Abstract
Drosophila Wingless (Wg) acts as a morphogen during development. Wg secretion is controlled by a seven-pass transmembrane cargo Wntless (Wls). We have recently identified retromer as a key regulator involved in Wls trafficking. As sorting nexin (SNX) molecules are essential components of the retromer complex, we hypothesized that specific SNX(s) is required for retromer-mediated Wnt secretion. Here, we generated Drosophila mutants for all of the eight snx members, and identified Drosophila SNX3 (DSNX3) as an essential molecule required for Wg secretion. We show that Wg secretion and its signaling activity are defective in Dsnx3 mutant clones in wing discs. Wg levels in the culture medium of Dsnx3-depleted S2 cells are also markedly reduced. Importantly, Wls levels are strikingly reduced in Dsnx3 mutant cells, and overexpression of Wls can rescue the Wg secretion defect observed in Dsnx3 mutant cells. Moreover, DSNX3 can interact with the retromer component Vps35, and co-localize with Vps35 in early endosomes. These data indicate that DSNX3 regulates Wg secretion via retromer-dependent Wls recycling. In contrast, we found that Wg secretion is not defective in cells mutant for Drosophila snx1 and snx6, two components of the classical retromer complex. Ectopic expression of DSNX1 or DSNX6 fails to rescue the Wg secretion defect in Dsnx3 mutant wing discs and in Dsnx3 dsRNA-treated S2 cells. These data demonstrate the specificity of the DSNX3-retromer complex in Wls recycling. Together, our findings suggest that DSNX3 acts as a cargo-specific component of retromer, which is required for endocytic recycling of Wls and Wg/Wnt secretion.
Similar content being viewed by others
Introduction
Wnt proteins are evolutionarily conserved glycoproteins that play profound roles in a variety of developmental processes as well as in adult homeostasis 1. Abnormal Wnt signaling is associated with many human diseases, ranging from cancers to degenerative diseases 2, 3. Drosophila Wingless (wg) encodes the homolog of vertebrate Wnt-1 4, 5. Wg acts as a short-range organizer and a long-range morphogen in a variety of developmental contexts 6, 7. How Wnt processing and secretion are regulated is an essential issue in the Wnt field, yet the underlying mechanisms are not fully understood 8.
Genetic screens in Drosophila identified Wntless (Wls, also known as Evenness Interrupted (Evi) or Sprinter (Srt)) as an essential regulator for Wg/Wnt secretion 9, 10, 11. Wls is a seven-pass transmembrane protein that is localized in the Golgi apparatus, at the cell surface and in early endosomes 9, 12, 13, 14. The function of Wls in Wg/Wnt secretion is conserved from Drosophila, C. elegans to vertebrates. In Drosophila wls mutants, Wg is not secreted and is accumulated inside Wg-producing cells 9, 10, 11. Recent studies have also shown that Gpr177, the mouse homolog of Drosophila wls, is essential for Wnt secretion during embryonic development 15, 16.
We and others have recently identified retromer as an essential regulator for Wg/Wnt secretion in Wg/Wnt-producing cells 12, 13, 14, 17, 18, 19, 20. Our studies as well as others demonstrate that Wls endocytosis is a clathrin-mediated process in which retromer plays an essential role in Wls retrograde recycling from early endosomes to the trans-Golgi network (TGN) 12, 13, 14, 17, 18. Retromer is an evolutionarily conserved multi-protein complex whose function is to sort cargo from early endosomes back to TGN 21, 22, 23. In yeast, the retromer complex consists of two independently assembled subcomplexes: the cargo selective VPS subcomplex, consisting of Vps35p, Vps26p, and Vps29p and the membrane-binding dimer formed by Vps5p and Vps17p. Vps5p and Vps17p are sorting nexins (SNXs), a protein family containing a phosphoinositide-binding PX (phox homology) domain that binds phosphatidylinositol 3-phosphate (PI3P) and other phosphoinositides 24, 25, 26. Some of the SNXs also have a BAR (Bin/amphiphysin/Rvs) domain, which is a dimerization and membrane-binding module capable of sensing membrane curvatures 24. In mammals, SNX1 and SNX2 are the orthologs of yeast Vps5p 27, 28, while SNX5 and SNX6 appear to be the functional orthologs of yeast Vps17p 29. SNX1/SNX2 (SNX1/2) and SNX5/SNX6 (SNX5/6) all contain BAR domains 24. The current model for the classic retromer complex is that one chain of SNX1 or SNX2 and one chain of SNX5 or SNX6 interact with the VPS subcomplex to form the functional retromer complex required for retrograde transport of cargo proteins such as the phagocytic receptor CED-1 in C. elegans 30, and the cation-independent mannose 6-phosphate receptor (CI-MPR) in mammals 23, 31, 32, 33, 34, 35. Recent studies further demonstrated that SNX5/6 can interact with the p150Glued subunit of the dynein/dynactin motor complex and that such interaction is important to drive the formation and movement of tubular retrograde intermediates 34, 35. In addition to the SNX-BAR proteins, previous studies have shown that yeast Grd19p, a SNX3 homolog lacking the BAR domain, can also function in retromer-dependent endosome-to-Golgi retrieval of the iron transporter Fet3p-Ftr1p, a cargo protein 36, 37, 38. Very recently, SNX27, another non-BAR SNX molecule, was shown to act as a retromer component for recycling the β2 adrenergic receptor to the plasma membrane 39. Currently, it is unknown whether different SNX molecules can interact with the VPS subcomplex to form a specific retromer subcomplex required for cargo selection and retrograde transport.
To identify specific SNX molecules involved in retromer-mediated Wg secretion, we generated mutants of individual Drosophila snx genes and analyzed their roles in Wg secretion and signaling. To our surprise, we found that Drosophila SNX3 (DSNX3), but not the SNX-BAR proteins SNX1/2 and SNX5/6, is required for Wg secretion. We show that Wg secretion is defective in the absence of DSNX3 activity. Moreover, DSNX3 interacts with Vps35 and Wls, and regulates Wls stability. Our data argue that DSNX3 interacts with the VPS subcomplex to form a retromer subcomplex required for endocytic recycling of Wls.
Results
Identification of Dsnx3 as an essential candidate gene involved in Wg secretion
Searches of Drosophila genome databases identified eight snx genes, including CG2774, Dsnx6/CG8282, CG6359, SH3PX1/CG6757, CG5734, CG3077, Snz/CG1514 and CG32758. To examine the roles of these snx genes in development, we generated null alleles of seven Drosophila snx genes by P element-mediated imprecise excision. Null mutant for Dsnx6 was obtained by the “Ends-out” gene targeting technique 40, as there was no available P element inserted near the Dsnx6 gene. DSNX6 is the homolog of mammalian SNX5/6. The protein product of CG2774 is highly homologous to both yeast Vps5p and mammalian SNX1/2, and therefore we referred to CG2774 as Drosophila snx1 (Dsnx1). The protein product of CG6359 is highly homologous to mammalian SNX3 or SNX12, therefore is referred to as DSNX3. Previous studies in mammalian systems have shown that SNX1/2 and SNX5/6 are the components of the classic retromer complex 27, 29, 34, 35. Therefore, we speculated that DSNX1 and DSNX6 may be the critical SNX molecules required for retromer-mediated Wg secretion. To our surprise, Dsnx1 null mutants were completely viable and fertile, and exhibited no detectable defects in Wg signaling determined by genetic mosaic analysis in wing discs (see data later). Similarly, the Dsnx6 null mutants were also completely viable and fertile, and exhibited no obvious defects (data not shown). In contrast, Dsnx3 null mutants were semi-lethal, and the fly wings of homozygous Dsnx3 mutants extended from the body axis. Using the “direct mosaic system” 41, we generated Dsnx3 mutant mosaic clones in developing wings by vg Q1206-Gal4 UAS-Flp, and found obvious notching at the margin region of the wing (Supplementary information, Figure S1B and S1B'), indicating that DSNX3 is involved in Wg signaling.
In the wing disc, the Wg protein is produced at the dorso/ventral (D/V) boundary and forms a gradient along the D/V axis 42 (Figure 1A). Wg induces senseless (sens) expression at a short range, whereas it activates distalless (dll) at a long range 43, 44, 45. We examined the requirement of DSNX3 for Wg secretion and signaling by genetic mosaic analysis in wing discs using the FLP-FRT method 46 or by RNA interference (RNAi) to deplete Dsnx3. Wg-producing cells mutant for Dsnx3 had higher levels of Wg compared with surrounding wild-type cells (Figure 1B). However, Wg levels were not altered in a mutant clone residing outside of the Wg-producing cells (marked by yellow dotted lines and an arrow in Figure 1B). Similarly, when UAS-Dsnx3RNAi was expressed in the entire posterior (P) compartment using enGal4, Wg protein accumulated in Wg-producing cells and was significantly reduced in Wg-receiving cells in the P compartment (Figure 1E). Furthermore, in a Dsnx3 homozygous mutant background, ectopic expression of DSNX3 using enGal4 can rescue the Wg secretion defect in the P compartment (Figure 1F). Wg accumulation in the mutant cells was not due to increased wg transcription, as no difference was observed in the activity of a WgLacZ reporter between mutant and wild-type cells (Figure 1G and 1G'). Furthermore, using an extracellular staining technique 47, we observed reduced levels of extracellular Wg in Dsnx3 mutant clones (Figure 1C and 1C'). Similarly, extracellular Wg levels were reduced in the P compartment of Wg-producing cells and Wg-receiving cells when Dsnx3 activity was eliminated by UAS-Dsnx3RNAi using enGal4 (Figure 1D). These data argue that Wg protein accumulated inside Wg-producing cells in the absence of DSNX3.
DSNX3 is required for Wg secretion and signaling. (A-D) Wing discs are oriented dorsal top-right, anterior top-left. (A) Wg staining in wild-type disc. Wg was detected in its producing cells along D/V boundary and outside of producing cells in punctate structures (arrowheads). (B-B'') Wg and Sens staining in a wing disc bearing Dsnx31 clones. Mutant clones are outlined by the dotted lines to mark the absence of GFP (B'', green). Wg was accumulated inside Dsnx3 mutant Wg-producing cells, but was not altered in a mutant clone residing outside of the Wg-producing cells marked by yellow dotted lines (arrow in B). Sens staining was dramatically reduced in Dsnx31 clones (arrowhead in B'). (B''') Enlarged view of clone in B shows accumulated Wg inside Dsnx3 mutant Wg-producing cells. Also noted is the reduced number of Wg punctate structures outside Wg-producing cells in the clone regions (arrowheads in B'''). (C-C') Extracellular Wg distribution was examined in Dsnx31 clones (C) marked by the dotted lines and by the absence of GFP (C', green). Extracellular Wg is reduced in the Dsnx31 mutant Wg-producing cells. (D) Extracellular Wg distribution in the P compartment of a wing disc expressing UAS-Dsnx3RNAi using enGal4. The extracellular Wg level was reduced in the P compartment where DSNX3 activity was eliminated by UAS-Dsnx3RNAi. (E-G) Wing discs are oriented anterior left, dorsal up. (E) UAS-Dsnx3RNAi was expressed in wing discs using enGal4 to deplete DSNX3 activity in the P compartment. Anterior/posterior (A/P) boundary is shown by the dotted line. Wg accumulated in Wg-producing cells of the P compartment and Wg punctate structures in surrounding cells were almost absent in the P compartment (arrowhead). (F) In Dsnx3 homozygous mutant background, UAS-Dsnx3-V5 was expressed in the P compartment using enGal4. Wg secretion appeared to be normal in the P compartment (arrowhead), which exhibited secreted Wg punctates outside Wg-producing cells and no accumulation of Wg in Wg-producing cells. (G-G') Wg expression was monitored by WglacZ in Dsnx31 mutant Wg-producing cells (G) marked by the dotted lines and by the absence of GFP (G', green). As shown in G (arrowhead), Wg expression was not altered. (H) The amount of Wg in the supernatant (S) was strongly reduced when S2+pMK-Wg cells were treated with dsRNAs targeted to different regions of Dsnx3 compared to the control (GFP dsRNA). However, the amount of Wg in cell lysates was markedly increased. Both RNAi1 and RNAi2 were targeted to Dsnx3 coding region. β-actin showed equal loading of lysate samples and was not altered by Dsnx3 RNAi treatment.
We further examined the requirement of DSNX3 function for sens expression in the wing disc. Sens was reduced in Dsnx3 mutant clones straddling the wg expression domain (Figure 1B'). However, Sens expression was still present in some Dsnx3 mutant cells when they neighbored wild-type Wg-producing cells (Supplementary information, Figure S2A and S2A'), arguing that DSNX3 is not essential for Wg signaling per se. This result also suggests that accumulated Wg protein inside Dsnx3 mutant cells failed to activate Wg signaling. Taken together, these data suggest that loss of DSNX3 activity causes a defect in Wg secretion, subsequently leading to defects in Wg target gene expression.
To further confirm the requirement of DSNX3 for Wg secretion, we examined the effect of DSNX3 on Wg secretion in S2R+ cells expressing Wg. As shown in Figure 1H, Wg was reduced in the supernatants of cells treated with Dsnx3 dsRNAs against two different regions of the Dsnx3 gene, whereas control cells treated with GFP dsRNA secreted Wg normally. Taken together, the above results strongly argue that DSNX3 activity is required for Wg secretion.
DSNX3 is required for Wg secretion in other tissues, but not essential for other signaling pathways
As Wg is required for the development of other larval imaginal discs, we examined the role of DSNX3 in Wg secretion in other tissues. As shown in Figure 2, Wg was accumulated in both leg and haltere discs in the absence of DSNX3 activity (Figure 2D-2F'). Similarly, Wg was also accumulated in the Dsnx3 mutant Wg-producing cells of the wing hinge region (arrowhead in Figure 2D). These data suggest that DSNX3 is critical for Wg secretion in other tissues.
DSNX3 is required for Wg secretion in other tissues, but not essential for other signaling pathways. (A-A') Wing discs are oriented dorsal top-right, anterior top-left. Dsnx31 clones are marked by dotted lines and by the absence of GFP (A'). Hh protein levels (A) were altered neither in clones residing in Hh-expression cells in P compartment nor in those of Hh-receiving cells in A compartment. (B-D') Wing discs are oriented anterior left, dorsal up. (B-B') Wing disc bearing Dsnx31 clones marked by dotted lines and by the absence of GFP (B'). Ptc (Hh receptor) levels were normal in Dsnx31 mutant cells (B). (C-C') Wing disc bearing Dsnx31 mutant clones marked by dotted lines and by the absence of GFP (C'). Sal (a downstream component of Dpp signaling) levels were not altered in Dsnx3 mutant clones (C). (D-D') Wg staining in wing disc bearing Dsnx31 clones marked by dotted lines and by the absence of GFP (D'). Wg protein was accumulated in its producing cells mutant for Dsnx3 but its level was not altered in a mutant clone residing outside of the Wg-producing cells marked by yellow dotted lines. Also note that Wg was accumulated in Dsnx31 mutant Wg-producing cells in the hinge region (arrowheads in D). (E-E') Dsnx31 clone in leg disc (E', marked by GFP absence). As in the wing disc, Wg was accumulated in Dsnx3 mutant Wg-producing cells. (F-F') Dsnx31 clone in haltere disc (F', marked by GFP absence). As in the wing and leg discs, Wg was also accumulated in Dsnx3 mutant Wg-producing cells.
To determine the specificity of DSNX3, we examined whether DSNX3 is required for the activity of two other morphogens, Hedgehog (Hh) and Decapentaplegic (Dpp). In contrast to Wg secretion, we found that Hh secretion was normal (Figure 2A and 2A'), and that Hh-induced Patched (Ptc) expression was not altered (Figure 2B and 2B'). Similarly, Dpp signaling as determined by Sal expression was not altered (Figure 2C and 2C'). As wg transcription in wing discs is controlled by Notch signaling 48 and was not affected in Dsnx3 mutant cells (Figure 1G and 1G'), we suggest that DSNX3 activity is also not required for Notch signaling. Taken together, our data suggest that DSNX3 is specifically required for Wg secretion.
Wls stability is regulated by DSNX3
As Wls is required for Wg secretion 9, 10, 11, one possibility is that DSNX3 may control Wg secretion by regulating Wls activity. We first examined whether DSNX3 interacts with Wls in vivo. When Dsnx3-GFP and wls-V5 were expressed in wing discs, Wls was present in vesicle-like punctate structures, and the majority of Wls co-localized with DSNX3 (Figure 3A-3A''). The majority of DSNX3 also co-localized with the early endosome marker GFP-2×FYVE 49 (Figure 3B-3B''). Previous studies have shown that Wls is located in early endosomes 12, 14. The above data suggest that DSNX3 may interact with Wls in early endosomes. Consistent with this view, DSNX3 co-localized with human Wls (hWls) and the early endosome marker early endosomal antigen 1 (EEA1) 50 when DSNX3 and hWls were co-expressed in HeLa cells (Figure 3C-3C''').
DSNX3 interacts with Wls and affects Wg secretion by controlling Wls stability. (A-A'') UAS-Dsnx3-GFP was expressed in the P compartment of the wing disc using enGal4 in the flies carrying hs-wls-V5 construct. Wls-V5 expression was induced by heat shock for 2 h. Another 2 h later, Wls-V5 protein distribution was examined. Wls-V5 levels were markedly increased in the P compartment. Both V5-tagged Wls and GFP-tagged DSNX3 were detected in punctate structures (arrowheads in A and A'), and they significantly co-localized with each other (arrowheads in A''). (B-B'') UAS-Dsnx3-V5 and UAS-GFP-2×FYVE were expressed in wing discs using enGal4. V5-tagged DSNX3 was detected in punctate structures (arrowheads in B'). DSNX3 punctate structures (B) co-localized with the early endosome marker GFP-2×FYVE (B'). Sites of co-localization are shown by arrowheads in B''. (C-C''') hWls-V5 and DSNX3-GFP expression vectors were co-transfected in HeLa cells. The subcellular localizations of hWls-V5 and DSNX3-GFP proteins were determined by immunostaining. DSNX3-GFP (C, green) co-localized with hWls-V5 (C', red) in early endosomes, which were marked by the early endosome marker EEA1 (C'', blue). Sites of co-localization are indicated by arrowheads in C”'. (D-F) Wing discs are oriented dorsal top-right, anterior top-left. (D) Endogenous Wls was ubiquitously expressed in the wing disc; however, Wls staining was increased at the D/V boundary. (E-E') Endogenous Wls staining in a wing disc bearing Dsnx31 clones. Mutant clones are outlined by the dotted lines to mark the absence of GFP (E', green). The accumulation of Wls in Wg-producing cells was markedly reduced in clones homozygous for Dsnx31 (arrowhead in E). (F) UAS-Dsnx3RNAi was expressed in wing discs using enGal4 to deplete DSNX3 activity in the P compartment. A/P boundary is shown by the dotted line. Wls staining in the apical section of Wg-producing cells was almost absent in the P compartment (arrowhead). (G-H) Wing discs are oriented anterior left, dorsal up. A/P boundary is shown by the dotted line. (G) UAS-HA-wls was expressed in wing discs using enGal4 in the P compartment. Note that Wg levels were somewhat enhanced when Wls was overexpressed (arrowhead). (H) UAS-wls-HA was expressed using enGal4 in the P compartment of Dsnx3 homozygous mutant discs. Wg secretion appeared to be enhanced in the P compartment compared with the A compartment (arrowhead). (I) V5-tagged Wls and GFP-tagged DSNX3 expression vectors were transfected individually or together into S2 cells. Upon immunoprecipitation of DSNX3-GFP protein from transfected cells, Wls-V5 was detected by western blotting in the immunoprecipitates.
Next, we intended to determine whether Wls trafficking was regulated by DSNX3. In the wing disc, Wls is ubiquitously expressed; however, increased Wls staining is observed in a stripe at the D/V boundary (Figure 3D), a region known to secrete and receive high levels of Wg 14. Endogenous Wls levels were strikingly reduced in Dsnx3 mutant clones (Figure 3E and 3E'). Similarly, we expressed UAS-Dsnx3RNAi in the posterior (P) compartment using enGal4 or hhGal4, and found that Wls staining was lost in the Wg-secreting cells (arrowhead in Figure 3F) and markedly reduced in the non-Wg-secreting cells (Supplementary information, Figure S3A-S3A''). Furthermore, in flies carrying the hs-wls-V5 construct, ectopically expressed Wls levels were strikingly reduced 5 h after induction, while significant levels of Wls protein were still maintained at this point in the wild-type cells (Supplementary information, Figure S3B-S3B''). Taken together, these data strongly argue that Wls stability is controlled by DSNX3.
We further asked whether overexpression of Wls could rescue the Wg secretion defect observed in Dsnx3 mutant cells. As shown previously 12, Wg protein levels could be enhanced in the P compartment expressing UAS-wls-HA (Figure 3G). In wing discs of Dsnx3 homozygous mutant, ectopic expression of Wls using enGal4 could produce more Wg particles in the P compartment (arrowheads in Figure 3H), suggesting that overexpression of Wls can restore the Wg secretion defect in Dsnx3 mutant cells.
Finally, we examined whether DSNX3 and Wls formed a complex in S2 cells. V5-tagged Wls and GFP-tagged DSNX3 expression vectors were transfected individually or together into S2 cells. Upon immunoprecipitation of the DSNX3-GFP protein from transfected cells, Wls-V5 was detected by western blotting in the immunoprecipitates (Figure 3I). Collectively, these data argue that DSNX3 interacts with Wls and that Wls stability is regulated by DSNX3.
DSNX3 forms a complex with retromer
Our results so far provided compelling evidence for the role of DSNX3 in regulating the stability of Wls. Previous studies have demonstrated the importance of retromer function for Wls stability 12, 13, 14. As SNX molecule(s) is an important component of the retromer complex, we speculated that DSNX3 either directly or indirectly controls retromer-mediated recycling of Wls. We performed co-localization experiments using HeLa cells. A GFP-tagged DSNX3 expression vector was transfected into HeLa cells, and the subcellular localization of the DSNX3-GFP protein was detected by immunostaining. As shown in Figure 4A-4A''', many of the DSNX3-GFP molecules co-localized with endogenous hVps35 in early endosomes marked by EEA1.
Subcellular localization and interaction of DSNX3 and Vps35. (A-A''') DSNX3-GFP expression vector was transfected into HeLa cells and DSNX3-GFP protein subcellular localization was determined by immunostaining. DSNX3-GFP (A, green) co-localized with endogenous Vps35 (hVps35, blue in A'') in early endosomes (arrowheads in A''') that were marked by the early endosome marker EEA1 (red in A'). (B-D') Wing discs are oriented dorsal top-right, anterior top-left. A/P boundary is shown by the dotted line. (B-B''') UAS-Dsnx3-GFP was overexpressed in the P compartment of the wing disc using enGal4. Wg secretion was markedly expanded in the P compartment (arrowheads in B). B''' shows the enlarged view of a segment of the posterior compartment from B'. The majority of DSNX3-GFP was detected in punctate structures in the apical section of the wing disc (arrowheads in B'''). (C-C') UAS-Dsnx3-GFP was expressed in the A compartment of wing discs using ciGal4. Wg secretion was markedly expanded in the A compartment (arrowheads in C). (D-D') UAS-Dsnx3-GFP was co-expressed with UAS-Vps35RNAi in the P compartment of wing discs using enGal4. Wg accumulated in Wg-producing cells of the P compartment and the gain of function phenotype of DSNX3 observed in B was dramatically repressed by the depletion of DVps35 (arrowhead in D). (E) Myc-tagged DVps35 and GFP-tagged DSNX3 expression vectors were co-transfected into Drosophila S2 cells. Cell lysates were immunoprecipitated and analyzed by western blotting with the antibodies indicated. IP: immunoprecipitation; IB: immunoblot.
We further examined whether DSNX3 formed a complex with retromer by co-immunoprecipitation assay in S2 cells. Myc-tagged DVps35 and GFP-tagged DSNX3 expression vectors were transfected individually or together into S2 cells. Upon immunoprecipitation of the DSNX3-GFP protein from transfected cells, Myc-DVps35 was detected by western blotting in the immunoprecipitates (Figure 4E). This data suggest that DSNX3 can interact with DVps35.
Finally, we further performed an epistatic analysis between DSNX3 and retromer. Enhanced levels of Wg were observed in the P or the anterior (A) compartment when DSNX3 was ectopically expressed by enGal4 or ciGal4, respectively (Figure 4B-4B''', 4C and 4C'). However, when Dsnx3 was co-expressed with Dvps35RNAi (Figure 4D and 4D') in the P compartment by enGal4, Wg levels were increased in the producing cells, but reduced outside of the Wg-producing cells. This data suggest that the loss of retromer activity can block the gain of function activity of DSNX3, arguing that DSNX3 acts upstream of, or in parallel with, retromer in regulating Wg secretion.
Taken together, our results demonstrate that DSNX3 interacts with DVps35 in early endosomes, supporting the view that DSNX3 is involved in retromer-mediated recycling of Wls.
The PI3P-binding motif of DSNX3 in the PX domain is essential for its function
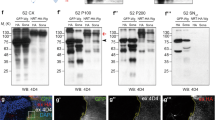
The PX domains of the SNX3 molecules are highly conserved among SNX3 molecules from yeast to mammals (Supplementary information, Figure S4). Previous studies identified the most conserved motif in the PX domain, RR(F/Y)S(D/E)F (single letter amino-acid abbreviations are used here and hereafter), to be very sensitive to mutations 51, 52. Point mutations within this region of SNX3 abolished both the interaction with PI3P and membrane targeting 51, 52. We therefore opted to determine whether membrane targeting of DSNX3 is essential for its activity in Wg secretion. We generated DSNX3(-RRY) in which all three potential PI3P attachment sites of DSNX3, R74, R75 and Y76, were converted to A74, A75 and A76, respectively. As shown earlier, overexpression of DSNX3 can promote Wg secretion (Figure 4B-B''', 4C and 4C'). Moreover, DSNX3-GFP was detected in vesicle-like punctate structures in the apical section of the P compartment (arrowheads in Figure 4B'''). When UAS-Dsnx3(-RRY)-GFP was expressed in wing discs using enGal4 in the P compartment, we observed no obvious difference in Wg levels between the P and A compartments (arrowheads in Figure 5A). The DSNX3(-RRY) was not detected in vesicle-like punctate structures, but rather accumulated throughout the cytoplasm (Figure 5A''). Furthermore, we expressed UAS-Dsnx3(-RRY)-GFP in a Dsnx3 homozygous mutant using enGal4 in the P compartment. The Wg secretion defect cannot be rescued by the expression of Dsnx3(-RRY)-GFP (Figure 5B). Collectively, these data strongly suggest that the PI3P-binding motif of DSNX3 is essential for the activity of DSNX3 in Wg secretion.
PI3P-binding motif of PX domain is essential for DSNX3 function. (A-B) Wing discs are oriented anterior left, dorsal up. (A-A'') UAS-Dsnx3(-RRY)-GFP was expressed in the P compartment of wing discs using enGal4. There was no obvious difference in Wg levels between the P and A compartments (arrowheads in A). DSNX3(-RRY)-GFP was distributed throughout the cytoplasm, and no vesicle-like punctate structures can be observed (A''). A'' shows the enlarged view of a segment of the posterior compartment from A'. (B) UAS-Dsnx3(-RRY)-GFP was expressed in the P compartment of Dsnx3 homozygous mutant wing discs using enGal4. There was no detectable difference in the Wg levels between A and P compartments, indicating that Wg secretion defect of the Dsnx3 mutant cannot be rescued by the expression of DSNX3(-RRY)-GFP. (C-C''') hWls-V5 and DSNX3(-RRY)-GFP expression vectors were co-transfected into HeLa cells. The subcellular localization of hWls-V5 and DSNX3(-RRY)-GFP proteins were determined by immunostaining. DSNX3(-RRY)-GFP was distributed in the cytoplasm (C, green), and lost the co-localization with hWls-V5 (C', red) and the early endosome marker EEA1 (C'', blue). (D-D''') DSNX3(-RRY)-GFP expression vector was transfected into HeLa cells. DSNX3(-RRY)-GFP protein was detected in the cytoplasm (D, green) and did not co-localized with early endosome marker EEA1 (D', red) and hVps35 (D'', blue).
We further asked whether DSNX3(-RRY) lost its interaction with both Wls and Vps35. To examine this, we first performed co-localization experiments in HeLa cells. V5-tagged hWls and GFP-tagged DSNX3(-RRY) expression vectors were co-transfected into HeLa cells and subcellular localizations were determined by immunostaining. DSNX3(-RRY)-GFP accumulated in the cytoplasm (Figure 5C, green), and did not co-localize with either hWls (Figure 5C', red) or the early endosome marker EEA1 (Figure 5C'', blue). DSNX3(-RRY)-GFP (Figure 5D, green) also did not co-localize with endogenous hVps35 (Figure 5D'', blue) in early endosomes marked by EEA1(Figure 5D', red). However, when we performed co-immunoprecipitation experiments between DSNX3(-RRY) and Wls or DVps35 (Supplementary information, Figure S5), we observed the interaction between Wls and DSNX3(-RRY) (Supplementary information, Figure S5A). Similarly, DVps35 could also form a complex with DSNX3(-RRY) (Supplementary information, Figure S5B). These data indicate that the PI3P-binding site in DSNX3 is not required for the interaction of DSNX3 with either Wls or DVps35. On the basis of the above data, we suggest that the membrane targeting of DSNX3 via the PI3P binding is essential for the activity of DSNX3.
DSNX1 and DSNX6 are not essential for retromer-mediated Wg secretion
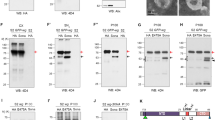
Various studies have demonstrated essential roles of SNX1/SNX2 and SNX5/SNX6 for retromer activities in a variety of cellular functions, including the recycling of cargo proteins Vps10p in yeast, the phagocytic receptor CED-1 in C. elegans and mammalian CI-MPR 30, 31, 32, 33, 53. As both Dsnx1 and Dsnx6 mutants are viable, they may not be essential for Wg secretion. In support of this view, Wg and Sens levels were not altered in Dsnx1 mutant clones (Figure 6A-6A''). Similarly, Wg and Sens levels were also not altered when Dsnx6 is depleted by Dsnx6RNAi (Figure 6B-6B''). Moreover, ectopic expression of DSNX1 failed to rescue Wg secretion defects in Dsnx3-depleted cells (Figure 6C-6C''). Similarly, ectopic expression of DSNX6 also failed to rescue Wg secretion defects in Dsnx3-depleted wing disc cells (Figure 6D-6D'') and in Dsnx3-depleted S2 cells (Figure 6E). These data argue that the activity of DSNX3 in retromer-mediated Wg secretion cannot be replaced by DSNX1 and/or DSNX6, further suggesting the specificity of DSNX3 in Wg secretion.
DSNX1 and DSNX6 are not required for Wg secretion and signaling. (A-C) Wing discs are oriented dorsal top-right, anterior top-left. (A-A'') Wg and Sens staining in a wing disc bearing Dsnx11 clones. Mutant clone is outlined by the dotted lines to mark the absence of GFP (A'', green). Wg levels were not altered in the absence of Dsnx1 (arrowhead in A). Sens staining was also not altered in the mutant clone (arrowhead in A'). (B-B'') UAS-Dsnx6RNAi was expressed in wing discs using hhGal4 to deplete Dsnx6 activity in the P compartment. A/P boundary is shown by the dotted line. Wg was secreted normally under these conditions (arrowhead in B), and the expression of Sens was also not reduced (arrowhead in B'). (C-C'') UAS-Dsnx3RNAi and UAS-V5-Dsnx1 were co-expressed in the P compartment using hhGal4. Wg accumulated in the P compartment (arrowhead in C), indicating that the Wg secretion defect caused by DSNX3 depletion cannot be rescued by the overexpression of DSNX1. (D-D'') UAS-Dsnx3RNAi and UAS-V5-Dsnx6 were co-expressed in the P compartment using hhGal4. Wg accumulated in the P compartment (arrowhead in D), indicating that the Wg secretion defect caused by DSNX3 depletion cannot be rescued by the overexpression of DSNX6. (E) The amount of Wg in the supernatant (S) was strongly reduced when S2+pMK-Wg cells were treated with dsRNAs targeted to the coding region of Dsnx3. However, the Wg secretion defect caused by DSNX3 depletion cannot be rescued by the overexpression of DSNX6.
Discussion
Roles of DSNX3 in Wg secretion
A main finding in this work is the demonstration that DSNX3 controls Wg secretion by regulating retromer-mediated Wls recycling. First, we showed that the loss of DSNX3 activity in the wing disc caused increased levels of Wg inside the producing cells, but reduced levels in the receiving cells. Cell culture experiments further demonstrated that secreted Wg in the conditioned medium was reduced when Wg-producing S2 cells were treated with Dsnx3 dsRNA. These data provide compelling evidence for a critical role of DSNX3 in Wg secretion. Second, we showed that the Wls level was strikingly reduced in the wing disc in the absence of DSNX3 activity. We further showed that DSNX3 co-localized with Wls and can be co-immunoprecipitated with Wls. Similarly, DSNX3 also co-localized with Vps35 and can be co-immunoprecipitated with Vps35. Thus, this work extends further our previous findings of a critical role for retromer in Wg/Wnt secretion by linking SNX3 into this regulatory pathway.
Interestingly, although DSNX3 is essential for Wg secretion, we observed no obvious defects in Dpp or Hh signaling. Previous data showed that loss of retromer activity did not affect Dpp or Hh signaling. In this regard, DSNX3 recapitulates the defects associated with retromer activity. On the other hand, the Dvps35 mutant is lethal, but the Dsnx3 mutant is viable. In addition, Dvps35 homozygous mutant larvae exhibited other defects such as melanotic tumor formation 13, 54. However, homozygous Dsnx3 mutant larvae did not exhibit any detectable melanotic tumors (data not shown). These data suggests that SNX molecule(s) other than DSNX3 are involved in other retromer-mediated activities. On the basis of the above observations, we propose that DSNX3 forms a specific retromer subcomplex that is required for Wls recycling and Wg secretion.
While DSNX3 is essential for Wg secretion, one important issue that remains to be resolved is whether DSNX3 is required for all retromer-mediated Wls recycling and Wg secretion. In addition to wing discs, we also observed Wg secretion defects in other tissues, including leg and haltere discs. Similarly, in cultured S2 cells, DSNX3 is critical for Wg secretion, as depletion of Dsnx3 by RNAi in cultured S2 cells also caused a Wg secretion defect. However, both wls and Dvps35 null mutant embryos are lethal, while Dsnx3 null embryos are viable. It is important to mention that Dsnx3 mutant had relatively weaker Wg secretion defects than wls and Dvps35 mutants. We also found that Dsnx3 mutant embryos derived from Dsnx3 homozygous females were normal, arguing that Wg secretion in the Dsnx3 mutant embryos is not defective (data not shown). These data suggest a possibility that mechanisms other than DSNX3-mediated Wls recycling might be involved in retromer-mediated Wg secretion in the embryos, and possibly in other tissues. Further experiments are needed to determine such mechanisms.
Specific role of DSNX3 in the function of the retromer complex in Wg secretion
Another important observation of this work is the specificity of DSNX3 in retromer-mediated Wls recycling. Various studies have demonstrated the importance of SNX1/SNX2 and SNX5/SNX6 in diverse functions of retromer, including retrograde transport of yeast Vps10p, the C. elegans phagocytic receptor CED-1 and the mammalian CI-MPR 30, 31, 33, 53. Therefore, DSNX3 is an atypical SNX molecule involved in the activity of retromer in Wg secretion. We showed that Wg secretion was normal in the absence of either DSNX1 or DSNX6 activity, arguing that DSNX1 and DSNX6 are not essential for Wg secretion. Importantly, we found that the activity of DSNX3 in Wg secretion cannot be simply replaced by overexpression of DSNX1 or DSNX6, further demonstrating that the specific activity of SNX3, rather than SNX1/SNX6, is involved in retromer-mediated Wg secretion. Among SNX molecules, a unique feature of SNX3 is the lack of a BAR domain, which has been shown to be essential for dimerization and membrane binding. The BAR domain preferentially interacts with membranes of positive curvature, drives membrane deformation and generates membrane tubules 55. Thus, one possibility is that a specific structural property of DSNX3 allows it to form a subcomplex with retromer that is distinct from other classic SNX-BAR-retromer complexes. Alternatively, as DSNX3 can interact with Wls, the association of DSNX3 with Wls may allow Wls to load onto the SNX3-retromer complex for retrograde transport. In this case, the function of DSNX3 is very similar to that of Grd19p, the yeast SNX3 that interacts with the cargo iron transporter Fet3p-Ftr1p, and regulates retromer-dependent retrieval of Fet3p-Ftr1p 36, 37, 38. Our results pinpoint that the specificity of retromer function can be attributed to SNX molecules.
During preparation of our manuscript, a paper from Cullen, Basler and Korswagen groups was published, in which they reported the identification of SNX3 as an essential retromer component involved in Wnt secretion 56. Both their and our independent observations reach the same conclusion that SNX3, rather than SNX1 and SNX6, is required for Wg/Wnt secretion and Wls recycling. In their study, they showed that C. elegans devoid of both SNX1 and SNX6 activities exhibited no defect in Wnt secretion 56. Consistent with their data, we also observed no Wg secretion defects in Drosophila Dsnx1 mutants and in Dsnx6 RNAi-depleted cells. Importantly, we showed that overexpression of either DSNX1 or DSNX6 failed to rescue Wg secretion defects observed in Dsnx3 mutant cells. These data suggest qualitative differences between SNX3-mediated retromer activity in Wg/Wnt secretion and SNX1/SNX6-mediated retromer activity in other cellular processes. Moreover, we observed specific interactions of DSNX3 with Wls in co-immunoprecipitation experiments, and such interactions are independent of the PI3P-binding motif in the PX domain of DSNX3, while their experiments failed to detect a Wls-SNX3 interaction. Further studies are needed to define detailed mechanisms by which SNX3 regulates Wls recycling in development.
Materials and Methods
Drosophila stocks
The following fly lines were used in this study: the transgenic lines: UAS-wls-HA, UAS-GFP-2×FYVE and hs-wls-V5 were described previously 12. enGal4, hhGal4 and WgLacZ were as described in FlyBase. vg Q1206-Gal4 UAS-Flp 41 was used to generate mosaic clones mutant for Dsnx31 in developing wings. The following RNAi lines were obtained from the Vienna Drosophila RNAi Center: UAS-Dsnx3RNAi (104494) and UAS-Dsnx6RNAi (24276). The Dsnx1 (CG2774) mutant was generated by imprecise excision of a P element (Bloomington stock number 27118). The mutant has a 1 566-bp deletion starting from 130 bp upstream of the Dsnx1 ATG start codon and ending in the middle of the third exon, and therefore is likely to be a null allele. UAS-Dsnx3-V5, UAS-Dsnx3-GFP, UAS-Dsnx3(-RRY)-GFP, UAS-V5-Dsnx1 and UAS-V5-Dsnx6 transgenic lines were generated in this study.
Generation of the Dsnx3 null allele, Dsnx31
The Dsnx3 (CG6359) gene is located on the right arm of the third chromosome. We identified a P element insertion Dsnx3EY (EY05688) 57 that resides in the first exon of the Dsnx3 gene and is a semi-lethal allele (data not shown). After mobilization of the EY05688 P element, we screened for Dsnx3 deletions and obtained two Dsnx31 null alleles, Dsnx31 and Dsnx32. Dsnx31 had 1 398 bp deletion that included the start codon and all the three exons. Dsnx32 deletion was 1 659 bp and was extended even further in 3′UTR of the Dsnx3 gene. Dsnx31 was used for all of the experiments in this study.
Generation of mutant clones and ectopic expression experiments
Clones of mutant cells were generated by the FLP-FRT method 46 and induced in first/second-instar larvae by heat shock at 37 °C for 2 h. We list the genotypes and conditions used in our analyses below:
(1) Dsnx31 clones marked by absence of GFP:
y w hsp70-flp/+ or Y; FRT82B ubiquitin-GFP/FRT82B Dsnx31 (Figures 1B-1B''', 1C and 1C', 2A-2F', 3E and 3E'; Supplementary information, Figure S2A and S2A').
y w hsp70-flp/+ or Y; WgLacZ/+; FRT82B ubiquitin-GFP/FRT82B Dsnx31 (Figure 1G-1G').
y w hsp70-flp/+ or Y; hs-wls-V5/+;FRT82B ubiquitin-GFP/FRT82B Dsnx31 (Supplementary information, Figure S3B and S3B'').
(2) Wing disc expression of UAS-Dsnx3RNAi using enGal4 or hhGal4:
enGal4/UAS-Dsnx3RNAi (Figures 1D, 1E and 3F), UAS-Dsnx3RNAi/+; hhGal4 (Supplementary information, Figure S3A-S3A''), UAS-Dsnx3RNAi/+; hhGal4/V5-Dsnx1 (Figure 6C-6C''), UAS-Dsnx3RNAi/+; hhGal4/V5-Dsnx6 (Figure 6D-6D'').
(3) Dsnx31 rescue experiments:
enGal4 UAS-GFP/UAS-Dsnx3-V5; Dsnx31/Dsnx31 (Figure 1F)
enGal4/UAS-wls-HA; Dsnx31/Dsnx31 (Figure 3H)
enGal4/Dsnx3(-RRY)-GFP; Dsnx31/Dsnx31 (Figure 5B)
(4) Co-localization of Wls with DSNX3:
enGal4/UAS-Dsnx3-GFP; hs-Wls-V5/+ (Figure 3A-3A'')
(5) Co-localization of DSNX3 with early endosome marker:
enGal4/UAS-Dsnx3-V5; UAS- GFP-2×FYVE/+ (Figure 3B-3B'')
(6) Dsnx31 clones marked by absence of GFP (Figure 6A-6A'')
y w hsp70-flp/+ or Y; FRT40A ubiquitin-GFP/FRT40ADsnx31
(7) Epistatic analysis of DSNX3 and Vps35 (Figure 4D-4D')
en Gal4 /UAS-vps35 RNAi ; UAS-Dsnx3-GFP/+
(8) Generation of Dsnx31 mosaic clones mutant in developing wings using “direct mosaic system” (Supplementary information, Figure S1B and S1B'')
vg Q1206-Gal4 UAS-Flp/+; FRT 82B /FRT 82B Dsnx3 1
(9) Wing disc expression of UAS-Dsnx6RNAi using hhGal4:
hhGal4/UAS-Dsnx6RNAi (Figure 6B-6B'')
Antibodies used for immunostaining, immunoprecipitation and western blotting
Antibody staining of wing imaginal discs or cells was performed using standard protocols. The following primary antibodies were used: mouse anti-Wg (4D4; DSHB), guinea pig anti-Sens 44, rabbit anti-Wls (made in our laboratory according to 14), rabbit anti-GFP Alexa Fluor 488 (Molecular Probe), mouse anti-lacZ (Abmart), mouse anti-V5 (Invitrogen), rabbit anti-V5 (Sigma), mouse anti-EEA1 (BD Biosciences), goat anti-hVps35 (IMGENEX), rabbit anti-Hh 58, mouse anti-Ptc (DSHB) and rabbit anti-Sal (made in our laboratory). The primary antibodies were detected by fluorescence-conjugated secondary antibodies from Jackson ImmunoResearch Laboratories, Inc. Confocal fluorescence imaging was performed with a Zeiss LSM 780 laser-scanning microscope (Carl Zeiss).
The primary antibodies used for immunoprecipitation and western blot analysis were rabbit anti-V5 (Sigma), guinea pig anti-GFP (made in our laboratory), mouse anti-V5 (Invitrogen), mouse anti-Wg (4D4; DSHB), mouse anti-Myc (Invitrogen) and mouse anti-β-actin (Abmart).
Transgenes and dsRNA generation
To generate V5 or GFP C-terminally tagged DSNX3, the complete Dsnx3 coding sequence fragment was amplified from Dsnx3 cDNA clone RE52028 and inserted into pUAST-V5-6xHis and pUAST-EGFP vectors (established by our laboratory), respectively. Dsnx3(-RRY) was generated using Invitrogen's Gene-tailor mutagenesis kit. All three potential PI3P-binding sites, R74, R75 and Y76 were converted to A74, A75 and A76, respectively. After that the mutated Dsnx3 fragment was inserted into pUAST-EGFP vector.
The dsRNAs for Drosophila S2 cells were generated from Dsnx3 cDNA using MEGAscript in vitro transcription kit from Ambion according to the protocol as described (http://flyrnai.org/all_protocols.html).
The RNAi1 dsRNA was generated using the primers:
5′-TAATACGACTCACTATAGGG-ggctgcgaaacgagctggag-3′
5′-TAATACGACTCACTATAGGG-aatccgggcaagcacacacatt-3′
The RNAi2 dsRNA was generated using the primers:
5′-TAATACGACTCACTATAGGG-tttagaccaatctgcccgtc-3′
5′-TAATACGACTCACTATAGGG-tttcctcttaaggattgaaacttt-3′
The control (GFP) dsRNA was generated from GFP cDNA using the primers:
5′-TAATACGACTCACTATAGGG G-acgtaaacggccacaagtt-3′
5′-TAATACGACTCACTATAGGG-tgttctgctggtagtggtcg-3′
Cell culture, transfection, co-immunoprecipitation and western blotting
Drosophila S2 cells were maintained at 25 °C in HyQ SFX-INSECT cell culture medium (Hyclone SH30278.01). Drosophila S2R+ cells were maintained at 25 °C in Schneider's medium (Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco). HeLa cells were maintained in Dulbecco's Modified Eagle Medium (Gibco) supplemented with 10% FBS.
For co-immunoprecipitation experiments, S2 cells were transfected using Effectene transfection reagent (QIAGEN) in 100 mm dishes with 4 μg of total DNA, including pUAST-Dsnx3-GFP, pUAST-Dwls-V5 (or pUAST-Myc-Dvps35) and pArmadillo-Gal4. For HeLa cells' transfection, we used Polyfect transfection reagent (QIAGEN) in the same experimental conditions. Cells were harvested 60 h later and lysed in 900 ml of 150 mM NaCl, 20 mM Tris-HCl pH 7.5, 1.5 % Triton X-100, 1 mM EDTA plus proteinase inhibitors (Roche) on ice for 1 h. After pre-clearance with protein G Sepharose 4 Fast Flow (Amersham) beads, the lysates were incubated with guinea pig anti-GFP (made in our laboratory) for 4 h at 4 °C, and then incubated for additional 2 h in the presence of 20 μl of beads. Beads were washed four times with lysis buffer, and eluted in Laemmli sample buffer. Eluted samples were analyzed by western blotting. The primary antibodies used for western blot are rabbit anti-GFP (Abmart), mouse anti-V5 (Invitrogen) and mouse anti-Myc (Invitrogen).
RNAi and Wg secretion assays
To examine the effect of Dsnx3 RNAi on Wg secretion, 5 × 106 S2R+ cells were seeded in a 60 mm dish and transfected the next day with 2 μg of pMK33-Wg, using Effectene (QIAGEN). At 24 h post transfection, cells were sub-seeded into six-well plates at a concentration of 2 × 106 cells/well in 0.5 ml serum-free media with 45 μg of Dsnx3 dsRNA and incubated at 25 °C for 1 h, then 0.5 ml of serum-containing medium was added. The remaining procedure was as described 10, 12.
References
MacDonald BT, Tamai K, He X . Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009; 17:9–26.
Clevers H . Wnt/beta-catenin signaling in development and disease. Cell 2006; 127:469–480.
Klaus A, Birchmeier W . Wnt signalling and its impact on development and cancer. Nat Rev Cancer 2008; 8:387–398.
Moon RT, Bowerman B, Boutros M, Perrimon N . The promise and perils of Wnt signaling through beta-catenin. Science 2002; 296:1644–1646.
Wodarz A, Nusse R . Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 1998; 14:59–88.
Tabata T, Takei Y . Morphogens, their identification and regulation. Development 2004; 131:703–712.
Vincent JP, Dubois L . Morphogen transport along epithelia, an integrated trafficking problem. Dev Cell 2002; 3:615–623.
Port F, Basler K . Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic 2010; 11:1265–1271.
Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K . Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 2006; 125:509–522.
Bartscherer K, Pelte N, Ingelfinger D, Boutros M . Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 2006; 125:523–533.
Goodman RM, Thombre S, Firtina Z, et al. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development 2006; 133:4901–4911.
Belenkaya TY, Wu Y, Tang X, et al. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell 2008; 14:120–131.
Franch-Marro X, Wendler F, Guidato S, et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol 2008; 10:170–177.
Port F, Kuster M, Herr P, et al. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol 2008; 10:178–185.
Fu J, Ivy Yu HM, Maruyama T, Mirando AJ, Hsu W . Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev Dyn 2011; 240:365–371.
Fu J, Jiang M, Mirando AJ, Yu HM, Hsu W . Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc Natl Acad Sci USA 2009; 106:18598–18603.
Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G . C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell 2008; 14:132–139.
Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC . Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell 2008; 14:140–147.
Coudreuse DY, Roel G, Betist MC, Destree O, Korswagen HC . Wnt gradient formation requires retromer function in Wnt-producing cells. Science 2006; 312:921–924.
Prasad BC, Clark SG . Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development 2006; 133:1757–1766.
Bonifacino JS, Hurley JH . Retromer. Curr Opin Cell Biol 2008; 20:427–436.
Seaman MN . Recycle your receptors with retromer. Trends Cell Biol 2005; 15:68–75.
McGough IJ, Cullen PJ . Recent advances in retromer biology. Traffic 2011; 12:963–971.
Cullen PJ . Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol 2008; 9:574–582.
Seet LF, Hong W . The Phox (PX) domain proteins and membrane traffic. Biochim Biophys Acta 2006; 1761:878–896.
Teasdale RD, Loci D, Houghton F, Karlsson L, Gleeson PA . A large family of endosome-localized proteins related to sorting nexin 1. Biochem J 2001; 358:7–16.
Rojas R, Kametaka S, Haft CR, Bonifacino JS . Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol Cell Biol 2007; 27:1112–1124.
Griffin CT, Trejo J, Magnuson T . Genetic evidence for a mammalian retromer complex containing sorting nexins 1 and 2. Proc Natl Acad Sci USA 2005; 102:15173–15177.
Wassmer T, Attar N, Bujny MV, Oakley J, Traer CJ, Cullen PJ . A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J Cell Sci 2007; 120:45–54.
Chen D, Xiao H, Zhang K, et al. Retromer is required for apoptotic cell clearance by phagocytic receptor recycling. Science 2010; 327:1261–1264.
Seaman MN . Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J Cell Sci 2007; 120:2378–2389.
Seaman MN . Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol 2004; 165:111–122.
Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS . Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol 2004; 165:123–133.
Hong Z, Yang Y, Zhang C, et al. The retromer component SNX6 interacts with dynactin p150(Glued) and mediates endosome-to-TGN transport. Cell Res 2009; 19:1334–1349.
Wassmer T, Attar N, Harterink M, et al. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell 2009; 17:110–122.
Strochlic TI, Setty TG, Sitaram A, Burd CG . Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling. J Cell Biol 2007; 177:115–125.
Zhou CZ, Li de La Sierra-Gallay I, Quevillon-Cheruel S, et al. Crystal structure of the yeast Phox homology (PX) domain protein Grd19p complexed to phosphatidylinositol-3-phosphate. J Biol Chem 2003; 278:50371–50376.
Voos W, Stevens TH . Retrieval of resident late-Golgi membrane proteins from the prevacuolar compartment of Saccharomyces cerevisiae is dependent on the function of Grd19p. J Cell Biol 1998; 140:577–590.
Temkin P, Lauffer B, Jager S, Cimermancic P, Krogan NJ, von Zastrow M . SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol 2011; 13:717–723.
Gong WJ, Golic KG . Ends-out, or replacement, gene targeting in Drosophila. Proc Natl Acad Sci USA 2003; 100:2556–2561.
Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J . pygopus Encodes a nuclear protein essential for wingless/Wnt signaling. Development 2002; 129:4089–4101.
Couso JP, Bishop SA, Martinez Arias A . The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development 1994; 120:621–636.
Neumann CJ, Cohen SM . Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development 1997; 124:871–880.
Nolo R, Abbott LA, Bellen HJ . Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 2000; 102:349–362.
Zecca M, Basler K, Struhl G . Direct and long-range action of a wingless morphogen gradient. Cell 1996; 87:833–844.
Xu T, Rubin GM . Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 1993; 117:1223–1237.
Strigini M, Cohen SM . Wingless gradient formation in the Drosophila wing. Curr Biol 2000; 10:293–300.
Rulifson EJ, Blair SS . Notch regulates wingless expression and is not required for reception of the paracrine wingless signal during wing margin neurogenesis in Drosophila. Development 1995; 121:2813–2824.
Gaullier JM, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H, Aasland R . FYVE fingers bind PtdIns(3)P. Nature 1998; 394:432–433.
Christoforidis S, McBride HM, Burgoyne RD, Zerial M . The Rab5 effector EEA1 is a core component of endosome docking. Nature 1999; 397:621–625.
Xu Y, Seet LF, Hanson B, Hong W . The Phox homology (PX) domain, a new player in phosphoinositide signalling. Biochem J 2001; 360:513–530.
Xu Y, Hortsman H, Seet L, Wong SH, Hong W . SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat Cell Biol 2001; 3:658–666.
Seaman MN, McCaffery JM, Emr SD . A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol 1998; 142:665–681.
Korolchuk VI, Schutz MM . Gomez-Llorente C, et al. Drosophila Vps35 function is necessary for normal endocytic trafficking and actin cytoskeleton organisation. J Cell Sci 2007; 120:4367–4376.
Gallop JL, McMahon HT . BAR domains and membrane curvature: bringing your curves to the BAR. Biochem Soc Symp 2005; 72:223–231.
Harterink M, Port F, Lorenowicz MJ, et al. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat Cell Biol 2011; 13:914–923.
Bellen HJ, Levis RW, Liao G, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 2004; 167:761–781.
Taylor AM, Nakano Y, Mohler J, Ingham PW . Contrasting distributions of patched and hedgehog proteins in the Drosophila embryo. Mech Dev 1993; 42:89–96.
Acknowledgements
We thank Dr Hugo J Bellen (Baylor College of Medicine, USA), Dr Susan Cumberledge (University of Massachusetts, USA) and the Iowa Developmental Studies Hybridoma Bank (IDSHB) for antibodies; the Bloomington Stock Center and the Vienna Drosophila RNAi Center for Drosophila stocks. This work was supported by grants from the National Basic Research Program of China (2011CB943901), the National Natural Science Foundation of China (31030049 and 31071284), the Chinese Academy of Sciences (KSCX2-YW-R-263), and by NIH grants (2R01 GM063891 and 1R01GM087517).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Dsnx3 null alleles generation and mosaic clone analysis in developing wings. (PDF 190 kb)
Supplementary information, Figure S2
Sens expression in wing disc bearing Dsnx31 clones. (PDF 51 kb)
Supplementary information, Figure S3
DSNX3 regulates Wg secretion by controlling Wls stability. (PDF 106 kb)
Supplementary information, Figure S4
Sequences alignment of the PX domains of Snx3 homologues from different species. (PDF 100 kb)
Supplementary information, Figure S5
DSNX3 interacts with Wls and DVps35 independent of the PI3P binding motif of the PX domain. (PDF 81 kb)
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0
About this article
Cite this article
Zhang, P., Wu, Y., Belenkaya, T. et al. SNX3 controls Wingless/Wnt secretion through regulating retromer-dependent recycling of Wntless. Cell Res 21, 1677–1690 (2011). https://doi.org/10.1038/cr.2011.167
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2011.167
Keywords
This article is cited by
-
EGF-SNX3-EGFR axis drives tumor progression and metastasis in triple-negative breast cancers
Oncogene (2022)
-
Sorting nexin 3 induces heart failure via promoting retromer-dependent nuclear trafficking of STAT3
Cell Death & Differentiation (2021)
-
(D620N) VPS35 causes the impairment of Wnt/β-catenin signaling cascade and mitochondrial dysfunction in a PARK17 knockin mouse model
Cell Death & Disease (2020)
-
Retromer stabilization results in neuroprotection in a model of Amyotrophic Lateral Sclerosis
Nature Communications (2020)
-
Structure of the membrane-assembled retromer coat determined by cryo-electron tomography
Nature (2018)