Abstract
This study investigated the contribution of blood oxidative stress (OS) to the development of hypertension, as well as sex differences in the antioxidant defense system (ADS) in genetic models of hypertension. Nine-week-old normotensive Wistar-Kyoto (WKY) rats, borderline hypertensive rats (BHR) and spontaneously hypertensive rats (SHR) of both sexes were used. Systolic blood pressure (SBP) was determined by tail-cuff plethysmography, the trolox equivalent antioxidant capacity (TEAC) and the concentration of lipid peroxides (LP) were determined in plasma. The activity of the antioxidant enzymes Cu/Zn–superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) was determined in erythrocytes. SBP was significantly elevated in BHR and SHR in both sexes. BHR and SHR males had a higher SBP than the respective females. Sex-dependent differences in the ADS were found only in SHR, in which TEAC, SOD and CAT were significantly higher in males than in females. No differences in TEAC, SOD, CAT and GPx were observed between BHR (males and females) and WKY controls. LP levels were similar in all the groups investigated. Significant positive correlations were observed between SBP and both SOD and CAT. TEAC correlated positively with SOD and LP. As no signs of oxidative damage to lipids were found in young BHR and SHR of either sex, OS in the blood does not seem to be causatively related to the development of hypertension in these rats. However, despite activated antioxidant defenses, the positive correlation between plasma TEAC and LP suggests that oxidative damage is progressing slowly and therefore it seems to be a consequence rather than the cause of hypertension.
Similar content being viewed by others
Introduction
Arterial hypertension is one of the most frequent health problems in the global population, and it is an important cause of morbidity and mortality in the developed countries. It is a major risk factor for atherosclerosis, stroke, heart attack and renal failure.1 Essential hypertension (EH) is a multi-factorial, polygenetic disease whose main cause is unknown. There are several genetic factors that significantly increase the risk of development and progression of the disease. In addition, there are lifestyle and environmental factors (smoking, obesity, a sedentary life style, a high salt and alcohol intake, stress) that have a role in the development of EH.1, 2
One of the factors that may be responsible for the development and progression of hypertension is oxidative stress (OS). OS results from increased levels of reactive oxygen species (ROS), caused either by increased production of ROS or by decreased elimination of ROS by antioxidants.3 It should be noted that optimal ROS production is required for normal cell signaling as ROS serve as second messengers in the activation and regulation of various transcription factors and kinases that have roles in the processes of cell growth, inflammation, apoptosis and cell differentiation.4, 5, 6
Regarding the association between OS and blood pressure (BP), OS has been observed in patients with EH, renovascular hypertension, malignant hypertension and pre-eclampsia, as well as in various animal experimental models of hypertension.7, 8, 9, 10, 11, 12, 13 It is not yet clear whether OS is a cause or a consequence of high BP.14, 15 In addition, there are sex differences in BP regulation in both humans and animals. In humans, elevated BP and the risk of cardiovascular diseases is much higher in men than in age-matched premenopausal women, but this difference diminishes after menopause.16 In rats, differences in BP regulation have been observed in various strains, such as spontaneously hypertensive rats (SHR), Dahl salt-sensitive rats and deoxycorticosterone-salt hypertensive rats.17, 18, 19 In these rats, males developed an earlier and more severe hypertension than females.20 Interestingly, in adult Wistar-Kyoto (WKY) rats, males had higher systolic but not diastolic BP (SBP and DBP, respectively) when compared with females.21 Several studies have demonstrated that sex differences in hypertensive adult rats are associated with OS.20, 22, 23 However, it is very difficult to distinguish if OS is a cause or a consequence of high BP as both pathologies are typically present simultaneously.
SHR are a commonly used model of human EH that allows the unravelling of the molecular mechanisms associated with hypertension. The disadvantages of using this rat strain result from fast development of hypertension soon after weaning (that is, between 4 and 7 weeks of life) rather than in middle or later life as seen in humans. Thus borderline hypertensive rats (BHR) are more suitable for studying the mechanisms of hypertension development, because the progression of BP increases in this strain is delayed to a later period of life than in SHR.24
The aim of this study was to investigate the role of OS in the development of hypertension in genetic models of hypertension, that is, in juvenile BHR and SHR. In addition, possible sex differences in the antioxidant defense system (ADS) in the blood of juvenile male and female rats were determined.
Methods
Animals
The rats used in this study were born in the animal facility of the Institute of Normal and Pathological Physiology of the Slovak Academy of Sciences in Bratislava, Slovak Republic. Normotensive WKY, BHR (offspring of spontaneously hypertensive dams and normotensive sires) and SHR (offspring of spontaneously hypertensive parents) were used. Animals were housed in the groups of four rats per cage at a temperature of 22–24 °C on a 12:12-h dark–light cycle and maintained on a standard pellet diet with tap water ad libitum. All procedures were performed in accordance with European Community and NIH guidelines for the use of experimental animals and were approved by the State Veterinary and Food Administration of the Slovak Republic.
After birth, the rats were kept together with their mothers until the end of the fifth week of life. Then, they were separated from the mothers and randomly divided according to sex. SBP, heart rate (HR) and body weight (BW) of rats were measured at the age of 9 weeks. SBP and HR were determined by non-invasive tail-cuff plethysmography as described previously.25 At the end of experiment, the rats were killed by decapitation after brief CO2 anesthesia.
Sample preparation
Blood was collected in heparin-coated tubes and centrifuged (850 g/10 min, 4 °C) to obtain plasma and erythrocytes. Then, plasma was aliquoted and stored at −20 °C until analysis. Total antioxidant capacity and concentration of lipid peroxides (LP) were determined in plasma. Isolated erythrocytes were washed three times with 0.15 mol l−1 NaCl solution. After centrifugation (900 g/5 min, 4 °C), erythrocytes were hemolyzed by adding a triple volume of cold distilled water and stored at −20 °C until further analyses. Activities of Cu/Zn–superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) and the concentration of hemoglobin (Hb) (measured using the Drabkin method) were determined in the hemolysate of erythrocytes.26
Methods
The activity of SOD was determined using a commercial kit according to the manufacturer’s instructions (Sigma-Aldrich, St Louis, MO, USA). The results are expressed in U SOD per mg Hb. The activity of GPx was determined by a commercial kit according to the manufacturer’s instructions (Enzo Life Sciences, New York, NY, USA). The results are expressed in μkat per g Hb. CAT activity was determined by a modified method based on that used by Bergmeyer,27 and the results are expressed in μkat per g Hb. The total antioxidant capacity of plasma was measured using the trolox equivalent antioxidant capacity (TEAC) assay according to Re et al.28 Quantification was performed using the dose–response curve for the reference antioxidant trolox, a water soluble form of vitamin E. The results are presented as mmol of trolox l−1. The level of LP in plasma was measured using the method previously described by El-Saadani et al.,29 and the results are presented in nmol ml−1 of plasma.
Statistical analysis
The data are presented as the mean±s.e.m. Results were analyzed by factorial analysis of variances. Two-way analysis of variance (with the sex and phenotype as categorical factors) and Bonferroni’s post-hoc test were used. There were n=6–8 for each parameter determined. Values of P<0.05 are considered statistically significant. Correlation between variables was determined using Pearson’s correlation coefficient (r). GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) and Statistica 7 (Stat Soft, Inc., Tulsa, OK, USA) were used for statistical analyses.
Results
Basic biometric and hemodynamic parameters (SBP, BW and HR), as well as the TEAC, antioxidant enzyme activities and LP concentrations, of WKY rats, BHR and SHR are shown in Table 1. Significant phenotype-dependent differences (in both hypertensive rat strains) compared with WKY rats were observed in BW (F2,34=76.9, P<0.00001) and BP (F2,34=136, P<0.00001) but not in HR. For antioxidant enzymes, significant phenotype-dependent differences were observed only in CAT (F2,34=18, P<0.0001) and SOD (F2,34=5, P<0.02) (Table 1a), which were elevated compared with the values found in WKY rats.
Characteristics that varied significantly by sex were BW (F1,34=613, P<0.00001), HR (F1,34=9.3 P<0.004), SBP (F1,34=25, P<0.0001), SOD (F1,34=5.8, P<0.02), CAT (F1,34=4.3, P<0.05) and GPx (F1,34=5.2, P<0.03). Of these parameters, SBP, SOD and CAT were significantly elevated in males compared with females, whereas GPx and HR were lower in males than in females.
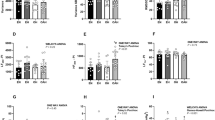
The interaction of both factors (phenotype and sex) was significant for BW (F2,34=27, P<0.0001, Table 1b), SBP (F2,34=12, P<0.0002, Figure 1a), HR (F2,34=9.3 P<0.004, Table 1b), SOD (F2,34=4.3, P<0.02, Figure 1c), CAT (F2,34=4.5, P<0.02, Figure 1d) and GPx (F2,34=3.6, P<0.04, Figure 1e). As depicted in Figure 1, the SBP in BHR and SHR males was significantly higher than that seen in females (Figure 1a). The TEAC, SOD and CAT activities were significantly elevated in SHR males over females (Figures 1b–d), whereas GPx was reduced (Figure 1e). However, despite a significantly elevated BP, there were no phenotype- and sex-dependent differences in TEAC, SOD, CAT and GPx between BHR and WKY rats. Furthermore, regardless of alterations in SBP, there were no phenotype- and sex-dependent changes observed in LP in individual groups of rats (Figure 1f), suggesting the absence of oxidative damage to lipids in young (pre)hypertensive rats.
Blood pressure (a), trolox equivalent antioxidant capacity (TEAC) of plasma (b), activity of Cu/Zn–superoxide dismutase (SOD) (c), catalase (CAT) (d), glutathione peroxidase (GPx) (e) and the level of lipid peroxides (LP) (f) in Wistar-Kyoto rats (WKY), borderline hypertensive rats (BHR) and spontaneously hypertensive rats (SHR). White columns—females, black columns—males, *P<0.05 vs. female;+P<0.05 vs. WKY of the same sex; xP<0.05 vs. BHR of the same sex. Data are presented as mean±s.e.m.
Significant positive correlations were observed between SBP and SOD (r=0.51, P<0.002, n=36) and between SBP and CAT (r=0.72, P<0.0001, n=36) (Figures 2b and c) but not between SBP and GPx (r=0.08, P=0.66, n=36) and between SBP and TEAC or LP (Figures 2a and d). There was a positive correlation between TEAC and SOD (r=0.54, P<0.0008, n=36, Figure 3a) but not between TEAC and CAT (r=0.25, P=0.13, n=36, Figure 3b). There was also a positive correlation between SOD and CAT (r=0.49, P<0.002, n=36). Interestingly, increased TEAC correlated negatively with GPx (r=−0.47, P<0.004, n=36, Figure 3c), whereas a positive correlation was found between TEAC and LP (r=0.35, P<0.04, n=36, Figure 3d).
Discussion
This study investigated the role of OS in the development of hypertension in juvenile borderline hypertensive and SHR, as well as sex-dependent differences in the activities of enzymes involved in the ADS. The main finding of this study is that no signs of oxidative damage to the lipids in the blood were present in young BHR and SHR of either sex despite elevated BP. However, we found positive correlations between SBP and both CAT and SOD, and a phenotype-dependent increase in CAT and SOD activities, suggesting the activation of the ADS in rats with elevated BP. Despite having an activated ADS, a positive correlation between TEAC and LP may suggest a lack of sufficient detoxification of ROS, followed by a slow development of oxidative damage to lipids in young rats with a positive genetic predisposition to hypertension.
Although sex differences in SBP control are well known, the underlying mechanisms are not clear. There is some evidence supporting the involvement of sex hormones in cardiovascular regulation. For example, estrogens have been shown to inhibit renin release and angiotensin-converting enzyme activity, whereas testosterone has been shown to stimulate the renin–angiotensin system.30, 31 Additionally, pharmacogenetic analysis has shown that, in males, BP was controlled by two loci on chromosomes 1 and 5 through the sympathetic nervous system. In contrast, baseline BP in females was controlled by two loci on chromosomes 3 and 7, and the effect of these loci was not mediated by the renin–angiotensin system, the sympathetic nervous system or the L-arginine/nitric oxide system.32 In our study, which used juvenile rats, there were significant sex-related differences in BP only in rats with a genetic predisposition to hypertension, which suggests the possible involvement of accentuated activity of the sympathetic system in these strains in conjunction with the effect of sex hormones. In addition, we observed sex differences in ADS activation in the blood of juvenile rats only in SHR, suggesting that elevated levels of ROS only have a role in SHR males. The fact that no activation of the ADS in SHR females was detected in our study is surprising, as we have recently found high superoxide production in the aorta of this particular strain of rats. Thus local tissue ROS production seems to be different from that in circulation.24 Other authors have also reported sex differences in oxidative status in SHR. Sullivan et al.23 reported that urinary hydrogen peroxide excretion was higher in 12- to 14-week-old SHR males than in females. Romero et al.22 also showed that adult female SHR exhibit lower SBP and less OS in the heart than male SHR. Similar results were also reported by Sartori-Valinotti et al.,33 who observed increased expression of antioxidant enzymes (SOD, GPx, CAT) in the kidney of 15-week-old male SHR compared with females. Interestingly, in our experiment, the level of LP in plasma was not significantly changed despite the elevated SBP and activated antioxidant enzymes, suggesting that the ADS in the circulation is able to eliminate the ROS and prevent oxidative damage to lipids even in 9-week-old SHR males. However, this does not exclude oxidative damage to nucleic acids or proteins, which may affect the function of various enzymes.
As described above, OS has been detected in humans with EH and in various experimental models of high BP.8, 9, 13, 15 Mechanisms leading to elevated ROS production have been described previously.6 With respect to the effect of OS on BP regulation, it has been shown that vascular OS has an important role in the development of endothelial dysfunction (ED), leading to elevated total peripheral resistance and thus to an increase in BP. One mechanism of OS-induced ED in the vasculature may be related to ROS-mediated NO deactivation with simultaneous accentuation of the influence of endothelium-derived constricting factors.34 Yet, despite these findings, it is still unclear whether OS-induced ED precedes the development of hypertension or if it is causatively related to the increase in BP. We used BHR, a model of human prehypertension, to elucidate this issue. In young BHR, BP is significantly elevated compared with WKY rats as early as 5 weeks of age.24, 35 However, despite a higher BP and vascular superoxide production, we did not observe ED at the age of 7 weeks24, 35 or an altered ADS in the blood of 9-week-old BHR (this study). In addition, there are numerous studies showing that elevated BP occurs in pubertal and young SHR (up to 10 weeks of age) in the absence of ED.36 Collectively, these studies in young rats do not support the idea that vascular OS and/or OS-induced ED precede hypertension in genetic models of hypertension. Instead, local disturbances in ROS levels, mainly in the brain, could trigger hypertension via sympathoexcitation.37
In this study, we also investigated relationships among BP, the antioxidant capacity of blood and the individual enzymes involved in the ADS in a population of rats with various BP. Interestingly, both SOD and CAT correlated positively with BP. Similar correlations between BP, SOD and CAT in the red blood cells of adult male SHR have been observed previously.38 Thus elevated SOD and CAT in the blood might serve as early markers of OS and/or hypertension development. However, no such relationship was observed between BP and GPx in this study, GPx was significantly reduced in male SHR (which concurrently developed the highest BP and the highest SOD and CAT activities) compared with females and GPx correlated negatively with TEAC. Other authors have also reported reduced GPx activity in the heart tissue or the kidneys of SHR males from the age of 8 weeks.39, 40, 41 This phenomenon may result from inactivation of GPx by several mechanisms. One cause of decreased GPx activity might lie in a lack of its substrates—hydrogen peroxide or LP. We found increased CAT activity in SHR males; as CAT catalyzes the cleavage of hydrogen peroxide into water and oxygen, it thus eliminates the substrate of GPx. As a result of CAT activity, LP are not formed and thus GPx activity is reduced. Another potential cause of the reduction in GPx activity might be a decrease in glutathione levels.42
In conclusion, this study showed increasing activity of the antioxidant enzymes SOD and CAT in the blood of young rats with a positive genetic predisposition to hypertension but no evidence of oxidative damage to their blood lipids. As no signs of oxidative damage to lipids were found in young BHR and SHR of either sex, while increased SBP was observed compared with WKY controls, it appears that OS in the circulation is not causatively related to the development of hypertension in these rats. However, despite having an activated antioxidant defense, the positive correlation between the TEAC and LP levels in the plasma suggests a slow progression of oxidative damage. This seems to be a consequence rather than a cause of high BP and may have a negative role in later periods of life.
References
Buttar HS, Li T, Ravi N . Prevention of cardiovascular diseases: role of exercise, dietary interventions, obesity and smoking cessation. Exp Clin Cardiol 2005; 10: 229–249.
Rosskopf D, Schürks M, Rimmbach C, Schäfers R . Genetics of arterial hypertension and hypotension. Naunyn Schmiedebergs Arch Pharmacol 2007; 374: 429–469.
Cutler RG . Oxidative stress profiling: part I. Its potential importance in the optimization of human health. Ann NY Acad Sci 2005; 1055: 93–135.
Touyz RM . Reactive oxygen species, vascular oxidative stress and redox signaling in hypertension: what is the clinical significance? Hypertension 2004; 44: 248–252.
Alfadda AA, Sallam RM . Reactive oxygen species in health and disease. J Biomed Biotechnol 2012; 2012: 936486.
Majzunova M, Dovinova I, Barancik M, Chan JY . Redox signaling in pathophysiology of hypertension. J Biomed Sci 2013; 20: 69.
Wang D, Strandgaard S, Iversen J, Wilcox CS . Asymetric dimethylarginine, oxidative stress and vascular nitric oxide synthase in essential hypertension. Am J Physiol Regul Integr Comp Physiol 2009; 296: 195–200.
Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K . Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med 2002; 346: 1954–1962.
Lip GY, Edmunds E, Nuttall SL, Landray MJ, Blann AD, Beevers DG . Oxidative stress in malignant and non-malignant phase hypertension. J Hum Hypertens 2002; 16: 333–336.
Lee VM, Quinn PA, Jennings SC, Ng LL . Neutrophil activation and production of reactive oxygen species in pre-eclampsia. J Hypertens 2003; 21: 395–402.
Kimura H, Kon N, Furukawa S, Mukaida M, Yamakura F, Matsumoto K, Sone H, Murakami-Murofushi K . Effect of endurance exercise training on oxidative stress in spontaneously hypertensive rats (SHR) after emergence of hypertension. Clin Exp Hypertens 2010; 32: 407–415.
Chen X, Mori T, Guo Q, Hu C, Ohsaki Y, Yoneki Y, Zhu W, Jiang Y, Endo S, Nakayama K, Ogawa S, Nakayama M, Miyata T, Ito S . Carbonyl stress induces hypertension and cardio-renal vascular injury in Dahl salt-sensitive rats. Hypertens Res 2013; 36: 361–367.
Lu Q, Yang Y, Villar VA, Asico L, Jones JE, Yu P, Li H, Weinman EJ, Eisner GM, Jose PA . D5 dopamine receptor decreases NADPH oxidase, reactive oxygen species and blood pressure via heme oxygenase-1. Hypertens Res 2013; 36: 684–690.
Harrison DG, Gongora MC . Oxidative stress and hypertension. Med Clin North Am 2009; 93: 621–635.
Bernatova I . Endothelial dysfunction in experimental models of arterial hypertension: cause or consequence? Biomed Res Int 2014; 2014: 598271.
Wiinber N, Hoegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW . 24-H ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens 1995; 8: 978–986.
Ganten U, Schroder G, Witt M, Zimmermann F, Ganten D, Stock G . Sexual dimorphism of blood pressure in spontaneously hypertensive rats: effect of anti-androgen treatment. J Hypertens 1989; 7: 721–726.
Rowland NE, Fregly MJ . Role of gonadal hormones in hypertension in the Dahl salt-sensitive rat. Clin Exp Hypertens 1992; 14: 367–375.
Ouchi Y, Share L, Crofton JT, Iitake K, Brooks DP . Sex difference in the development of deoxycorticosterone-salt hypertension in the rat. Hypertension 1987; 9: 172–177.
Dantas APV, Franco MC, Silva-Antonialli MM, Tostes RCA, Fortes ZB, Nigro D, Carvalho MH . Gender differences in superoxide generation in microvessels of hypertensive rats: role of NAD(P)H-oxidase. Cardiovasc Res 2004; 61: 22–29.
Maris ME, Melchert RB, Joseph J, Kennedy RH . Gender differences in blood pressure and heart rate in sponaeously hypertensive and Wistar-Kyoto rats. Clin Exp Pharmacol Physiol 2005; 32: 35–39.
Romero M, Caniffi C, Bouchet G, Elesgaray R, Laughlin MM, Tomat A, Arranz C, Costa MA . Sex differences in the beneficial cardiac effects of chronic treatment with atrial natriuretic peptide in spontaneously hypertensive rats. PLoS ONE 2013; 8: e71992.
Sullivan JC, Sasser JM, Pollock JS . Sexual dimorphism in oxidant status in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 2007; 292: R764–R768.
Slezak P, Puzserova A, Balis P, Sestakova N, Majzunova M, Dovinova I, Kluknavsky M, Bernatova I . Genotype-related effect of crowding stress on blood pressure and vascular function in young female rats. Biomed Res Int 2014; 2014: 413629.
Puzserova A, Slezak P, Balis P, Bernatova I . Long-term social stress induces nitric oxide-independent endothelial dysfunction in normotensive rats. Stress 2013; 16: 331–339.
Drabkin DL, Austin JH . Spectrophotometric studies: I. Spectrophotometric constants for common hemoglobin derivates in human, dog and rabbit blood. J Biol Chem 1932; 98: 719–733.
Bergmeyer HU . Methods of Enzymatic Analysis, Volume III: Enzymes 1: Oxidoreductases, Transferases. Verlag Chemie: Weiheim, Germany. 1987, 277 pp.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C . Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26: 1231–1237.
El-Saadani M, Esterbauer H, El-Sayed M, Goher M, Nassar AY, Jürgens G . A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J Lipid Res 1989; 30: 627–630.
Reckelhoff JF, Zhang H, Granger JP . Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 1998; 31: 435–439.
Reckelhoff JF . Gender difference in the regulation of blood pressure. Hypertension 2001; 37: 1199–1208.
Ueno T, Tremblay J, Kunes J, Zicha J, Dobesova Z, Pausova Z, Deng AY, Sun Y, Jacob HJ, Hamet P . Gender-specific genetic determinants of blood pressure and organ weight: pharmacogenetic approach. Physiol Res 2003; 52: 689–700.
Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF . Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin Exp Pharmacol Physiol 2007; 34: 938–945.
Vanhoutte PM, Feletou M, Taddei S . Endothelium-dependent contractions in hypertension. Br J Pharmacol 2005; 144: 449–458.
Bernatova I, Puzserova A, Balis P. Sex-related differences in cardiovascular action of crowding stress in young normotensive and hypertensive rats. In Physiology 2014, The Queen Elizabeth II Conference Centre, London, UK. 30 June–2 July 2014. The Physiological Society: London, pp 28–29.
Bernatova I, Conde MV, Kopincova J, Gonzalez MC, Puzserova A, Arribas SM . Endothelial dysfunction in spontaneously hypertensive rats: focus on methodological aspects. J Hypertens Suppl 2009; 27: S27–S31.
Kishi T, Hirooka Y . Oxidative stress in the brain causes hypertension via sympathoexcitation. Front Physiol 2012; 3: 335.
Yuan YV, Kitts DD, Godin DV . Heart and red blood cell antioxidant status and plasma lipid levels in the spontaneously hypertensive and normotensive Wistar-Kyoto rat. Can J Physiol Pharmacol 1996; 74: 290–297.
Alvarez MC, Caldiz C, Fantinelli JC, Garciarena CD, Console GM, Chiappe de Cingolani GE, Mosca SM . Is cardiac hypertrophy in spontaneously hypertensive rats the cause or the consequence of oxidative stress? Hypertens Res 2008; 31: 1465–1476.
Lee SK, Arunkumar S, Sirajudeen KN, Singh HJ . Glutahione system in young spontaneously hypertensive rats. J Physiol Biochem 2010; 66: 321–327.
Sundaram A, Siew Keah L, Sirajudeen KN, Singh HJ . Upregulation of catalase and downregulation of glutathione peroxidase activity in the kidney precede the development of hypertension in pre-hypertensive SHR. Hypertens Res 2013; 36: 213–218.
Wang X, Desai K, Clausen JT, Wu L . Increased methylglyoxal and advanced glycation end products in kidney from spontaneously hypertensive rats. Kidney Int 2004; 66: 2315–2321.
Acknowledgements
This study was supported by grants of the Slovak Research and Development Agency No. APVV-0523-10 and the Scientific Grant Agency of the Ministry of Education of Slovak Republic and the Academy of Sciences VEGA 2/0084/14. Infrastructure used in this study was partially supported by the ‘ITMS 26240120020-Establishment of the Centre for the Research on Composite Materials for Structural, Engineering and Medical Applications-CEKOMAT II’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Horvathova, M., Zitnanova, I., Kralovicova, Z. et al. Sex differences in the blood antioxidant defense system in juvenile rats with various genetic predispositions to hypertension. Hypertens Res 39, 64–69 (2016). https://doi.org/10.1038/hr.2015.117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.117
Keywords
This article is cited by
-
Effects of a catechins-enriched diet associated with moderate physical exercise in the prevention of hypertension in spontaneously hypertensive rats
Scientific Reports (2022)
-
Oxidative Stress Induced by 30 Days of Mercury Exposure Accelerates Hypertension Development in Prehypertensive Young SHRs
Cardiovascular Toxicology (2022)
-
Put “gender glasses” on the effects of phenolic compounds on cardiovascular function and diseases
European Journal of Nutrition (2018)
-
The NOX2-derived reactive oxygen species damaged endothelial nitric oxide system via suppressed BKCa/SKCa in preeclampsia
Hypertension Research (2017)