensity in a large group of healthy subjects: a ventricular-arterial coupling parameter. Heart Vessels 2016; 31: 360–369.
Sugawara M, Niki K, Furuhata H, Ohnishi S, Suzuki S . Relationship between the pressure and diameter of the carotid artery in humans. Heart Vessels 2000; 15: 49–51.
Vriz O, Driussi C, La Carrubba S, Di Bello V, Zito C, Carerj S, Antonini-Canterin F . Comparison of sequentially measured Aloka echo-tracking one-point pulse wave velocity with SphygmoCor carotid–femoral pulse wave velocity. SAGE Open Med 2013; 1: 2050312113507563.
Henry RM, Kostense PJ, Spijkerman AM, Dekker JM, Nijpels G, Heine RJ, Kamp O, Westerhof N, Bouter LM, Stehouwer CD . Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation 2003; 107: 2089–2095.
Peterson LH, Jensen RE, Parnell J . Mechanical properties of arteries in vivo. Circ Res 1960; 8: 622–639.
Roman MJ, Pini R, Pickering TG, Devereux RB . Non-invasive measurements of arterial compliance in hypertensive compared with normotensive adults. J Hypertens Suppl 1992; 10: S115–S118.
Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cifkova R, Cosentino F, De Carlo M, Gallino A, Landmesser U, Laurent S, Lekakis J, Mikhailidis DP, Naka KK, Protogerou AD, Rizzoni D, Schmidt-Trucksäss A, Van Bortel L, Weber T, Yamashina A, Zimlichman R, Boutouyrie P, Cockcroft J, O’Rourke M, Park JB, Schillaci G, Sillesen H, Townsend RR . The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015; 241: 507–532.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F . 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31: 1281–1357.
Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, von Allmen RS, Vrints CJM . 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014; 35: 2873–2926.
Paini A, Boutouyrie P, Calvet D, Tropeano AI, Laloux B, Laurent S . Carotid and aortic stiffness: determinants of discrepancies. Hypertension 2006; 47: 371–376.
Cusmà-Piccione M, Zito C, Khandheria BK, Pizzino F, Di Bella G, Antonini-Canterin F, Vriz O, Bello VA, Zimbalatti C, La Carrubba S, Oreto G, Carerj S . How arterial stiffness may affect coronary blood flow: a challenging pathophysiological link. J Cardiovasc Med (Hagerstown) 2014; 15: 797–802.
Zito C, Mohammed M, Todaro MC, Khandheria BK, Cusmà-Piccione M, Oreto G, Pugliatti P, Abusalima M, Antonini-Canterin F, Vriz O, Carerj S . Interplay between arterial stiffness and diastolic function: a marker of ventricular-vascular coupling. J Cardiovasc Med (Hagerstown) 2014; 15: 788–796.
Mohammed M, Zito C, Cusmà-Piccione M, Di Bella G, Antonini-Canterin F, Taha NM, Di Bello V, Vriz O, Pugliatti P, Carerj S . Arterial stiffness changes in patients with cardiovascular risk factors but normal carotid intima-media thickness. J Cardiovasc Med (Hagerstown) 2013; 14: 622–628.
Yang EY, Chambless L, Sharrett AR, Virani SS, Liu X, Tang Z, Boerwinkle E, Ballantyne CM, Nambi V . Carotid arterial wall characteristics are associated with incident ischemic stroke but not coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study. Stroke 2012; 43: 103–108.
Störk S, van den Beld AW, von Schacky C, Angermann CE, Lamberts SW, Grobbee DE, Bots ML . Carotid artery plaque burden, stiffness, and mortality risk in elderly men: a prospective, population-based cohort study. Circulation 2004; 110: 344–348.
Haluska BA, Jeffries L, Carlier S, Marwick TH . Measurement of arterial distensibility and compliance to assess prognosis. Atherosclerosis 2010; 209: 474–480.
Dijk JM, Algra A, van der Graaf Y, Grobbee DE, Bots ML . Carotid stiffness and the risk of new vascular events in patients with manifest cardiovascular disease. The SMART study. Eur Heart J 2005; 26: 1213–1220.
Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME . Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol 2001; 37: 1374–1380.
Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 2010; 31: 2338–2350.
Vermeersch SJ, Rietzschel ER, De Buyzere ML, De Bacquer D, De Backer G, Van Bortel LM, Gillebert TC, Verdonck PR, Segers P . Age and gender related patterns in carotid-femoral PWV and carotid and femoral stiffness in a large healthy, middle-aged population. J Hypertens 2008; 26: 1411–1419.
Mendoza N, Juliá MD, Galliano D, Coronado P, Díaz B, Fontes J, Gallo JL, García A, Guinot M, Munnamy M, Roca B, Sosa M, Tomás J, Llaneza P, Sánchez-Borrego R . Spanish consensus on premature menopause. Maturitas 2015; 80: 220–225.
Kallikazaros I, Tsioufis C, Zambaras P, Stefanadis C, Toutouzas P . Conjugated estrogen administration improves common carotid artery elastic properties in normotensive postmenopausal women. Clin Cardiol 2002; 25: 167–172.
Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S . Influences of age and gender on results of noninvasive brachial–ankle pulse wave velocity measurement—a survey of 12517 subjects. Atherosclerosis 2003; 166: 303–309.
Stern R, Tattersall MC, Gepner AD, Korcarz CE, Kaufman J, Colangelo LA, Liu K, Stein JH . Sex differences in predictors of longitudinal changes in carotid artery stiffness: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2015; 35: 478–484.
Athyros VG, Pagourelias ED, Gossios TD, Vasilikos VG . Treating heart failure with preserved ejection fraction related to arterial stiffness. Can we kill two birds with one stone? Curr Vasc Pharmacol 2015; 13: 368–380.
Diaz A, Bourassa MG, Guertin MC, Tardif JC . Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J 2005; 26: 967–974.
Mangoni AA, Mircoli L, Giannattasio C, Ferrari AU, Mancia G . Heart rate-dependence of arterial distensibility in vivo. J Hypertens 1996; 14: 897–901.
Palatini P, Julius S . Heart rate and the cardiovascular risk. J Hypertens 1997; 15: 3–17.
Nichols WW, O’Rourke MF (eds). McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles, 5th edn. Hodder Arnold: London, UK, 2005.
Coutinho T, Bailey KR, Turner ST, Kullo IJ . Arterial stiffness is associated with increase in blood pressure over time in treated hypertensives. J Am Soc Hypertens 2014; 8: 414–421.
Brunner EJ, Shipley MJ, Ahmadi-Abhari S, Tabak AG, McEniery CM, Wilkinson IB, Marmot MG, Singh-Manoux A, Kivimaki M . Adiposity, obesity, and arterial aging: longitudinal study of aortic stiffness in the Whitehall II cohort. Hypertension 2015; 66: 294–300.
Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR . Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000; 102: 1270–1275.
Huynh QL, Blizzard CL, Raitakari O, Sharman JE, Magnussen CG, Dwyer T, Juonala M, Kähönen M, Venn AJ . Vigorous physical activity and carotid distensibility in young and mid-aged adults. Hypertens Res 2015; 38: 355–360.
Rajkumar S, Schmidt-Trucksäss A, Wellenius GA, Bauer GF, Huynh CK, Moeller A, Röösli M . The effect of workplace smoking bans on heart rate variability and pulse wave velocity of non-smoking hospitality workers. Int J Public Health 2014; 59: 577–585.
Ciftçi O, Günday M, Calişkan M, Güllü H, Güven A, Müderrisoğlu H . Light cigarette smoking and vascular function. Acta Cardiol 2013; 68: 255–261.
Ciccone MM, Balbarini A, Teresa Porcelli M, Santoro D, Cortese F, Scicchitano P, Favale S, Butitta F, De Pergola G, Gullace G, Novo S . Carotid artery intima–media thickness: normal and percentile values in the Italian population (camp study). Eur J Cardiovasc Prev Rehabil 2011; 18: 650–655.
Stoner L, Lambrick DM, Faulkner J, Young J . Guidelines for the use of pulse wave analysis in adults and children. J Atheroscler Thromb 2013; 20: 404–406.
Hirata C, Miyai N, Idoue A, Utsumi M, Hattori S, Iwahara A, Uematsu Y, Shiba M, Arita M . Effect of metabolic syndrome components and their clustering on carotid atherosclerosis in a sample of the general Japanese population. Hypertens Res 2016; 39: 362–366.
Jarauta E, Mateo-Gallego R, Bea A, Burillo E, Calmarza P, Civeira F . Carotid intima–media thickness in subjects with no cardiovascular risk factors. Rev Esp Cardiol 2010; 63: 97–102.
Claridge MW, Bate GR, Hoskins PR, Adam DJ, Bradbury AW, Wilmink AB . Measurement of arterial stiffness in subjects with vascular disease: are vessel wall changes more sensitive than increase in intima–media thickness? Atherosclerosis 2009; 205: 477–480.
Yildirim A, Kosger P, Ozdemir G, Sahin FM, Ucar B, Kilic Z . Carotid intima–media thickness and elastic properties of aortas in normotensive children of hypertensive parents. Hypertens Res 2015; 38: 621–626.
Magda SL, Ciobanu AO, Florescu M, Vinereanu D . Comparative reproducibility of the noninvasive ultrasound methods for the assessment of vascular function. Heart Vessels 2013; 28: 143–150.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Supplementary information
Rights and permissions
About this article
Cite this article
Vriz, O., Aboyans, V., Minisini, R. et al. Reference values of one-point carotid stiffness parameters determined by carotid echo-tracking and brachial pulse pressure in a large population of healthy subjects. Hypertens Res 40, 685–695 (2017). https://doi.org/10.1038/hr.2017.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2017.24
Keywords
This article is cited by
-
Compliant vascular models 3D printed with the Stratasys J750: a direct characterization of model distensibility using intravascular ultrasound
3D Printing in Medicine (2021)
-
Augmentation index predicts mortality in patients with aortic stenosis: an echo-tracking study
The International Journal of Cardiovascular Imaging (2021)
-
Age related structural and functional changes in left ventricular performance in healthy subjects: a 2D echocardiographic study
The International Journal of Cardiovascular Imaging (2019)