Abstract
Hypertension is associated with cognitive decline in elderly persons. We studied asymptomatic hypertensive subjects using brain magnetic resonance (MR) spectroscopy to evaluate metabolite impairments before the appearance of symptoms in patients with different treatment outcomes. In all, 14 healthy controls and 37 asymptomatic hypertensive patients (17 controlled and 20 resistant) underwent brain structural MR and MR spectroscopy of the posterior paralimbic (PPL) area and left frontal white matter. Ischemic burden (IB), global cortical atrophy and microbleeds were analyzed with visual scales. Metabolite ratios involving N-acetyl-aspartate (NAA), creatine (Cr), choline (Cho) and myoinositol (mI) were computed. Ultrasound measurements, including intima-media thickness, plaques and hemodynamic ratios, were obtained. Intergroup differences in IB, atrophy and metabolite ratios, and the atrophy and IB relationship were assessed with parametric and nonparametric statistical tests. In addition, the impacts of demographic, analytic and clinical factors, ischemia and atrophy, and ultrasound measurements on metabolite ratios were assessed. The significance level was set at P⩽0.05. Higher atrophy scores presented with higher total or frontal IB (P<0.05). However, there was no intergroup difference in atrophy and IB. PPL mI/Cr was increased in resistant hypertension (P<0.021), whereas frontal NAA/Cr (P<0.007) showed opposite trends between controlled (increased ratios) and resistant (decreased ratios) hypertension. Unlike PPL mI/Cr, frontal NAA/Cr showed significant correlations with the lipid profile and ultrasound measurements. PPL mI/Cr increases in resistant hypertension, and frontal NAA/Cr diverges between controlled and resistant hypertension before physical and neuropsychological symptoms appear.
Similar content being viewed by others
Introduction
Cognitive impairment and hypertension are highly prevalent in the elderly.1, 2 The relationship between hypertension and cognition is controversial. The majority of longitudinal studies suggest an association between high blood pressure (BP) and cognitive decline.3 Nevertheless, although antihypertensive treatment has proven beneficial in preventing cardiac disease and stroke, it is not clear that it prevents cognitive impairment. This may be due to the effects of antihypertensive treatments on cerebral perfusion, especially in patients with significant small-vessel disease.3 A recent systematic review shows that there is no evidence that lowering BP lowers the risk of or prevents dementia or cognitive decline in hypertensive patients with no apparent previous cerebrovascular disease.2 As cognitive impairment related to vascular disease is not readily identified by the commonly used neuropsychological measures,3 it is necessary to search for other surrogate biomarkers that could identify preclinical changes in hypertension and the effects of antihypertensive treatment. Magnetic resonance spectroscopy (MRS) is a widely available imaging technique that has increasingly shown effectiveness in assessing dementia, even in its early stages.4 However, only a few studies have focused on the effects of hypertension on brain metabolites. Consequently, our purpose was to investigate, through conventional MRS, hypertension-related changes in brain metabolite ratios in asymptomatic patients and to determine the differences depending on the level of success of BP control.
Methods
Sample size calculation
To estimate an appropriate sample size, we first assumed that the proportion of control cases would be substantially smaller than the fraction of hypertensive patients, considering the amount of clinical, analytical, neuropsychological and imaging examinations that patients were expected to undergo. Accordingly, a 2.5:1 patients/controls ratio was established. Increases in MRS myoinositol (mI)/creatine (Cr) ratio have been reported in pre-dementia and in the very early stages of Alzheimer's disease,4 as well as in asymptomatic hypertension.5 We took into account the mean and s.d. (0.56±0.04) of the control group ratio reported in this paper5 and a mI/Cr s. d. measurement of 0.036 found after a pilot random assessment involving 15 hypertensive patients from our sample. Assuming 5 and 20% α and β errors, respectively, 14 control subjects and 35 hypertensive patients were estimated to be adequate to obtain a 0.036 deviation difference between normotensive and hypertensive subjects. The analysis was carried out with GRANMO 5.2 software for Windows (Institut Municipal d'Investigació Médica, Barcelona, Spain).6
Patients
The study was conducted within an institutionally financed project and approved by the institutional review committee. A total of 54 patients were recruited consecutively by a convenience sampling of hypertensive subjects under treatment in the Vascular Risk Control Unit (VRCU) of the Department of Internal Medicine, provided they were asymptomatic and diagnosed with either controlled or resistant hypertension, depending on the medical treatment outcome. On the basis of the medical records of the VRCU, all subjects had been previously diagnosed with hypertension, with hypertension defined as a systolic or diastolic BP constantly >140 or >90 mm Hg, respectively, or both, with or without the use of BP-lowering drugs. Furthermore, hypertension was classified as ‘controlled’ when successfully managed with three or fewer drugs, with no difference in the type of drugs used in each group. Hypertension was considered to be ‘resistant’ when a combination of more than three drugs was required for control (hard-control hypertension) or remaining uncontrolled even with the use of five or more drugs, after excluding those with poor adherence to treatment and the white-coat effect.7 Furthermore, at the time of the imaging study, BP was measured with a sphygmomanometer with the patient in a sitting position after 5 min of rest. The first and the fourth Korotkoff sounds were used for the systolic and diastolic BP measurements, respectively. Values were measured twice in succession, taking the second value for further analysis in this study. A control group of 20 normotensive, asymptomatic subjects was recruited from among healthy relatives. Both patients and control subjects were considered asymptomatic when no previous neurological signs and symptoms were reported, when they or their relatives did not report symptoms of cognitive impairment and when coexisting diseases were ruled out. All subjects were then referred to and interviewed in the Regional Unit for Dementia, where cognitive performance was assessed by administering the validated Spanish version of the Mini-Mental State Examination, adjusted for age and education (a-MMSE),8, 9 and a complete neuropsychological battery. Dementia was ruled out according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV).10 Given that mild cognitive impairment can confound metabolite measurements, we excluded subjects (6 normotensive, 17 hypertensive) with a-MMSE scores <25, in accordance with the recommendations of the International Working Group on Mild Cognitive Impairment,8, 9, 11 even when cognition had not been reported as impaired in the patients' VRCU charts. Major psychiatric and metabolic diseases leading to dementia were also ruled out with an exhaustive clinical and analytic study. The final sample consisted of 14 normotensive and 37 hypertensive (17 controlled, 20 resistant) patients. All participants gave written informed consent.
Data acquisition and imaging assessment
Structural magnetic resonance imaging (MRI) was used both to insert the MRS voxels and for the analysis of hypertension-related brain ischemia, atrophy and microbleeding. The protocol consisted of (1) a sagittal three-dimensional spoiled gradient-recall T1-weighted (SPGR-T1W) sequence (TR 30 ms, TE 6 ms, flip angle 45°, thickness 1.3 mm). Symmetry was assured with three orthogonal scout fast T1-weighted sequences; (2) an axial double-echo long TR FSE sequence (TR 3000 ms, TE 25/90 ms, thickness 5.0 mm); and (3) an axial T2-weighted gradient-echo sequence (TR 600 ms, TE 15 ms, flip angle 20°, thickness 5.0 mm), with both axial sequences parallel to the inferior callosal plane.
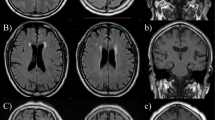
A single rater (JMGS), a neuroradiologist with 20 years of experience, assessed all images at a separate workstation, blinded to the clinical information. Ischemic burden (IB) was evaluated in the double-echo sequence. Following a simple scale,12 supratentorial age-related white matter (ARWM) changes were classified in one of four severity levels (Figure 1): 0, no ischemic lesions; 1, punctate lesions (focal lesions <10 mm in size, or groups of lesions <20 mm in diameter); 2, beginning confluent lesions (focal lesions 10–20 mm in diameter, or group of lesions >20 mm in diameter); and 3, confluent lesions (single lesions or confluent areas of hyperintensity >20 mm in diameter), in the frontal, parieto-occipital and temporal lobes. In the basal ganglia, IB was classified in four severity levels (0, no ischemic lesions; 1, one lesion >5 mm; 2, two or more lesions >5 mm; and 3, confluent lesions). All lobar ischemic scores plus the basal ganglia ischemic scores plus the posterior fossa score (same scale as supratentorial white matter) made up the total IB, for a maximum score of 27.
(a) Atrophy scoring. Axial reformatted 3-mm thickness T1-weighted images. From left to right are images from three different patients showing no atrophy (1), some atrophy (2) and substantial atrophy (3). No patient in our sample met the criteria for end-stage atrophy. (b) Ischemia scoring. Axial long TR double-echo images. From left to right are images from three different patients with punctate lesions (1), beginning confluent lesions (2, white arrowhead) and confluent lesions (3, open arrowhead). (c). Microbleeds. Axial gradient-echo T2-weighted image. Tiny hypointense brain lesion (arrow) corresponding to a small hemorrhage.
Global cortical atrophy analysis was carried out with a reported simple scale.13 Atrophy scoring was mainly carried out by means of 3-mm thickness, reformatted, axial SPGR-T1W images, although they were interactively assessed with sagittal and coronal reconstructions. Each subject was assigned to one of the four global severity steps according to the Pasquier scale (0, no atrophy; 1, some atrophy; 2, substantial atrophy; and 3, end-stage atrophy) (Figure 1). The presence or absence of brain microbleeds was assessed in gradient-echo T2W images. A positive diagnosis was considered when very hypointense, round, parenchymal foci >2 mm and <10 mm were identified (Figure 1).14 When appropriate, confounding flow voids were distinguished using the axial, double-echo, long TR FSE images for comparison.
Magnetic resonance spectroscopy was performed in a standard MRI system with a standard quadrature head coil for both transmission and reception of the MR signal and an automated single-voxel proton MRS commercial package (PROBE/SV; MRI LX 1.5T, GE Medical Systems, Milwaukee, WI, USA). The cingulate gyri and inferior precunei (posterior paralimbic, PPL) have shown frequent metabolite imbalances in patients with cognitive impairment and, at the same time, have shown higher magnetic field homogeneity, providing better diagnostic accuracy than other brain areas.15 On the other hand, hypertension is associated with higher frontal IB.16 Accordingly, a point-resolved spectroscopy sequence (TR/TE 1500/35 ms; 2048 data points, 128 averages) was carried out with 2 × 2 × 2 cm voxels, placed (1) in the mid-sagittal SPGR-T1W image, covering both PPL, and (2) in an axial FSE intermediate-weighted image covering the white matter of the left frontal lobe (Figure 2). The prescan algorithm of PROBE automatically adjusted the transmitter and receiver gains and center frequency. The local magnetic field homogeneity was optimized with the three-plane auto-shim procedure, and the flip angle of the third water-suppression pulse was adjusted for chemical-shift water suppression (CHESS) before PRESS acquisition. N-acetyl-aspartate (NAA), mI, Cr and choline (Cho) peak resonances were automatically integrated and quantitative values were obtained. From these values, metabolite intensity ratios, NAA/Cr, Cho/Cr, NAA/Cho, mI/Cr and NAA/mI, were calculated. As MRS measurements, especially mI ratios, are easily distorted by subject movement and physiological motion,17 Cr signal-to-noise ratio (SNR) was also automatically measured in each case to support the consistency of those measurements.
(a) Sagittal T1W image. The voxel involves both medial posterior parietal cortices. (b) Axial intermediate-weighted axial image. Taking the intermediate-weighted axial image at the level of the calcarine cortex as a reference (not shown), the frontal voxel was systematically drawn in two upper axial slices, involving mainly the frontal white matter. (c) Posterior paralimbic magnetic resonance (MR) spectroscopy in three different subjects belonging to the different clinical groups. Spectra are scaled to the creatine peak (dashed line). Arrows denote the myoinositol peak, which is higher in resistant hypertension. ppm, parts per million; BP, blood pressure; cHT, controlled hypertension; rHT, resistant hypertension.
Carotid Doppler ultrasonography was carried out by two neuroradiologists (JMGS, STR) with 20 and 9 years of experience, respectively, using the same protocol and ultrasound equipment (Esaote Technos MPX, Genoa, Italy). Two intima-media thickness measurements were carried out at the distant wall of each common carotid artery, one at the mid-third of the artery and the other just proximal to the carotid division, always at the widest point of the wall (Figure 3). The two measurements were averaged to give an overall thickness. The presence of carotid plaques was reported and classified as mainly soft or mainly calcified when soft or calcium components, respectively, were undoubtedly dominant from a visual point of view (Figure 3). When both types of plaques appeared in the same patient, it was classified as a mixture of plaques. Doubtful cases were decided by consensus between the two raters. Peak systolic and diastolic velocities were obtained in the common carotid artery, close to the carotid division, and in the internal carotid artery, immediately distal to the carotid bulb or at the level of a plaque-related lumen stenosis (Figure 3), with the Doppler box in the middle of the arterial lumen, always with the same size and at an angle of 60°. Systolic and diastolic internal carotid artery to common carotid artery ratios were automatically calculated and were used for analysis.
(a) Intima-media thickness measurement at the level of the mid stretch of the common carotid artery. Measurements were always carried out between the free edge of the intima and the transition from the hypoechogenic intima-media complex to the echogenic adventitia, and averaged with the same measurement in the distal common carotid artery. (b) Mainly soft carotid plaques (stars), one of them (arrow) with a nondominant calcification. (c) Mainly calcified internal carotid plaques (stars). (d) Internal carotid measurements were carried out just distal to the carotid bulb unless a plaque-related lumen narrowing was detected. In that case, flow velocities were acquired at the level of the stenosis. Dashed line: calcified plaque.
Statistical analysis
Reliability assessment.
For visual assessment of IB and atrophy, the observer had been previously certified for visual rating of aging and dementia by the Image Analysis Center (IAC), Vrije Universiteit Medical Center (Amsterdam, The Netherlands). Accordingly, the inter-rater variability was determined by comparing the score of the rater with the IAC gold standard with the following weighted κ-values acceptable for the IAC standards: (1) ARWM scale, IAC κ⩾0.85; (2) global cortical atrophy, IAC κ⩾0.70. The inter-rater variability for detection of microbleeds was analyzed by comparing the assessments of the main observer and a second rater (STR) in a random group of 25 subjects within the sample. Intra-rater reliability was evaluated by scoring global cortical atrophy, IB and microbleeds twice in the same group. The two raters also carried out a measurement of intima-media thickness at the distal third of both common carotid arteries two times in a sample of eleven consecutive patients. Inter- and intra-rater agreements for microbleeds and intima-media thickness measurements and intra-rater agreement for atrophy and IB were assessed with Cohen's κ and intra-class correlation coefficients for qualitative and quantitative variables, respectively, with 95% confidence intervals (CIs). The analysis was carried out with the EPIDAT 3.1 software for Windows (Servicio de Información sobre Saúde Pública de la Dirección Xeral de Saúde Pública de la Consellería de Sanidade (Xunta de Galicia), Santiago de Compostela, Spain).
Data analysis. Intergroup differences:
All variables were expressed as means and s.d., and categorical variables were also classified in proportions. First, clinical, analytical, demographic and imaging data (ultrasound measurements, Cr SNR, metabolite ratios, total and frontal IB, microbleeds, and atrophy scores) were compared among the three clinical groups. For atrophy analysis, cutoff scores of 0, 1 and ⩾2 were used. As the IB was low, a cutoff score of 3 (⩽3 or >3) was established for total IB, and 0–1, 2 and ⩾2 scores for frontal IB.
Metabolite changes depending on demographic, clinical, analytic, ultrasound and structural imaging features:
As hypertension is associated with higher total and frontal ischemia,16 more atrophy18 and microbleeds,14 we also analyzed their impact on metabolite ratios. For this purpose, we assessed the differences in ratio measurements between groups of white matter disease (total and frontal) and atrophy using the same cutoff scores as before. Correlations linking metabolite ratios on one hand and atrophy, IB, ultrasound measurements, systolic and diastolic BPs, clinical and analytical data and a-MMSE score on the other were also analyzed. The influences of sex, age (both dichotomized by the median of ⩽67 years and considered as a continuous variable) and duration of disease (both dichotomized by the median of ⩽8 years and considered as a continuous variable) were also analyzed. All assessments were carried out for metabolite ratios showing intergroup differences in the first analysis.
After the Kolmogorov-Smirnov test, continuous variables considered to be dependent were evaluated either with a two-tailed Student's t-test and one-factor analysis of variance test or Mann–Whitney and Kruskal–Wallis test, depending on sample distribution. Multiple comparisons were carried out by Bonferroni correction. Categorical frequency distributions were compared with the χ2 Pearson's test, χ2-test of lineal trend or Fisher's exact test depending on the variables tested and proportions. Relationship assessments were carried out with the Pearson's correlation coefficient. Statistical significance was set at P⩽0.05. The analysis was carried out with SPSS 15.0 software for Windows (Chicago, IL, USA).
Results
Reliability
Inter-rater reliability for IB and atrophy assessment was satisfactory as the weighted κ-values exceeded the acceptable IAC limits both for the ARWM scale (rater κ=0.89) and global cortical atrophy assessment (rater κ=0.78). Inter-rater concordance was substantial for microbleeds detection (κ=0.79, CI 0.50–1.00) and excellent for intima-media thickness measurements (κ=0.970, CI 0.932–0.987). Intra-rater reliability was excellent for global cortical atrophy (κ=0.82, CI 0.59–1.00), frontal (κ=0.89, CI 0.76–1.00) and global ischemia scores (κ=0.91, CI 0.83–0.96), microbleeds detection (κ=0.90, CI 0.70–1.00) and intima-media thickness (rater 1: κ=0.995, CI 0.987–0.998; rater 2: κ=0.981, CI 0.957–0.992).
Participant characteristics
All groups were similar in age, sex distribution, duration of disease, a-MMSE scores, tobacco consumption, diabetes mellitus and cardiovascular disease (Table 1). Except for low-density lipoprotein (LDL)-cholesterol, which was higher in resistant hypertension, there were no significant intergroup differences in the lipid profile. Likewise, fibrinogen and homocysteine levels were similar among the three clinical groups. At the time of imaging, mean systolic and diastolic BPs were higher in resistant patients. However, group differences did not reach statistical significance.
Ultrasound, atrophy, microbleeds and IB analysis
Ultrasound examination was carried out in all but one patient (controlled). There was no intergroup difference in any of the measurements carried out (Table 1). Global cortical atrophy and microbleeds were assessed in all participants. Comparisons among the three clinical groups showed similar proportions of subjects with and without microhemorrhages and within each level of atrophy. Cerebral ischemia could be analyzed in all but one patient (resistant), also without significant intergroup differences in the proportions of subjects scoring ⩽3 and >3 for total IB and 0–1, 2 and ⩾2 for frontal IB. When considering the association between total IB and atrophy, the proportion of subjects with total ischemia scores >3 increased significantly as atrophy scores rose. However, frontal ischemia and global cortical atrophy did not show a significant relationship (Figure 4).
Relationships between total and frontal ischemia and atrophy scores. Proportions of subjects with total ischemia scores ⩽3 and >3 were significantly different depending on the atrophy score. Unlike patients with no or some atrophy, patients with atrophy scores of 2 also scored higher in brain total ischemia. However, frontal ischemia and global atrophy did not show a significant relationship. TIS, total ischemia score; FIS, frontal ischemia score; GCA, global cortical atrophy; AS, atrophy score.
Metabolite analysis
Intergroup differences
Magnetic resonance spectroscopy was performed in all subjects, but in two patients the frontal spectrum quality was poor and no metabolite ratio could be calculated. Accordingly, the final MRS samples consisted of PPL from 14 normal, 17 controlled and 20 resistant BP subjects, and the frontal lobe from 13 normal, 17 controlled and 19 resistant subjects.
Among all metabolite ratios, only PPL mI/Cr and frontal NAA/Cr showed intergroup differences (Table 2). PPL mI/Cr was higher in hypertensive patients and progressively increased from normal BP to uncontrolled patients (Figure 2). However, the difference was significant only in resistant hypertension. Both hypertensive groups showed significant differences in frontal NAA/Cr. NAA/Cr was higher in controlled patients and lower in resistant subjects. The mean and s.d. of Cr SNR in the PPL was 33.72±6.94, and 16.30±6.41 for the frontal voxel. The two measurements were statistically different (P<0.001). In the PPL, the vast majority of patients (46 of 51) showed SNR values over the cutoff point of 25 established by Okada et al.17 for this location. All normotensive subjects, 16 of 17 of the controlled hypertensive patients and 16 of 20 of the resistant hypertensive subjects had Cr SNR values exceeding a threshold of 25. When removing mI/Cr values corresponding to the five hypertensive patient under the SNR threshold, resistant hypertension mI/Cr mean moved slightly away from those of normotensive and controlled hypertensive patients.
Metabolite changes depending on demographic, clinical, analytic, ultrasound and structural imaging features
In spite of the metabolite differences among clinical groups, neither systolic nor diastolic BP measurements showed significant correlations with any of the four significant metabolite ratios. PPL mI/Cr and frontal NAA/Cr were similar across the different levels of IB and atrophy. Frontal NAA/Cr showed significant inverse correlations with ultrasound right systolic (P=0.045) and diastolic ratios (P=0.041), left intima-media thickness (P=0.044), total cholesterol levels (P=0.031) and LDL levels (P=0.001). Unlike frontal metabolite ratios, PPL mI/Cr did not change or correlate with any of the analytical parameters and ultrasound measurements. Neither PPL mI/Cr nor frontal NAA/Cr showed differences depending on the characteristics of carotid plaques.
Microbleeds, diabetes mellitus, cardiovascular disease, smoking, sex, age, duration of disease and a-MMSE score did not have an impact on any of these ratios.
Discussion
These results show that mI/Cr in the PPL was augmented in subjects with resistant hypertension. However, it remained unrelated to atrophy, IB, ultrasound findings, and demographic, analytic and clinical data. Instead, frontal NAA/Cr proved to be different between the two hypertensive groups, but not between normotensive and hypertensive patients. Moreover, this variable showed relationships with ultrasound parameters and the lipid profile. Owing to the high Cr SNR, the PPL mI/Cr results seem to be highly reliable.17 Cr SNR was significantly lower in the frontal lobe voxel. However, the effect of the lower SNR on the NAA/Cr variability was smaller than that observed for the mI/Cr ratio, and reproducibility of frontal measurements has been previously found to be consistent.17, 19 In addition, the SNR means of both voxels in our sample were quite similar to those previously reported for an adequate MRS signal quality (PPL 34.6; frontal 14.0),20 and the lack of significant differences in PPL and frontal Cr SNR among groups also lends support to the reliability of our results.
Brain MRS studies of hypertension are scarce and their results are variable. The increase in PPL mI/Cr in our series agrees with a previous report showing higher mI/Cr values in the peritrigonal white matter of patients with asymptomatic hypertension.5 Regarding the changes in frontal NAA/Cr ratio, the ratio drop in our resistant hypertensive patients is in agreement with two recent chemical-shift imaging reports involving the white matter of the centrum semiovale21 and particular areas in the white and gray matters.22 These studies showed decreasing NAA concentrations associated with increasing severity of disease21 and NAA/Cr decreases in the gray matter of hypertensive patients.22 Contrary to these results, NAA/Cr was not found to differ between treated and untreated hypertensive patients in a recent related study.23 The different outcomes might be due to the different methods used. In the Hannesdottir et al.23 study, NAA/Cr ratio was averaged from the ratio values across all voxels measured within a field of view of 22 cm centered in the centrum ovale, including the white and gray matters. In the other studies, the authors carried out either an absolute quantification of white matter metabolites in a similar field of view21 or metabolite ratio measurements in very specific brain areas (insular cortex, thalami and parieto-occipital peritrigonal white matter),22 as we did with our single-voxel analysis of the frontal lobe and the PPL. As sensitivity of metabolite ratios to abnormalities has been reported to be less than absolute quantification,24 the added effects of different quantification methods and differences in the regions of interest possibly accounted for the conflicting results. In this setting, what makes our results more interesting is our observation of a combined imbalance of NAA/Cr and mI/Cr ratios in two different brain areas, with different variation trends. In addition, the originality of these results also dwells in the sample characteristics. Although, in the other quoted papers, comparisons were carried out between groups of normal vs asymptomatic hypertensive patients,5, 22 symptomatic vs asymptomatic hypertensive patients,21 or treated vs untreated patients,23 we focused on the effect of medical treatment by comparing two groups of treated asymptomatic hypertensive patients with opposing outcomes.
Our sample composed of subjects with few microbleeds and with minor ischemic involvement whose global cortical atrophy was significantly higher with higher IB, but without a significant difference in atrophy between hypertensive and normotensive subjects. Moreover, although BP was higher in resistant hypertension, BP measurements were not significantly different among the three clinical groups at the time of imaging. Although the reason for the absence of differences could be, in part, the size of the sample, these results were not surprising considering the characteristics of the selected patients. Thus, the fact that our patients were asymptomatic, free of severe diseases and under intense medical control might have allowed us to reduce the expected effects of brain lesions (IB or atrophy) and other variables, and to better observe the possible effects of hypertension on metabolite measurements depending on whether or not it was controlled with medical treatment. Moreover, NAA depletion could be independent of the level of atrophy.25 Given that clinical groups did not differ in IB, atrophy, age, sex ratio, duration of disease, diabetes and cardiovascular disease, it seems unlikely that these variables influenced the metabolite imbalances in our patients. However, as periventricular and subcortical white matter lesions are more severe in hypertensive than in normal elderly persons,26 it is still reasonable to suggest that frontal and PPL metabolic changes could be induced directly or indirectly by white matter disease. Although IB was not different between our normotensive and hypertensive patients, the pattern of change of white matter disease from normotensive to resistant hypertensive patients was quite analogous to the lesion load reported by Hannesdotir et al.23 In that study, the lesion load proved to be significantly higher in treated relative to untreated hypertensive patients. In our case, differences in IB perhaps failed to be significant because our whole-hypertensive sample composed of patients under treatment. The significant differences we observed in frontal NAA/Cr, being highest in controlled patients and lowest in resistant subjects, resembled the IB patterns in the same groups. However, in our series the NAA/Cr ratio changed with treatment outcome but not with IB. In addition, the increase of NAA/Cr in controlled hypertension points to a favorable outcome of antihypertensive treatment22, 23 and not to the expected deleterious effect of white matter disease. Therefore, we hypothesize that, beyond white matter disease, frontal NAA/Cr changes could be caused by something else. Increased LDL is a major cause of arteriosclerosis and coronary heart disease,27 and it was significantly higher in our resistant hypertensive patients. Furthermore, although they did not differ among our three clinical groups, other arteriosclerosis-related parameters, such as right systolic and diastolic carotid ratios, intima-media thickness and total cholesterol, showed inverse significant correlations with frontal NAA/Cr, as well as LDL levels. Altogether, the increase of LDL and the negative relationships in resistant hypertensive patients support the hypothesis that changes in brain perfusion related to carotid arteriosclerosis were accountable for the observed frontal NAA/Cr drop in resistant hypertension. On the other hand, it has been suggested that antihypertensive drugs, especially aggressive treatments, could account for an unexpectedly higher volume of leukoaraiosis,26, 28 probably because of the effects on cerebral perfusion.3 Accordingly, drug responders could be more sensitive to both beneficial and unwanted treatment effects, which, in the frontal lobes of our controlled hypertensive patients, would be particularly perceptible in the form of NAA/Cr ratio increases, as well as in the trend to a higher IB.
The PPL mI/Cr ratio also failed to relate to IB, considering that it was highest in our resistant subjects. In contrast, the highest ischemia scores, although not statistically different, were seen in controlled patients. Moreover, the PPL mI/Cr ratio did not show significant correlations with arteriosclerosis-related ultrasound parameters or the lipid profile that could support a relationship with changes in the brain blood flow similar to that found in frontal NAA/Cr ratio. In the PPL, the mI/Cr ratio increase with an unchanged NAA/Cr ratio reproduces the metabolite pattern of pre-Alzheimer's mild cognitive impairment.5, 29 Similar to mild cognitive impairment and Alzheimer's disease,30 perfusion imaging has shown relative cerebral blood flow (rCBF) drops involving the PPL and frontal lobes in asymptomatic hypertension that could contribute to cognitive deficits without imaging evidence of white matter disease.31 Increasing mI or impaired high-energy metabolism were present in patients with reduced brain perfusion,32, 33 but a PPL mI/Cr increase is also an accepted neurodegenerative marker.29 Whether rCBF drops represent a direct hypertension flow effect or the results of secondary neurodegeneration is a matter of debate, but our results raise the interesting possibility that both phenomena could explain why frontal NAA/Cr was related to ultrasound parameters and the lipid profile, whereas PPL mI/Cr was not. Unlike many previous reports,5, 21, 22, 23 we compared two groups of asymptomatic hypertensive patients with different treatment outcomes, and mI/Cr increased only with failing treatment. Considering that PPL flow defects have been regarded as dementia predictors in mild cognitive impairment,34 and in view of the present results and previous research,5, 35 MRS could have a similar role, taking into account that metabolite disturbances emerged before deficits in cognition arose, especially when medical treatment failed. Nevertheless, whether this finding implies that successful medical treatment could delay neurodegeneration should be considered with caution given that, currently, we do not know whether mild cognitive impairment will eventually develop in our resistant hypertensive patients. Furthermore, as reported by Catani et al.,5 we cannot show that mI/Cr imbalances are definitively linked to neurodegeneration in hypertensive patients, nor has lowering BP been shown to prevent cognitive decline.2
This study has some limitations. First, the median duration of disease was only 8 years. More long-lasting hypertension would have allowed us to further explore its effects on the brain. Second, MRS measurements were carried out with conventional software with a conventional MRI system. Although we did not acquire measurements of absolute concentrations, but instead of metabolite ratios, this is an easy, common way to work in a clinical environment. As a result, our data can be used as an adequate reference in clinical practice. Finally, measurements of ischemia and atrophy were not automated, but were achieved following widely accepted imaging scales for clinical research and were carried out by a single trained observer. As with metabolite measurements, we believe that this method of scoring ischemia and atrophy is currently more applicable in medical practice, providing our results with clinical validity.
In summary, in asymptomatic hypertension, changes in brain metabolites, mainly mI/Cr ratio in the PPL, are similar to those reported in mild cognitive impairment, increase with failing treatment, and can be detected in neuropsychologically unaffected patients with different treatment outcomes. Whether metabolite ratio imbalances are linked to hypertension brain perfusion effects or neurodegeneration, whether successful treatment could decrease hypertension-related cognitive impairment, and whether MRS could be a valuable tool to identify subjects at risk must be further investigated with a follow-up of our sample and longitudinal studies.
Conflict of interest
The authors declare no conflict of interest.
References
Tormo MJ, Navarro C, Chirlaque MD, Pérez-Flores D . Prevalence and control of arterial hypertension in the south-east of Spain: a radical but still insufficient improvement. Eur J Epidemiol 1997; 13: 301–308.
McGuinness B, Todd S, Passmore P, Bullock R . The effects of blood pressure lowering on development of cognitive impairment and dementia in patients without apparent prior cerebrovascular disease. Cochrane Database Systematic Reviews 2006 Issue 2. Article no.: CD004034.
Birns J, Markus H, Kalra L . Blood pressure reduction for vascular risk: is there a price to be paid? Stroke 2005; 36: 1308–1313.
Kantarci K . 1H magnetic resonance spectroscopy in dementia. Br J Radiol 2007; 80 (Spec No 2): S146–S1152.
Catani M, Mecocci P, Tarducci R, Howard R, Pelliccioli GP, Mariani E, Metastasio A, Benedetti C, Senin U, Cherubini A . Proton magnetic resonance spectroscopy reveals similar white matter biochemical changes in patients with chronic hypertension and early Alzheimer's disease. J Am Geriatr Soc 2002; 50: 1707–1710.
Marrugat J, Vila J, Pavesi M, Sanz F . Estimation of the sample size in clinical and epidemiological investigations. Med Clin (Barc) 1998; 111: 267–276.
Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM . Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51: 1403–1419.
Lobo A, Saz P, Marcos G, Día JL, de la Cámara C, Ventura T, Morales Asín F, Fernando Pascual L, Montañés JA, Aznar S . Revalidación y normalización del Mini-Examen Cognoscitivo (primera versión en castellano del Mini-Mental Status Examination) en la población general geriátrica. Med Clin (Barc) 1999; 112: 767–774.
Blesa R, Pujol M, Aguilar M, Santacruz P, Bertran-Serra I, Hernández G, Sol JM, Peña-Casanova J . Normalisation of cognitive and functional instruments for dementia. Clinical validity of the ‘mini-mental state’ for Spanish speaking communities. Neuropsychologia 2001; 39: 1150–1157.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edn. American Psychiatric Association: Washington, DC, 1994.
Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC . Mild cognitive impairment-beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256: 240–246.
Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T, Scheltens P . European task force on age-related white matter changes. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001; 32: 1318–1322.
Pasquier F, Leys D, Weerts JG, Mounier-Vehier F, Barkhof F, Scheltens P . Inter- and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol 1996; 36: 268–272.
Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM . Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009; 8: 165–174.
Kantarci K, Jack Jr CR . Neuroimaging in Alzheimer's disease: an evidence-based review. Neuroimag Clin N Am 2003; 13: 197–209.
Gouw AA, van der Flier WM, Fazekas F, van Straaten EC, Pantoni L, Poggesi A, Inzitari D, Erkinjuntti T, Wahlund LO, Waldemar G, Schmidt R, Scheltens P, Barkhof F . Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the leukoaraiosis and disability study. Stroke 2008; 39: 1414–1420.
Okada T, Sakamoto S, Nakamoto Y, Kohara N, Senda M . Reproducibility of magnetic resonance spectroscopy in correlation with signal-to-noise ratio. Psychiatry Res 2007; 156: 169–174.
Wiseman RM, Saxby BK, Burton EJ, Barber R, Ford GA, O'Brien JT . Hippocampal atrophy, whole brain volume, and white matter lesions in older hypertensive subjects. Neurology 2004; 63: 1892–1897.
Mullins PG, Rowland R, Bustillo J, Bedrick EJ, Lauriello J, Brooks WM . Reproducibility of 1H-MRS measurements in schizophrenic patients. Magn Reson Med 2003; 50: 704–707.
Valenzuela MJ, Sachdev PS, Wen W, Shnier R, Brodaty H, Gillies D . Dual voxel proton magnetic resonance spectroscopy in the healthy elderly: subcortical-frontal axonal N-acetylaspartate levels are correlated with fluid cognitive abilities independent of structural brain changes. Neuroimage 2000; 12: 747–756.
Nitkunan A, Charlton RA, McIntyre DJ, Barrick TR, Howe FA, Markus HS . Diffusion tensor imaging and MR spectroscopy in hypertension and presumed cerebral small vessel disease. Magn Reson Med 2008; 59: 528–534.
Ben Salem D, Walker PM, Bejot Y, Aho SL, Tavernier B, Rouaud O, Ricolfi F, Brunotte F . N-acetylaspartate/creatine and choline/creatine ratios in the thalami, insular cortex and white matter as markers of hypertension and cognitive impairment in the elderly. Hypertens Res 2008; 31: 1851–1857.
Hannesdottir K, Nitkunan A, Charlton RA, Barrick TR, MacGregor GA, Markus HS . Cognitive impairment and white matter damage in hypertension: a pilot study. Acta Neurol Scand 2009; 119: 261–268.
Simister RJ, Woermann FG, McLean MA, Bartlett PA, Barker GJ, Duncan JS . A short-echo-time proton magnetic resonance spectroscopic imaging study of temporal lobe epilepsy. Epilepsia 2002; 43: 1021–1031.
Valenzuela MJ, Sachdev P . Magnetic resonance spectroscopy in AD. Neurology 2001; 56: 592–598.
van Dijk EJ, Breteler MM, Schmidt R, Berger K, Nilsson LG, Oudkerk M, Pajak A, Sans S, de Ridder M, Dufouil C, Fuhrer R, Giampaoli S, Launer LJ, Hofman A . The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension 2004; 44: 625–630.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497.
Schwartz GL, Bailey KR, Mosley T, Knopman DS, Jack Jr CR, Canzanello VJ, Turner ST . Association of ambulatory blood pressure with ischemic brain injury. Hypertension 2007; 49: 1228–1234.
Kantarci K, Jack Jr CR, Xu YC, Campeau NG, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC . Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: a 1H MRS Study. Neurology 2000; 55: 210–217.
Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N . Pattern of cerebral hypoperfusion in Alzheimer's disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 2005; 234: 851–859.
Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM . Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke 2008; 39: 349–354.
Ashwal S, Holshouser BA, del Rio MJ, Tong KA, Applegate RL, Bailey LL . Serial proton magnetic resonance spectroscopy of the brain in children undergoing cardiac surgery. Pediatr Neurol 2003; 29: 99–110.
Hattingen E, Lanfermann H, Menon S, Neumann-Haefelin T, de Rochement RD, Stamelou M, Höglinger GU, Magerkurth J, Pilatus U . Combined (1)H and (31)P MR spectroscopic imaging: impaired energy metabolism in severe carotid stenosis and changes upon treatment. MAGMA 2009; 22: 43–52.
Hirao K, Ohnishi T, Hirata Y, Yamashita F, Mori T, Moriguchi Y, Matsuda H, Nemoto K, Imabayashi E, Yamada M, Iwamoto T, Arima K, Asada T . The prediction of rapid conversion to Alzheimer's disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage 2005; 28: 1014–1021.
Ding B, Chen KM, Ling HW, Zhang H, Chai WM, Li X, Wang T . Diffusion tensor imaging correlates with proton magnetic resonance spectroscopy in posterior cingulate region of patients with Alzheimer's disease. Dement Geriatr Cogn Disord 2008; 25: 218–225.
Acknowledgements
This study was supported by Grants SEJ2005-01223/PSIC and CSD2008-00048 from the Spanish Ministry of Science and Innovation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García Santos, J., Fuentes, L., Vidal, J. et al. Posterior paralimbic and frontal metabolite impairments in asymptomatic hypertension with different treatment outcomes. Hypertens Res 33, 67–75 (2010). https://doi.org/10.1038/hr.2009.176
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2009.176
Keywords
This article is cited by
-
Elevated cerebral glutamate and myo-inositol levels in cognitively normal middle-aged adults with metabolic syndrome
Metabolic Brain Disease (2010)