Abstract
Cardiovascular disease mortality is reduced following smoking cessation but the reversibility of specific atherogenic risk factors such as endothelial dysfunction is less established. We assessed brachial artery flow-mediated dilation (FMD) in 57 chronic smokers and 15 healthy controls, alone and after oral tetrahydrobiopterin (BH4) administration, to assess the extent to which reduced bioactivity of BH4, a cofactor for the endothelial nitric oxide synthase enzyme (eNOS), contributes to smoking-associated reductions in FMD. Thirty-four smokers then ceased cigarette and nicotine use for 1 week, after which FMD (±BH4 administration) was repeated. Brachial artery FMD was calculated as the peak dilatory response observed relative to baseline (%FMD). Endothelium-independent dilation was assessed by measuring the dilatory response to sublingual nitroglycerin (%NTG). Chronic smokers exhibited reduced %FMD relative to controls: (5.6±3.0% vs. 8.1±3.7%; P<0.01) and %NTG was not different between groups (P=0.22). BH4 administration improved FMD in both groups (P=0.03) independent of smoking status (P=0.78) such that FMD was still lower in smokers relative to controls (6.6±3.3% vs. 9.8±3.2%; P<0.01). With smoking cessation, FMD increased significantly (from 5.0±2.9 to 7.8±3.2%;P<0.01); %NTG was not different (P=0.57) and BH4 administration did not further improve FMD (P=0.33). These findings suggest that the blunted FMD observed in chronic smokers, likely due at least in part to reduced BH4 bioactivity and eNOS uncoupling, can be restored with smoking cessation. Post-cessation BH4 administration does not further improve endothelial function in chronic smokers, unlike the effect observed in nonsmokers, indicating a longer-term impact of chronic smoking on vascular function that is not acutely reversible.
Similar content being viewed by others
Introduction
Nearly 18% of American adults currently smoke cigarettes, making it the leading cause of preventable disease and death in the United States.1 Moreover, smoking is a major risk factor for atherosclerotic disease with manifestations ranging from angina to coronary artery disease, stroke and peripheral vascular disease.2, 3
Diminished vascular endothelial function is thought to be one of the mechanisms underlying the progression of atherosclerosis and cardiovascular disease in smokers.4, 5, 6 The influence of smoking on the vascular endothelium is attributable in part to the reactive oxygen species generated by tobacco use, which diminish nitric oxide (NO) bioavilability, increase inflammation and augment oxidative stress.7, 8, 9 Specifically, free radicals arising either directly (from the gas or tar phase of cigarette smoke) or indirectly (from stimulation of endogenous sources) from smoking oxidize important metabolites of vascular endothelial function, including tetrahydrobiopterin (BH4).10 BH4 is a cofactor for the endothelial NO synthase enzyme (eNOS) that modulates production of NO. When BH4 is oxidized, eNOS no longer produces NO (a process termed eNOS uncoupling), thus reducing endothelium-dependent dilation.11 The long-term combination of endothelial dysfunction and heightened oxidative stress also triggers the creation of foam cells in the smooth muscle cell wall, which initiates eventual atheromatic plaque formation.12 Notably, brachial smooth muscle dilation to an exogenous NO donor (that is, endothelium-independent function) may also be reduced in apparently healthy and relatively young chronic smokers,6, 13, 14 indicating that endothelial dysfunction can quickly progress into more advanced manifestations of atherosclerotic disease in this susceptible population.
Large population-based studies have established that general cardiovascular disease mortality risk is reduced with smoking cessation15, 16, 17 and there is a direct relationship between the time since quitting and slowing of atherosclerotic progression in former smokers.18 However, the reversibility of specific vascular atherogenic risk factors such as endothelial function following smoking cessation is less well established. Short-term smoking cessation augments venous hand vein endothelial function19 and brachial artery endothelial function20, 21 in healthy young smokers. By contrast, 24 h of smoking cessation with nicotine replacement therapy does not improve endothelial function unless co-administered with vitamin E,22 and some cross-sectional studies of former smokers show that endothelial function is either similarly attenuated5 or only marginally improved13 relative to current smokers. These disparate findings may be attributable to the extent to which smoking-related oxidative stress evokes reversible endothelial dysfunction or whether there are additional vascular decrements contributing to the atherogenic progression.23
Accordingly, the purpose of the present investigation was to assess brachial artery endothelial function in chronic smokers, alone and with BH4 administration, before and after smoking cessation, to determine whether the diminished vascular function associated with smoking and associated reductions in the bioactivity of BH4 are acutely reversible with 1 week of complete tobacco and nicotine cessation.
Methods
Study population
Both chronic smokers (>10 years of pack per day smoking) and healthy controls, ages 30–50 years, were recruited for the study. Potential subjects were excluded from enrollment based on known criteria that influence vascular function and confound interpretation of data including the following: diagnosed chronic disease (cardiovascular, pulmonary, metabolic, neurological and/or psychiatric), stage I or higher blood pressure (>140/90 mm Hg), medications affecting hemodynamic variables (cholesterol-lowering medications, blood pressure-lowering medications, use of hormone therapy or oral contraceptives in the last 12 months), regular exercise >2 days a week, pregnancy, use of antioxidants (for example, vitamins C and E) within the last 6 weeks or current/past illicit drug use. Subjects were recruited via flyers, direct mailers targeting smokers in surrounding towns, hospital employee announcements and by word of mouth. The study was approved by the Institutional Review Board at Hartford Hospital and procedures were followed in accordance with the Declaration of Helsinki. Subjects gave their informed consent for participation on enrollment into the study.
Overview of study procedures
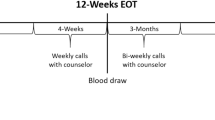
Smokers and healthy controls participated in three initial visits: a screening visit, a study visit consisting of brachial artery flow-mediated dilation (FMD) and nitroglycerin (NTG)-induced dilation, and an additional study visit (FMD and NTG after oral administration of BH4; Figure 1). The order of the two study visits was randomized and conducted on separate days, to ensure that there was no drug order effect for BH4 administration. Smokers were then asked to quit smoking for 7 days, in order for the vascular testing visits to be completed. To assist with cessation, smokers were offered free structured smoking cessation counseling with a trained counselor and established program,24 and/or free nicotine replacement therapy (4 and 2 mg nicotine gum or 21, 14 and 7 mg nicotine patch). Subjects were called on a weekly basis to follow-up and assess quit status. Following 7 days of self-reported smoking cessation, subjects were asked to report back to the laboratory. Post testing included carbon monoxide (CO) measurement and serum cotinine testing via venous blood draw. Confirmation of successful smoking cessation was affirmed if, and only if, subjects met the following three criteria: (1) no cigarette use in the last 7 days (that is, 7-day point cessation smoking status), (2) a CO measurement <10 p.p.m. and (3) a serum cotinine level <3 ng ml−1. The study visits were repeated, again in randomized order with all participants who stopped smoking. All follow-up measurements were performed 1 week after the subject’s last use of nicotine replacement therapy, as transdermal25 and oral26 nicotine acutely influence endothelial function and confound serum cotinine measurements.
Screening procedures
Subjects who passed the initial phone screen for inclusion/exclusion criteria reported to Hartford Hospital having fasted (no food or beverage other than water) for at least 12 h. Subject’s vital signs (blood pressure and heart rate), height, weight and waist circumference were measured. Detailed information regarding medical history, including allergies and diagnosed medical conditions, was obtained. Subjects were asked to blow into a hand-held monitor (Vitalograph, Lexus, KS, USA) to measure breath CO levels. Past and current smoking behavior such as cigarettes smoked per day, number of years smoking and longest quit attempt was assessed. Subjects were also queried on prior use of pharmacotherapy for smoking cessation and past history of other drug use and dependence. The Fagerstrom Test for Nicotine Dependence27 was used to measure dependence on nicotine, with 0 indicating no dependence and 10 indicating the greatest level of dependence.
Subjects then underwent a venous blood draw to assess blood lipids and serum cotinine levels. Plasma lipids (total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and triglycerides) were measured using spectrophotometry and cotinine values were measured via enzyme-linked immunosorbescent assay at the Clinical Laboratory Partners, Hartford, CT, USA.
Arterial stiffness and central blood pressure amplification were also assessed using the SphygmoCor CPV Central Blood Pressure/Pulse Wave Velocity System (AtCor Medical, Sydney, NSW, Australia). Briefly, a tonometer was held on the radial artery to obtain readings of the pulse waveform over 10 s. The tonometer transduced dynamic changes in arterial force and volume into a complete pressure waveform calibrated using systolic and diastolic pressure values generated from brachial cuff measurement. A generalized transfer function gain was then applied to the pulse wave derived from the radial artery to reconstruct the aortic pulse and determine the aortic pulse pressure amplification between the aorta and the radial artery. Augmentation index was calculated as the difference in pressure between the systolic shoulder of the ascending pressure curve and the systolic peak, expressed as an absolute value (Augmentation Pressure) and relative to a heart rate of 75 b.p.m. (Augmentation Index @ HR 75). For the measurement of central pulse wave velocity (PWV), the operator located both the right carotid and right femoral pulses and placed the applanation tonometer on each site sequentially to take multiple readings of the pulse wave at those sites. The distance between the points of measurement of the carotid and femoral pulses were recorded by taking measurements on the surface of the body from the suprasternal notch to the point where the right carotid pulse was found, and from the suprasternal notch to the right femoral pulse via the umbilicus; PWV was calculated as the propagation of the pulse wave over the known distance. For peripheral PWV, the same procedure was repeated to assess propagation of the pulse wave between the femoral and posterior tibial artery.
Based on data gathered at the initial screening session, classification of a subject as a smoker was confirmed if, and only if, the subject met the following three criteria: (1) a self-reported chronic smoking habit of at least one pack per day for >10 years, (2) a CO level >10 p.p.m.28 and (3) a serum cotinine value >3 ng ml−1.29 Subjects who did not currently self-report smoking or tobacco use, had no previous history of smoking or tobacco use and exhibited a CO <10 p.p.m. and serum cotinine <3 ng ml−1 were classified as healthy controls.
Standardization of conditions
For all subsequent study visits, subjects were fasted and asked to refrain from exercise, caffeine, migraine medicine, alcohol, aspirin, ibuprofen, phosphodiesterase inhibitors (for example, Viagra) or herbal supplements for at least 24 h before testing.
Brachial FMD test
Brachial FMD of the nondominant arm of each subject was measured with the subject lying supine with the arm extended 80° from the torso at heart level. Blood pressure in the dominant arm was measured every minute with an automated blood pressure monitor (Vital Signs Monitor; WelchAllyn, Skaneateles, NY, USA). A rapid-inflation/deflation pneumatic cuff (Hokanson, Bellevue, WA, USA) was placed around the forearm immediately distal to the olecranon process.30 The artery was imaged 1–3 inches proximal to the olecranon process using a 5- to 12-MHz multifrequency linear-array transducer attached to a high-resolution ultrasound machine (Terason t3000; TeraTech Corp, Burlington, MA, USA). Once a satisfactory image using optimal B-mode imaging was obtained, the placement of the probe was fixed on the upper arm to ensure that the site of measurement did not change. Doppler velocity was also measured continuously with the Terason using a 60° angle of insonation held constant throughout the study. After the subject rested in the supine position for 10 min, resting brachial artery diameter and velocity were measured for 1 min before inflation of the pneumatic cuff. The cuff was then inflated to suprasystolic pressure (300 mm Hg) for 5 min; diameter and velocity recordings resumed 30 s before cuff deflation and continued for 3 min after deflation.
Sublingual NTG administration
After a 10-min rest period, endothelium-independent dilation of the arm was assessed with administration of 0.4 mg sublingual NTG (Nitrostat, Parke-Davis, Morris Plains, NJ, USA). After 1 min of baseline diameter measurements, diameters were measured continuously for 10 min after administration of NTG.
Oral BH4 administration and FMD/NTG test
The morning of the study visit, subjects took an oral dose (10 mg per kg body weight) of BH4 (Schirks Laboratories, Jona SG, Switzerland). The selection of this single dose increases plasma biopterin levels by 50-fold,31 has been used in previous studies32 and is commercially available to treat special forms of phenylketonuria with minimal side effects.33, 34 To ensure compliance with the BH4 treatment, subjects were contacted by phone the night before the experimental session. The subject was instructed to take the BH4 3 h before the scheduled FMD test in the morning, as this dose of BH4 reaches its maximal plasma concentration 3 h after oral administration.31 On the study visit, the FMD test and sublingual NTG administration were performed as described above.
Data analysis
Ultrasound images were recorded at five frames per second using Camtasia software (TechSmith, Okemos,MI, USA) and saved as AVI files. Diameters and velocities were analyzed using Brachial Analyzer software (Medical Imaging Applications LLC, Coralville, IA, USA). A technician, blinded to any subject information, selected a region of interest along the arterial wall and the edge of the intima-lumen interface was detected by pixel density and represented by a line of best fit. Multiple perpendicular lines were fit between the near and far walls, and averaged to report a composite diameter measurement for each frame. Diameter analyses were triggered by the corresponding Doppler waveform to capture only end diastolic diameters. Resting diameters were calculated as the average of images taken over the 1-min baseline. The peak diameter was calculated as the highest 3-s average observed post occlusion. FMD was expressed as the percent dilation (% FMD) relative to the baseline measurement for each trial. For velocity analysis, a region of interest was selected around the Doppler waveform and the trace of the velocity-time integral was used to calculate mean velocity for each cardiac cycle. Velocity was matched to the corresponding diameter and shear rate was calculated using the formula 4V/D, where D is artery diameter (cm) and V is in cm s−1. The shear rate post-occlusion area under the curve was then calculated from cuff release up until the time of peak diameter30 and FMD was normalized to the shear rate post-occlusion area under the curve with the latter divided by an arbitrary value of 1000, to simplify data presentation. Our previous work established the coefficient of variation for this measurement and analysis technique for two repeated trials of brachial FMD as 3.5% for men and 3.7% for women.35 For NTG measurements, diameters were analyzed as described above, with the resting diameter calculated as the average of images taken over the 1-min baseline and the peak diameter calculated as the highest 3-s average observed post NTG administration. NTG dilation (% NTG) was calculated as the percent change in dilation relative to baseline.
Sample size calculations
Major comparisons of interest in the study were: (1) baseline differences in FMD and NTG dilation between chronic smokers and healthy controls, (2) baseline differences in improvement in FMD following administration of BH4 in chronic smokers vs. healthy controls, (3) alterations in FMD and NTG dilation in chronic smokers after smoking cessation and (4) improvement in FMD following administration of BH4 in chronic smokers before and after smoking cessation. Sample size calculations were based on cross-sectional differences in vascular function between smokers and nonsmokers (sample size estimated for independent samples t-test with an unequal ratio of control to experimental subjects) and smokers and former smokers (sample size estimated for paired t-test in smokers). All calculations assumed a power of 0.80 and a significance level of 0.05. Based on a review of existing literature with these comparisons,13, 36, 37 we estimated effects between and within groups in outcomes of FMD % dilation and NTG % dilation ranging from 1.8 to 5.0% with s.d. of 1.2–5.2%, yielding a desired sample size of 30 smokers and 15 controls for baseline comparisons and 34 smokers for pre-post smoking comparisons. Experiences at Hartford Hospital with smoking cessation programs suggested that abstinence rates would be about 45–50% immediately following the end of the financially incentivized program,24 in agreement with other published studies;38 hence, we aimed to recruit 50–75 smokers at baseline.
Statistical analyses
All statistical analyses were performed with SPSS 15.0 (SPSS, Chicago, IL, USA). Differences in subject characteristics between smokers and nonsmokers were determined by an independent t-test. A paired t-test was used to examine differences in subject characteristics in smokers before and after smoking cessation. Statistical comparisons for group differences and changes in FMD and NTG dilation before and after BH4 administration and smoking cessation were performed by a repeated-measures analysis of variance followed by Bonferroni post-hoc analysis, to identify significant differences among mean values when F-values were significant. Gender was included as a fixed factor to investigate sex differences and analysis of covariance was used to investigate whether any independent continuous predictor variables (at baseline or as changes with smoking cessation) modulated observed relationships.
Results
Subject characteristics
Subject characteristics at baseline and after smoking cessation in smokers are shown in Table 1; smokers differed from controls minimally at baseline, exhibiting a slightly older age, as well as higher resting systolic blood pressure and heart rate. Forty smokers self-reported total smoking cessation for at least the past 7 days but 6 were excluded from analysis due to serum cotinine levels >3 ng ml−1. Study visits were conducted on average 10±5 days after the last day of smoking. Among the 34 subjects who successfully quit smoking with cotinine verification, body mass index (from 29.7±8.3 to 30.2±8.5 kg m−2), total cholesterol (from 184±42 to 190±41 mg dl−1) and central PWV (from 7.3±1.8 to 8.0±2.1 m s−1) all increased significantly (P=0.02, 0.04 and<0.05, respectively) with smoking cessation.
Baseline brachial artery reactivity
Smokers had significantly lower FMD at baseline (P<0.01; Figure 2) than controls, which persisted after normalization to the shear stimulus (0.25±0.17 vs. 0.36±0.12 s−1; P=0.02), and when relevant covariates that were different between groups (age, systolic blood pressure and heart rate) were taken into account all (P<0.01). NTG-induced dilation was not different between groups (23.2±7.1% vs. 26.0±8.2%; P=0.22). BH4 administration improved FMD similarly in both groups (P=0.03 for drug effect) independent of smoking status (P=0.78 for group × drug interaction) such that FMD was still lower in smokers relative to controls (P<0.01; Figure 2) as was shear-normalized FMD (0.27±0.16 vs. 0.44±0.22 s−1; P<0.01). However, FMD and shear-normalized FMD measured after BH4 administration in smokers were not significantly different than baseline FMD (non-BH4 FMD) exhibited by controls (P=0.15 and 0.06, respectively). BH4 administration had no effect on nitroglycin-induced dilation (P=0.93) and again there was no group difference between smokers and controls in this condition (23.3±6.9% vs. 24.9±8.1%; P= 0.49).
Group means±s.d. of flow-mediated dilation (FMD; expressed as a % dilation above baseline) in smoking subjects (black bars) vs. healthy nonsmoking controls (white bars) with and without administration of BH4. *(P<0.05) difference between groups within each condition (baseline or BH4 treatment); †(P<0.05) difference within a group with BH4 treatment.
Effect of smoking cessation on brachial artery reactivity
With smoking cessation, FMD (Figure 3) and shear-normalized FMD (from 0.21±0.12 to 0.36±0.20 s−1) increased significantly (both P<0.01), although NTG-induced dilation was not different (from 23.5±6.9% to 24.4±6.0%; P=0.57). The change in cholesterol was the only significant covariate in models, as it was inversely related to the impact of smoking cessation on shear-normalized FMD (P=0.01), but this did not remarkably change the nature of the observed improvement pre-to post study (P=0.05 for effect of smoking cessation on shear-normalized FMD). All other baseline or change variables such as smoking dose or duration of smoking cessation investigated in the model were nonsignificant. In addition, FMD (Figure 3) and shear-normalized FMD (from 0.28±0.18 to 0.36±0.18 s−1) were also greater following BH4 administration after smoking cessation compared with BH4 administration at baseline (P<0.01 and P=0.01, respectively), and again NTG-induced dilation was not significantly different (from 22.9±7.9% to 26.1±7.7% P=0.06). However, after smoking cessation, BH4 administration did not further improve FMD, shear-normalized FMD or NTG-induced dilation relative to the non-BH4 condition (P=0.33, 0.94 and 0.14, respectively). Moreover, after smoking cessation, no dilatory variables (FMD, shear-normalized FMD or NTG-induced dilation), both with and without BH4 administration, were different than values exhibited by controls at baseline (all P>0.20).
Group means±s.d. of flow-mediated dilation (FMD; expressed as a % dilation above baseline) in smoking subjects (black bars) vs. the same subjects after successful smoking cessation (white bars) with and without administration of BH4. *(P<0.05) difference with smoking cessation; †(P<0.05) difference with BH4 treatment.
Discussion
Many studies have demonstrated that smokers exhibit blunted endothelial function relative to healthy controls.21, 37 One such mechanism underlying the blunted vasodilator function is a process termed eNOS uncoupling, a condition in which the eNOS enzyme produces superoxide rather than NO.39 Uncoupling is stimulated by, among other factors, a decrease in the bioavailability of BH4, as BH4 is an essential cofactor for eNOS function and healthy endothelial function. Cigarette smoking causes a concurrent oxidation of BH4 and inactivation of eNOS that is restored by supplementation of BH4.7, 40 For example, blunted forearm vasodilatory responses and brachial artery FMD in smokers can be improved by intra-arterial infusion41 and oral administration of BH4,37 respectively. In the current study, we did indeed observe that brachial artery FMD was ~25% lower in smokers relative to healthy controls and was improved by oral administration of BH4. Interestingly, despite exhibiting higher levels of FMD at baseline, healthy controls also exhibited improved FMD with administration of BH4. These findings suggest that even apparently healthy individuals may incur a chronic level of oxidative stress and inflammation, leading to the oxidation of BH4 and eNOS uncoupling, which diminishes endothelial function.
Most available data indicate that smoking cessation improves and/or restores endothelial function,42 although the mechanisms by which this occurs are less clear. For example, Kato et al.20 found that the use of varenicline to support smoking cessation reduced oxidative biomarkers and there was a correlation between reduced oxidative stress and improved endothelial function. By contrast, Sugiura et al.43 found that blunted endothelial function in smokers was inversely correlated to plasma serotonin levels; interestingly, 8 weeks smoking cessation improved endothelial function in smokers with no change in plasma serotonin. To the best of our knowledge, no study has directly tested the hypothesis that eNOS uncoupling and reduced BH4 bioactivity are restored by smoking cessation by directly administering BH4 before and after smoking cessation. In the current study, FMD was improved by smoking cessation to dilatory levels comparable to control nonsmokers and there were no statistically significant further improvements in FMD with BH4 administration after smoking cessation. This supports that smoking cessation improved FMD by reducing oxidative stress and restoring BH4 levels such that administration of BH4 did not have a further impact on endothelial function. As noted previously, nonsmokers exhibited improved FMD with BH4 administration even despite having greater FMD at baseline than smokers. This unexpected observation suggests that there may be mechanisms besides oxidative stress, diminished BH4 bioavailability and eNOS uncoupling,44, 45 which contribute to the blunted FMD observed in chronic smokers. In line with this hypothesis, we observed that central arterial stiffness (central PWV) also acutely increased with smoking cessation (Table 1). Moreover, Mah et al.21 found that additive α-tocopherol supplementation combined with 1 week smoking cessation improved endothelial function to a greater extent than smoking cessation alone in smokers, suggesting that systemic inflammation and oxidative stress is not fully restored by smoking cessation alone. Collectively, these observations indicate that endothelial dysfunction may not be fully reversible with acute smoking cessation and could explain the equivocal findings regarding FMD and endothelial function with short-term and long-term smoking cessation.
Interestingly, emerging data are showing that endothelium-independent function may be attenuated in smokers.14 We did not observe blunted endothelium-independent dilation in smoking subjects, as the dilatory response to NTG was not different between smokers and nonsmokers at baseline, and did not improve with smoking cessation. However, we administered a single, maximal dose of sublingual NTG (0.04 mg). Lanza et al.14 recently constructed a dose–response curve in smokers and nonsmokers of the brachial artery vasodilatory response to increasing doses of NTG (10–40 μg), reporting that impaired endothelial-independent dilation in smokers reached statistical significance only at lower levels of NTG administration (0.03 mg). These investigators hypothesized that a maximal dose of NTG may have masked abnormal submaximal endothelium-independent dilator function. Therefore, it is possible that the smokers in the current study did have attenuated endothelium-independent vasodilator function that we did not detect with one large dose of NTG and a blunted submaximal endothelium-independent vasodilator capacity could provide an alternative explanation as to why BH4 administration did not further increase FMD after smoking cessation as it did in the nonsmoking control group.
There are several limitations to the current study. We did not directly measure markers of oxidative stress, inflammation or reactive oxygen species, nor did we directly measure NO metabolites in the serum. However, rigorous data support that BH4 oxidation and eNOS uncoupling in smokers is caused primarily by the generation of reactive oxygen species associated with cigarette smoke.37, 41 In addition, we examined the effects of short-term smoking cessation on endothelium-dependent and -independent functions, and thus our discussion regarding the differences between acute and longer-term smoking cessation is speculative. For example, circulating endothelial progenitor cells are restored to nonsmoking levels after a month of smoking cessation.46 Further research is necessary to better define the time course of vascular adaptations following smoking cessation.
The current study provides, to the best of our knowledge, the first assessment of FMD with and without BH4 administration, before and after smoking cessation, to assess the extent to which smoking cessation restores the reduced BH4 bioactivity associated with chronic smoking. Our findings indicate that even short-term smoking cessation restores endothelium-dependent vasodilation to levels exhibited by control nonsmokers, such that administration of BH4 does not further augment FMD. These results are promising in that they suggest that a large portion of the endothelial dysfunction associated with chronic smoking is reversible, likely due to the influence of oxidative stress, free radical formation, BH4 depletion and eNOS uncoupling.41 However, given that we did not observe a similar enhancement in FMD with BH4 administration in smokers after smoking cessation as we did with nonsmoking controls, there may be additional detrimental vascular effects of chronic smoking that require longer durations of time and/or lifestyle or pharmacological interventions to be fully mitigated.
References
Current Cigarette Smoking Among Adults—United States, 2005–2013; c2014 (cited 2015 January 22). Available from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6347a4.htm.
LaCroix AZ, Lang J, Scherr P, Wallace RB, Cornoni-Huntley J, Berkman L, Curb JD, Evans D, Hennekens CH . Smoking and mortality among older men and women in three communities. N Engl J Med 1991; 324: 1619–1625.
Ambrose JA, Barua RS . The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 2004; 43: 1731–1737.
Wright JL, Churg A . Short-term exposure to cigarette smoke induces endothelial dysfunction in small intrapulmonary arteries: analysis using guinea pig precision cut lung slices. J Appl Physiol 2008; 104: 1462–1469.
Barr RG, Mesia-Vela S, Austin JH, Basner RC, Keller BM, Reeves AP, Shimbo D, Stevenson L . Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the emphysema and cancer action project (EMCAP) study. Am J Respir Crit Care Med 2007; 176: 1200–1207.
Thomas GN, Chook P, Yip TW, Kwong SK, Chan TY, Qiao M, Huang XS, Guo DS, Feng JZ, Chan SW, Leong HC, Celermajer DS, Woo KS . Smoking without exception adversely affects vascular structure and function in apparently healthy chinese: Implications in global atherosclerosis prevention. Int J Cardiol 2008; 128: 172–177.
Barua RS, Ambrose JA, Srivastava S, DeVoe MC, Eales-Reynolds LJ . Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: an in vitro demonstration in human coronary artery endothelial cells. Circulation 2003; 107: 2342–2347.
Barua RS, Ambrose JA, Eales-Reynolds LJ, DeVoe MC, Zervas JG, Saha DC . Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation 2001; 104: 1905–1910.
Messner B, Bernhard D . Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014; 34: 509–515.
Bernhard D, Wang XL . Smoking, oxidative stress and cardiovascular diseases—do anti-oxidative therapies fail? Curr Med Chem 2007; 14: 1703–1712.
Alp NJ, Channon KM . Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 2004; 24: 413–420.
Antoniades C, Tousoulis D, Tentolouris C, Toutouzas P, Stefanadis C . Oxidative stress, antioxidant vitamins, and atherosclerosis. from basic research to clinical practice. Herz 2003; 28: 628–638.
Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE . Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 1993; 88: 2149–2155.
Lanza GA, Spera FR, Villano A, Russo G, Di Franco A, Lamendola P, Crea F . Effect of smoking on endothelium-independent vasodilatation. Atherosclerosis 2015; 240: 330–332.
Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA . Smoking and smoking cessation in relation to mortality in women. JAMA 2008; 299: 2037–2047.
Rosenberg L, Kaufman DW, Helmrich SP, Shapiro S . The risk of myocardial infarction after quitting smoking in men under 55 years of age. N Engl J Med 1985; 313: 1511–1514.
Lao XQ, Jiang CQ, Zhang WS, Adab P, Lam TH, Cheng KK, Thomas GN . Smoking, smoking cessation and inflammatory markers in older chinese men: the Guangzhou Biobank Cohort Study. Atherosclerosis 2009; 203: 304–310.
McEvoy JW, Nasir K, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, Barr RG, Budoff MJ, Szklo M, Navas-Acien A, Polak JF, Blumenthal RS, Post WS, Blaha MJ . Relationship of cigarette smoking with inflammation and subclinical vascular disease: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol 2015; 35: 1002–1010.
Moreno H Jr, Chalon S, Urae A, Tangphao O, Abiose AK, Hoffman BB, Blaschke TF . Endothelial dysfunction in human hand veins is rapidly reversible after smoking cessation. Am J Physiol 1998; 275: H1040–H1045.
Kato T, Umeda A, Miyagawa K, Takeda H, Adachi T, Toyoda S, Taguchi I, Inoue T, Node K . Varenicline-assisted smoking cessation decreases oxidative stress and restores endothelial function. Hypertens Res 2014; 37: 655–658.
Mah E, Pei R, Guo Y, Ballard KD, Barker T, Rogers VE, Parker BA, Taylor AW, Traber MG, Volek JS, Bruno RS . Gamma-tocopherol-rich supplementation additively improves vascular endothelial function during smoking cessation. Free Radic Biol Med 2013; 65: 1291–1299.
Mah E, Pei R, Guo Y, Masterjohn C, Ballard KD, Taylor BA, Taylor AW, Traber MG, Volek JS, Bruno RS . Greater gamma-tocopherol status during acute smoking abstinence with nicotine replacement therapy improved vascular endothelial function by decreasing 8-iso-15(S)-prostaglandin F2alpha. Exp Biol Med (Maywood) 2015; 240: 527–533.
Yamagishi S, Matsui T, Nakamura K . Possible involvement of tobacco-derived advanced glycation end products (AGEs) in an increased risk for developing cancers and cardiovascular disease in former smokers. Med Hypotheses 2008; 71: 259–261.
Dornelas EA, Sampson RA, Gray JF, Waters D, Thompson PD . A randomized controlled trial of smoking cessation counseling after myocardial infarction. Prev Med 2000; 30: 261–268.
Yugar-Toledo JC, Ferreira-Melo SE, Sabha M, Nogueira EA, Coelho OR, Consolin Colombo FM, Irigoyen MC, Moreno H Jr . Blood pressure circadian rhythm and endothelial function in heavy smokers: acute effects of transdermal nicotine. J Clin Hypertens (Greenwich) 2005; 7: 721–728.
Sarabi M, Lind L . Short-term effects of smoking and nicotine chewing gum on endothelium-dependent vasodilation in young healthy habitual smokers. J Cardiovasc Pharmacol 2000; 35: 451–456.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO . The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict 1991; 86: 1119–1127.
Stookey GK, Katz BP, Olson BL, Drook CA, Cohen SJ . Evaluation of biochemical validation measures in determination of smoking status. J Dent Res 1987; 66: 1597–1601.
Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J . Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the united states between 1999 and 2004. Am J Epidemiol 2009; 169: 236–248.
Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ . Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011; 300: H2–H12.
Fiori L, Fiege B, Riva E, Giovannini M . Incidence of BH4-responsiveness in phenylalanine-hydroxylase-deficient italian patients. Mol Genet Metab 2005; 86: S67–S74.
Eskurza I, Myerburgh LA, Kahn ZD, Seals DR . Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 2005; 568: 1057–1065.
Niederwieser A, Curtius HC, Wang M, Leupold D . Atypical phenylketonuria with defective biopterin metabolism. monotherapy with tetrahydrobiopterin or sepiapterin, screening and study of biosynthesis in man. Eur J Pediatr 1982; 138: 110–112.
Kure S, Hou DC, Ohura T, Iwamoto H, Suzuki S, Sugiyama N, Sakamoto O, Fujii K, Matsubara Y, Narisawa K . Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. J Pediatr 1999; 135: 375–378.
Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ . Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. Am J Physiol Heart Circ Physiol 2011; 301: H1118–H1126.
Esen AM, Barutcu I, Acar M, Degirmenci B, Kaya D, Turkmen M, Melek M, Onrat E, Esen OB, Kirma C . Effect of smoking on endothelial function and wall thickness of brachial artery. Circ J 2004; 68: 1123–1126.
Ueda S, Matsuoka H, Miyazaki H, Usui M, Okuda S, Imaizumi T . Tetrahydrobiopterin restores endothelial function in long-term smokers. J Am Coll Cardiol 2000; 35: 71–75.
Oncken C, Cooney J, Feinn R, Lando H, Kranzler HR . Transdermal nicotine for smoking cessation in postmenopausal women. Addict Behav 2007; 32: 296–309.
Yang YM, Huang A, Kaley G, Sun D . eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 2009; 297: H1829–H1836.
Lowe ER, Everett AC, Lee AJ, Lau M, Dunbar AY, Berka V, Tsai AL, Osawa Y . Time-dependent inhibition and tetrahydrobiopterin depletion of endothelial nitric-oxide synthase caused by cigarettes. Drug Metab Dispos 2005; 33: 131–138.
Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Munzel T . Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res 2000; 86: E36–E41.
Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, Baker TB, Fiore MC, Stein JH . Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol 2010; 55: 1988–1995.
Sugiura T, Dohi Y, Hirowatari Y, Yamashita S, Ohte N, Kimura G, Fujii S . Cigarette smoking induces vascular damage and persistent elevation of plasma serotonin unresponsive to 8 weeks of smoking cessation. Int J Cardiol 2013; 166: 748–749.
Suarez-Varela MM, Llopis-González A, González Albert V, López-Izquierdo R, González- Manzano I, Cháves J, Biosca VH, Martin-Escudero JC . Zinc and smoking habits in the setting of hypertension in a Spanish populations. Hypertens Res 2015; 38: 149–154.
Nezu T, Hosomi N, Aoki S, Kubo S, Araki M, Mukai T, Takahashi T, Maruyama H, Higashi Y, Matsumoto M . Endothelial dysfunction is associated with the severity of cerebral small vessel disease. Hypertens Res 2015; 38: 291–297.
Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T . Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004; 24: 1442–1447.
Acknowledgements
We gratefully acknowledge the assistance of Jeremy Barbagallo, M.S., Brenda Foxen, R.N., and Donna Polk, M.D. This study was funded by CT Department of Public Health #2010-0089 Biomedical Research Award (BAT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
PDT has received research support from Genomas, Roche, Sanolfi, Regeneron, Esperion, Amarin and Pfizer; has served as a consultant for Amgen, Regeneron, Merck, Genomas, Runners World, Sanolfi, Esperion and Amarin; has received speaker honoraria from Merck, Astra Zenica, Kowa and Amarin; owns stock in Abbvie, Abbott Labs, General Electric, J&J; and has provided expert legal testimony on exercise-related cardiac events and statin myopathy. BAT served on the Pharmacovigilance Monitoring Board for Amgen, Inc. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Taylor, B., Zaleski, A., Dornelas, E. et al. The impact of tetrahydrobiopterin administration on endothelial function before and after smoking cessation in chronic smokers. Hypertens Res 39, 144–150 (2016). https://doi.org/10.1038/hr.2015.130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.130
Keywords
This article is cited by
-
Varied effects of tobacco smoke and e-cigarette vapor suggest that nicotine does not affect endothelium-dependent relaxation and nitric oxide signaling
Scientific Reports (2023)
-
Smoking cessation and vascular endothelial function
Hypertension Research (2023)
-
Exhaled nitric oxide in early rheumatoid arthritis and effects of methotrexate treatment
Scientific Reports (2022)
-
Association between passive smoking and hypertension in Chinese non-smoking elderly women
Hypertension Research (2017)
-
Impact of mild-to-moderate alcohol consumption and smoking on kidney function
Hypertension Research (2017)