Abstract
Plant protein Trichosanthin (Tk) has been shown in our previous experiments to suppress antigenic response of T cells. Here we explored its inhibitory mechanisms on the proliferation of human Jurkat leukemia T cell triggered by anti-CD3 McAb. By examination of tyrosine phosphorylation of cell lysate, we were able to show that Tk could interfere with the PTK-related activity in the TCR/CD3initiated signal transduction in addition to blocking the phosphorylation of PKC. As shown in our experiment, the expression intensity of ZAP-70, a kind of protein tyrosine kinase, was not changed but its phosphorylation could be inhibited. When physical link between CD3 ζchain and ZAP-70 was further examined by using coimmunoprecipitation after pluse-treatment of the cell line with Tk, the anti-CD3 McAb-induced recruitment of ZAP-70 to CD3ζchain was observed to be blocked in some extent. This may account for, at least in part, how Trichosanthin was able to inhibit the TCR-triggered T cell proliferation.
Similar content being viewed by others
Introduction
Trichosanthin (Tk), a plant protein isolated from a Chinese medicinal herb Trichosanthes kirilowii Maximovich, has been used as an abortifacient for several decades in China. Tk is a linear polypeptide of 247 amino acid residues, the encoding gene of which has been cloned and sequenced. As a ribosome inactivating protein (RIP), Tk is believed to exert its biological activity in inducing cytotoxicity1. However, at lower doses, Tk could inhibit HIV replication and suppress in vitro immune responses of both mouse and human lymphocytes2, 3. Several reports have been released dealing with treatment of AIDS with Tk in clinical trails 4. And for some Tk preparations, the HIV inhibition has been reported to be quite free from their toxic property5. The mechanisms of this intriguing phenomenon remains puzzle. In our previous study, we showed that the Tk-induced hyporeaction is irrelevant to direct cytolysis in the dose range used. In the lymphoproliferation to purified protein derivative (PPD), tetanus toxoid (TT) and anti-CD3 McAb, Tk induced suppression with great extent. However, only a little Tk's immunosuppression was observed in PMA-induced proliferation system in presence of Tk. Since the differential effects of PMA and other stimuli in activation of T cells mainly reflect that they use distinguishable signaling pathways, we propose that Tk inhibits T cell activation might be via interference of signal transduction.
Triggering of TCR/CD3 complex activates the complex-coupled intracellular protein tyrosine kinases (PTKs) to phosphorylated phospholipase C, PLCγ1, and the guanine nucleotide binding protein p21ras. The src family PTKs p56lck and p59fyn are thought to be responsible for this early phosphorylation event. The tyrosine phosphorylation of the CD3 andζchain leads to recruitment of the ZAP-70 and Syk tyrosine kinase by binding of their two SH2 domains to the tyrosine-phophorylated immunorecepter tyrosine-activation motif (ITAM) of CD3ζchain. Subsequently, the activated ZAP-70 phosphorylates PLCγ16.
In our previous study, we were able to show that Tk could inhibit soluble or alloantigen-induced lymphoproliferation by a CD8+ T cell-mediated pathway, and this was genetically controlled by HLA-DQ genes3, 7. In this paper, we examined the ZAP-70 and CD3ζchain in human T cells after Tk treatment. Our results indicated that, under the influence of Tk, the intracellular pathway of signal transduction initiated by triggering of TCR/CD3 complex was interrupted in an essential step, the recruitment of ZAP-70 to CD3ζchain.
Materials and Methods
Cell culture
Jurkat leukemia T cell lines were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated newborn calf serum, 2 mM L- glutamine, 100U/ml Penicillin and 100 μg/ml Streptomycin. The cells were washed three times with PBS before use. 1 x 107/ml cells were pulsed with 10 μg/ml Trichosanthin (Jingshan Pharmaceutical, Shanghai) for 3 h at 37°C and washed three times with ice-cold PBS. The Tk-pulsed cells were stimulated with anti-CD3 McAb (ATCC, USA) at a dose 10 μg/ml for 5 min and crosslinked with anti-mouse antibody for 5 min. When PTK was assayed for quantitative expression, Tk was subjected to be co -cultured with cells for 24 h.
Immunoblotting
Cells were lysed in lysis buffer (20 mM Tris-HC1 pH 7. 5, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% NP-40, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 100 mM NaF, 10 mM Na4P2O7, 1 mM Na3VO4) for 30 min at ice-bath. Lysates were precleared by centrifugation at 12,000 × g for 10 min at 4 °C and the postnuclear supernatants were collected. Protein concentration was carefully measured using the Bradford assay8 and the samples were adjusted to be able to load 50 μg on each lane for electrophoresis. Proteins were separated by 10% SDS-PAGE and transferred to nitrocellulus membrane (Schleicher and Schuell, USA) in 20 mM Tris, 150 mM glycine and 20% methanol at 250 mA for 2 h6. The membrane were saturated in blocking buffer (0.1% Tween-20 TBS) with blocking reagent (Amersham, UK) for 2 h at room temperature and then probed with 1:1000 antiphosphotyrosine antibody (6G9, Life Technologies) or 1:1000 anti-ZAP-70 antisera (kindly gifted by Dr. Weiss, A., UCSF), followed by incubation with 1:1000 peroxidase-conjugated rabbit anti-mouse Ig for 1 h, then washed and developed by enhanced chemiluminesence (ECL, Amersham, UK) according to the manufacturer's instructions.
Immunoprecipitation
107cells were stimulated with saturated amounts of anti-CD3 McAb as described above, then pelleted and lysed in lysis buffer. The cells were precleared with protein A sepharose CL-4B 50% w/v slurry. ZAP-70 or CD3ζchain was immunoprecipitated with anti ZAP-70 antibody or anti-CD3ζchain antibody (Santa Cruz, USA). Immunoprecipitaed protein were washed, subjected to 10% SDS-PAGE, and immunoblotted with antiphosphotyrosine antibody or anti-ZAP-70 antibody.
Results and discussion
Inhibition of CD3 McAb-induced T cell proliferation by Tk
We described previously that the soluble antigen-induced lymphoproliferation could be strongly inhibited by Tk in vitro3. When assayed by proliferation of Jurkat leukemia T cells, there was a significant difference observed between the groups with or without stimulation of anti-CD3 antibody. However, if T cells were pulsetreated with Trichosanthin in advance, the difference was disappeared (Fig 1). It suggested that TCR/CD3 complex-initiated signaling could be interrupted by Tk treatment in some way. For a further assessment of the mechanism underlying, some essential links of the pathways in signal transduction have been examined under the influence of Tk. The mobilization of cytosolic calcium driven by combining of TCR/CD3 with anti-CD3 McAb, for example, was firstly observed to be blocked in our experiment (data have been separately submitted for publication). And it has been shown that the translocation of PKCαand the autophosphorylation of PKC was also inhibited by Tk treatment9. These implicates that, in addition to PKC, protein tyrosine phosphorylation may need to be examined.
The profile of antiphosphotyrosine after Tk treatment
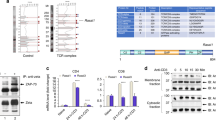
Protein tyrosine phosphorylation was critical upon T cell activation. Triggering of TCR/CD3 complex induces the phosphorylation of many PTK-related signal transduction events in T cells10. As shown on the line 3 of Fig 2, the anti-CD3 McAb-activated T cells showed dark bands when their cell lysates were analysed by probing with antiphosphotyrosine antibody. However, if T cells were pretreated with Trichosanthin and then pulsed with anti-CD3 McAb, only some of tyrosine phosphorylation bands could be detected (line 2), which were much weaker than those not only in group treated with CD3 McAb (line 3) but also in the T cells without Tk treatment (line 1). It implicates that the poor tyrosine phosphorylation was resulted from Tk pretreatment.
Inhibition of ZAP-70 for tyrosine phosphorylation but not for expression by Tk treatment
To know what kind of phosphArylation-related protein or kinase was effected by Tk, we examined the activation of ZAP-70 kinase by using immunoprecipitation and immunoblotting assay. ZAP-70 was a protein tyrosine kinase which functions at the downstream of CD3ζchain activation12. When TCR/CD3 complex was triggered, the change of structure conformation initiated phosphorylation ofζchain. Phosphorylated CD3ζchain recruited ZAP-70 kinase by interaction between two ITAMs ofζchain and SH2 domain of the kinase12. As first step, the expression intensity of ZAP-70 was determined. Jurkat leukemia T cells were co-cultured with Trichosanthin for 24 h. Cell lysate was dissolved by 10% SDS-PAGE and probed with anti-ZAP-70 antibody. As shown in Fig 3A, the band of 70 kD ZAP in the Tk cultured group shows same darkness as that in control, suggesting that the expression of ZAP-70 were not affected by Trichosanthin. Then, we examined the phosphorylation of ZAP-70. We immunoprecipitated ZAP-70 from cell lysate with anti-ZAP-70 antibody. As detected with antiphosphotyrosine antibody for the phosphorylated tyrosine in ZAP-70 immunoprecipitant, the darkness of phosphotyrosine band in Tk-treated experiment group was weaker than that in normal cells (Fig 3B). The results suggested a decreasing phosphotyrosine after Trichosanthin treatment.
Dissociation between CD3ζchain and ZAP-70 after Tk treatment
Jurkat leukemia T cells were pulsed with Trichosanthin and then stimulated with anti-CD3 McAb for 5 mim. CD3ζchain were immunoprecipitated from cell lysate with rabbit anti-CD3ζchain antibody. This immunoprecipitant was then probed with anti-ZAP-70 antisera. As a results, the ZAP-70 protein band was visualized much less obviously in the CD3ζchain immunoprecipitant of Tk-pulsed cells when compared to that of normal cells (Fig 4). It suggested that the recruitment of ZAP-70 to CD3ζchain was damaged by Trichosanthin pretreatment in TCR/CD3 complex-initiated signal transduction.
Usually, the studies on RIPs function of Trichosanthin were carried out by cell lysate. Here, we examined its function on intact T cells using the Tk at a dose range much lower than that causing cytotoxicity. According to our results, Tk was capable of blocking the physical link between CD3ζchain and ZAP-70, leading to a dysfunction of T cells. Obviously, the exact mechanism needs to be explored by further experiments. There are some similar studies with regard to the defective TCR-related signaling caused by external agents13, 14. How the extracellular Tk could exert its activity on the intracellular biochemical events of T cells was still not clear although there was evidence presented in our work that Tk was able to enter the antigen-presenting cells by exocytosis15.
References
Zhang JS, Lui WY . The mechanism of action of Trichosanthin on eukaryotic ribosomes-RNA N- glychsidase activity of the cytotoxin. Nucleic Acid Research 1992; 20:1271–5.
Yeh M, Chi YY, Shen RY, Lin GM . The study of in vitro and in vivo response to Trichosanthin in mouse. Acta Biologiae Experimentalis Sinica 1986; 19:81–6.
Chou KY, Zhang DQ, Xue BH . Human immune suppression is inducible by trichosanthin via CD8 cell-mediated pathway. Cell Research 1994; 4:17–29.
Mayer RA, Sergios PA, Coonan K, O'Brien L . Trichosanthin treatment of HIV-induced immune dysfunction. Eur J Clin Invest 1992; 22:113–22.
Lee-Huang S, Huang PL, Kung HF, Li BQ, Huang PL, Huang PH, Huang HI, Chen HC . TAP29: an anti-human immunodeficiency virus protein from Trichosanthin kirolowii that is non-toxic to intact cells. Proc Natl Acad Sci, USA 1991; 88:6570–4.
Rudd CE, Janssen O, Cai YC, daSilva AJ, Raab M, Prasad KV . Two-step TCR ζ/CD3-CD4 and CD28 signaling in T cells SH2/SH3 domains, protein-tyrosine and lipid kinases. Immunol Today 1994; 15:225–34.
Chou KY, Chan M, Bias WB . Differential expression of the down-regulatory function of CD8 cells in Trichosanthin-induced immunosuppression and its genetic control in humans. Eur J Immunogenetics 1996; 23:29–40.
Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W . Current protocols in immunology, by John Wiley and Sons, Inc.. 1994; 11.0.1-11.5.5.
Hong J, Lu PH, Shen ZY, Fu SL, Chou KY . Trichosanthin suppresses the anti-CD3 mAb-induced activation of PKCα in lymphocytes, Shanghai J Immunol 1997; 17:327–9.
June CH, Fletcher MC, Ledbetter JA, Weiss A . Inhibition of tyrosine phosphorylation prevents T cell receptor-mediated signal transduction. Proc Natl Acad Sci USA 1990; 87:7722–6.
Chan AC, Lwashima M, Turck CW . ZAP-70: 70 kD protein-tyrosine that associates with the TCRζchain. Cell 1992; 71:649–62.
Romeo C, Amiot M, Seed B . Sequence requirements for induction of cytolysis by the T cell antigen/Fc receptorζchain. Cell 1992; 68:889–97.
Migita K, Eguchi K, Kawabe Y, Tsukada T, Ichinose Y, Nagataki S . Defective TCR-mediated signaling in anergic T cells. J Immunol 1995; 155:5038–87.
Kanner SB, Haffer O . HIV-1 down-regulates CD4 costimulation of TCR/CD3 -directed tyrosine phosphorylation through CD4/p56lck dissociation. J Immunol 1995; 154:2996–3005.
Li NL, Zhen ZX, Shen BH, Chou KY . Modulation of T cell-mediated immune responses by Trichosanthin via antigen processing and presentation. Acta Biol Exp Sinica 1997; 30:165–71.
Acknowledgements
We thank Prof. Arthur Weiss of University of California, San Francisco, for kindly gifted with anti-ZAP-70 antisera. This study was supported by the National Natural Science Foundation of China (grant No: 39330190)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, J., Fu, S., Shen, Z. et al. Trichosanthin inhibits T cell activation by interfering with the recruitment of ZAP-70 to CD3 ζ chain. Cell Res 8, 33–39 (1998). https://doi.org/10.1038/cr.1998.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.1998.4
Keywords
This article is cited by
-
Induced apoptotic action of recombinant trichosanthin in human stomach adenocarcinoma MCG803 cells
Molecular Biology Reports (2009)